Individual and Additive Effects of Insecticide and Mating Disruption in Integrated Management of Navel Orangeworm in Almonds
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Plot Arrangement
2.2. Evaluation of Navel Orangeworm Damage
2.3. Individual and Additive Effects of Mating Disruption and Insecticide (2006 and 2007)
2.4. Mating Disruption Dispensers per Hectare (2008–2011)
2.5. Mating Disruption Active Ingredient Per Hectare (2012–2014)
2.6. Time of Start of Mating Disruption (2015)
2.7. Data Analysis
3. Results
3.1. Comparison of Huller and Windrow Samples
3.2. Comparison of Overall Damage over the 10-Year Study
3.3. Comparison of Damage in Specific Experiments
- →
- For the experiment from 2008 to 2011, there were numerical differences among all levels of the factorial comparison of 2.5 or 5 mating disruption dispensers with or without insecticide (Table 5). The GLMM analysis of fixed effects revealed significant effects due to insecticide (F1,13.75 = 11.34, p = 0.0047), not quite significant effects due to dispenser density (F1,13.76 = 3.33, p = 0.0896), and no significant interaction (F1,24.59 = 0.42, p = 0.52).
- →
- For the experiment from 2012 to 2014, percent navel orangeworm damage in mating disruption plots was, respectively, 1.6 ± 0.57 and 1.1 ± 0.39 for half and full label concentration mating disruption from 5 dispensers per ha in the absence of insecticide treatments. In the presence of insecticide, these figures were, respectively, 0.9 ± 0.61 and 0.7 ± 0.38 percent damage. There were no significant effects from either insecticide or mating disruption release rate, and the interaction was not significant (p > 0.1).
3.4. Relationship Between Damage in Early-Harvested Nonpareil and a Later Harvested Pollinizer Variety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardé, R.T.; Minks, A.K. Control of moth pests by mating disruption: Successes and constraints. Annu. Rev. Entomol. 1995, 40, 559–585. [Google Scholar] [CrossRef]
- Miller, J.R.; Gut, L.J. Mating disruption for the 21st century: Matching technology with mechanism. Environ. Entomol. 2015, 44, 427–453. [Google Scholar] [CrossRef]
- Abd El-Ghany, N.M. Semiochemicals for controlling insect pests. J. Plant Prot. Res. 2019, 59, 1–11. [Google Scholar]
- Evenden, M. Mating disruption of moth pests in integrated pest management. In Pheromone Communication in Moths. Evolution, Behavior, and Application; Allison, J.D., Carde, R.T., Eds.; University of California Press: Oakland, CA, USA, 2016; pp. 365–393. [Google Scholar]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex pheromones and their impact on pest management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef]
- Peterson, R.K.D.; Higley, L.G.; Pedigo, L.P. Whatever Happened to IPM? Am. Entomol. 2018, 64, 146–150. [Google Scholar] [CrossRef]
- UC ANR Statewide IPM Program. What Is Integrated Pest Management. 2020, Describes and Defines IPM. Available online: https://www2.ipm.ucanr.edu/What-is-IPM/ (accessed on 31 May 2020).
- Wilson, H.; Daane, K.M. Review of ecologically-based pest management in California vineyards. Insects 2017, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Lucchi, A.; Suma, P.; Ladurner, E.; Iodice, A.; Savino, F.; Ricciardi, R.; Cosci, F.; Marchesini, E.; Conte, G.; Benelli, G. Managing the vine mealybug, Planococcus ficus, through pheromone-mediated mating disruption. Environ. Sci. Pollut. Res. 2019, 26, 10708–10718. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, C.R.; Polk, D.; Holdcraft, R.; Koppenhöfer, A.M. Long-term evaluation of field-wide oriental beetle (Col., Scarabaeidae) mating disruption in blueberries using female-mimic pheromone lures. J. Appl. Entomol. 2014, 138, 120–132. [Google Scholar] [CrossRef]
- Arakaki, N.; Nagayama, A.; Kobayashi, A.; Hokama, Y.; Sadoyama, Y.; Mogi, N.; Kishita, M.; Adaniya, K.; Ueda, K.; Higa, M.; et al. Mating disruption for control of Melanotus okinawensis (Coleoptera: Elateridae) with synthetic sex pheromone. J. Econ. Entomol. 2008, 101, 1568–1574. [Google Scholar] [CrossRef]
- Mahroof, R.M.; Phillips, T.W. Mating disruption of Lasioderma serricorne (Coleoptera: Anobiidae) in stored product habitats using the synthetic pheromone serricornin. J. Appl. Entomol. 2014, 138, 378–386. [Google Scholar] [CrossRef]
- Martini, A.; Baldassari, N.; Baronio, P.; Anderbrant, O.; Hedenström, E.; Högberg, H.E.; Rocchetta, G. Mating disruption of the pine sawfly Neodiprion sertifer (Hymenoptera: Diprionidae) in isolated pine stands. Agric. For. Entomol. 2002, 4, 195–201. [Google Scholar] [CrossRef]
- McGhee, P.S.; Miller, J.R.; Thomson, D.R.; Gut, L.J. Optimizing aerosol dispensers for mating disruption of codling moth, Cydia pomonella L. J. Chem. Ecol. 2016, 42, 612–616. [Google Scholar] [CrossRef]
- Burks, C.S.; Thomson, D.R. Optimizing efficiency of aerosol mating disruption for navel orangeworm (Lepidoptera: Pyralidae). J. Econ. Entomol. 2019, 112, 763–771. [Google Scholar] [CrossRef]
- Wilson, H.; Burks, C.S.; Reger, J.E.; Wenger, J.A. Biology and management of navel orangeworm (Lepidoptera: Pyralidae) in California. J. Integr. Pest. Manag. 2020, 11, 25. [Google Scholar] [CrossRef]
- Wade, W.H. Biology of the navel orangeworm, Paramyelois transitella (Walker), on almonds and walnuts in northern California. Hilgardia 1961, 31, 129–171. [Google Scholar] [CrossRef][Green Version]
- Andrews, K.L.; Barnes, M.M. Invasion of pistachio orchards by navel orangeworm moths from almond orchards. Environ. Entomol. 1982, 11, 278–279. [Google Scholar] [CrossRef]
- Higbee, B.S.; Siegel, J.P. New navel orangeworm sanitation standards could reduce almond damage. Calif. Agric. 2009, 63, 24–28. [Google Scholar] [CrossRef]
- Sappington, T.W.; Burks, C.S. Patterns of flight behavior and capacity of unmated navel orangeworm adults (Lepidoptera: Pyralidae) related to age, gender, and wing size. Environ. Entomol. 2014, 43, 696–705. [Google Scholar] [CrossRef][Green Version]
- Hamby, K.; Gao, L.W.; Lampinen, B.; Gradziel, T.; Zalom, F. Hull split date and shell seal in relation to navel orangeworm (Lepidoptera: Pyralidae) infestation of almonds. J. Econ. Entomol. 2011, 104, 965–969. [Google Scholar] [CrossRef]
- Rosenheim, J.A.; Higbee, B.S.; Akerman, J.; Meisner, M.H. Ecoinformatics can infer causal effects of crop variety on insect attack by capitalizing on ‘pseudoexperiments’ created when different crop varieties are interspersed: A case study in almonds. J. Econ. Entomol. 2017, 110, 2647–2654. [Google Scholar] [CrossRef] [PubMed]
- Rice, R.E. A comparison of monitoring techniques for the navel orangeworm. J. Econ. Entomol. 1976, 69, 25–28. [Google Scholar] [CrossRef]
- Sanderson, J.P.; Barnes, M.M.; Seaman, W.S. Synthesis and validation of a degree-day model for navel orangeworm (Lepidoptera: Pyralidae) development in California almond orchards. Environ. Entomol. 1989, 18, 612–617. [Google Scholar] [CrossRef]
- Sanderson, J.P.; Barnes, M.M. Ability of egg traps to detect the onsect of second-generation navel orangeworm (Lepidoptera: Pyralidae) moth activity in almond orchards. J. Econ. Entomol. 1990, 83, 570–573. [Google Scholar] [CrossRef]
- Pathak, T.B.; Maskey, M.L.; Rijal, J.P. Impact of climate change on navel orangeworm, a major pest of tree nuts in California. Sci. Total Environ. 2020, 755, 142657. [Google Scholar] [CrossRef]
- Siegel, J.P.; Bas Kuenen, L.P.S.; Ledbetter, C. Variable development rate and survival of navel orangeworm (Lepidoptera: Pyralidae) on wheat bran diet and almonds. J. Econ. Entomol. 2010, 103, 1250–1257. [Google Scholar] [CrossRef]
- Kuenen, L.P.S.; Siegel, J.P. Protracted emergence of overwintering Amyelois transitella (Lepidoptera: Pyralidae) from pistachios and almonds in California. Environ. Entomol. 2010, 39, 1059–1067. [Google Scholar] [CrossRef]
- Higbee, B.S.; Siegel, J.P. Field efficacy and application timing of methoxyfenozide, a reduced-risk treatment for control of navel orangeworm (Lepidoptera: Pyralidae) in almond. J. Econ. Entomol. 2012, 105, 1702–1711. [Google Scholar] [CrossRef]
- Siegel, J.P.; Strmiska, M.M.; Niederholzer, F.J.A.; Giles, D.K.; Walse, S.S. Evaluating insecticide coverage in almond and pistachio for control of navel orangeworm (Amyelois transitella) (Lepidoptera: Pyralidae). Pest. Manag. Sci. 2019, 75, 1435–1442. [Google Scholar] [CrossRef]
- Demkovich, M.R.; Siegel, J.P.; Walse, S.S.; Berenbaum, M.R. Impact of agricultural adjuvants on the toxicity of the diamide insecticides chlorantraniliprole and flubendiamide on different life stages of the navel orangeworm (Amyelois transitella). J. Pest. Sci. 2018, 91, 1127–1136. [Google Scholar] [CrossRef]
- Bagchi, V.A.; Siegel, J.P.; Demkovich, M.R.; Zehr, L.N.; Berenbaum, M.R. Impact of pesticide resistance on toxicity and tolerance of hostplant phytochemicals in Amyelois transitella (Lepidoptera: Pyralidae). J. Insect Sci. 2016, 16, 62. [Google Scholar] [CrossRef][Green Version]
- Demkovich, M.; Siegel, J.P.; Higbee, B.S.; Berenbaum, M.R. Mechanism of resistance acquisition and potential associated fitness costs in Amyelois transitella (Lepidoptera: Pyralidae) exposed to pyrethroid insecticides. Environ. Entomol. 2015, 44, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Reger, J.; Wenger, J.; Brar, G.; Burks, C.; Willson, H. Evaluating response of mass-reared and irradiated navel orangeworm, Amyelois transitella (Lepidoptera: Pyralidae) to crude female pheromone extract. Insects 2020, 11, 703. [Google Scholar] [CrossRef]
- Haviland, D.R.; Rijal, J.P.; Rill, S.M.; Higbee, B.S.; Gordon, C.A.; Burks, C.S. Management of navel orangeworm (Lepidoptera: Pyralidae) using four commercial mating disruption systems in California almonds. J. Econ. Entomol. 2020. [Google Scholar] [CrossRef]
- Niu, G.; Pollock, H.S.; Lawrance, A.; Siegel, J.P.; Berenbaum, M.R. Effects of a naturally occurring and a synthetic synergist on toxicity of three insecticides and a phytochemical to navel orangeworm (Lepidoptera: Pyralidae). J. Econ. Entomol. 2012, 105, 410–417. [Google Scholar] [CrossRef]
- Niu, G.; Rupasinghe, S.G.; Zangerl, A.R.; Siegel, J.P.; Schuler, M.A.; Berenbaum, M.R. A substrate-specific cytochrome P450 monooxygenase, CYP6AB11, from the polyphagous navel orangeworm (Amyelois transitella). Insect Biochem. Mol. Biol. 2011, 41, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Hamby, K.A.; Nicola, N.L.; Niederholzer, F.J.A.; Zalom, F.G. Timing spring insecticide applications to target both Amyelois transitella (Lepidoptera: Pyralidae) and Anarsia lineatella (Lepidoptera: Gelechiidae) in almond orchards. J. Econ. Entomol. 2015, 108, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Rosenheim, J.A.; Higbee, B.S.; Ackerman, J.; Meisner, M. Predicting nut damage at harvest using different in-season density estimates of Amyelois transitella: Analysis of data from commercial almond production. J. Econ. Entomol. 2017, 110, 2692–2698. [Google Scholar] [CrossRef]
- Shorey, H.H.; Gerber, R.G. Use of puffers for disruption of sex pheromone communication among navel orangeworm moth (Lepidoptera: Pyralidae) in almonds, pistachios, and walnuts. Environ. Entomol. 1996, 25, 1154–1157. [Google Scholar] [CrossRef]
- Higbee, B.S.; Burks, C.S. Effects of mating disruption treatments on navel orangeworm (Lepidoptera: Pyralidae) sexual communication and damage in almonds and pistachios. J. Econ. Entomol. 2008, 101, 1633–1642. [Google Scholar] [CrossRef]
- Burks, C.S.; Higbee, B.S.; Beck, J.J. Traps and attractants for monitoring navel orangeworm (Lepidoptera: Pyralidae) in the presence of mating disruption. J. Econ. Entomol. 2020, 113, 1270–1278. [Google Scholar] [CrossRef]
- Higbee, B.S.; Burks, C.S.; Cardé, R.T. Mating disruption of the navel orangeworm (Lepidoptera: Pyralidae) using widely spaced aerosol dispensers: Is the pheromone blend the most efficacious disruptant? J. Econ. Entomol. 2017, 110, 2056–2061. [Google Scholar] [CrossRef]
- Benelli, G.; Lucchi, A.; Thomson, D.; Ioriatti, C. Sex pheromone aerosol devices for mating disruption: Challenges for a brighter future. Insects 2019, 10, 308. [Google Scholar] [CrossRef]
- Burks, C.S.; Thomson, D.R. Factors affecting disruption of navel orangeworm (Lepidoptera: Pyralidae) using aerosol dispensers. J. Econ. Entomol. 2020, 113, 1290–1298. [Google Scholar] [CrossRef]
- USDA Agricultural Research Service. An areawide control program for navel orangeworm. 2012. Available online: https://portal.nifa.usda.gov/web/crisprojectpages/0412631-an-areawide-control-program-for-navel-orangeworm.html (accessed on 13 December 2020).
- Wikipedia contributors. Section (United States Land Surveying). Wikipedia, The Free Encyclopedia. 2020. Available online: https://en.wikipedia.org/w/index.php?title=Section(United_States_land_surveying)&oldid=992229483 (accessed on 13 December 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- SAS Institute Inc. SAS/STAT 14.2 User’s Guide; SAS Institute, Inc.: Cary, NC, USA, 2016. [Google Scholar]
- Kenward, M.G.; Roger, J.H. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997, 53, 983–997. [Google Scholar] [CrossRef]
- University of California State-Wide, I.P.M. Degree-Days: Navel Orangeworm. 2021. Available online: http://www.ipm.ucdavis.edu/calludt.cgi/DDMODEL?MODEL=NOW (accessed on 6 February 2021).
- CIMIS. California Irrigation Management Information System. 2021. Available online: https://cimis.water.ca.gov/Default.aspx (accessed on 6 February 2021).
- Gut, L.J.; Stelinski, L.L.; Thomson, D.R.; Miller, J.R. Behaviour-modifying chemicals: Prospect and constraints in IPM. In Integrated Pest. Management: Potential, Constraints and Challenges; Koul, O., Dhaliwal, G.S., Cuperus, G.W., Eds.; CAB International: Wallingford, UK, 2004; pp. 73–121. [Google Scholar]
- Almond Board of California. Almond Orchard 2025 Goals. 2019, p. 8. Available online: http://www.almonds.com/sites/default/files/GoalsRoadmap_2019_web.pdf (accessed on 6 December 2020).
- Beck, J.J.; Higbee, B.S. Volatile Blends and the Effects Thereof on the Navel Orangeworm Moth. U.S. Patent No 9,220,261, 29 December 2015. [Google Scholar]
- Beck, J.J.; Light, D.M.; Gee, W.S. Electrophysiological responses of male and female Amyelois transitella antennae to pistachio and almond host plant volatiles. Entomol. Exp. Appl. 2014, 153, 217–230. [Google Scholar] [CrossRef]
- Burks, C.S. Combination phenyl propionate/pheromone traps for monitoring navel orangeworm (Lepidoptera: Pyralidae) in almonds in the vicinity of mating disruption. J. Econ. Entomol. 2017, 110, 438–446. [Google Scholar] [CrossRef]
- Lima, C.F.M.; Leandro, M.E.D.d.A.; Valero, C.; Coronel, L.C.P.; Bazzo, C.O.G. Automatic detection and monitoring of insect pests—A review. Agriculture 2020, 10, 161. [Google Scholar] [CrossRef]
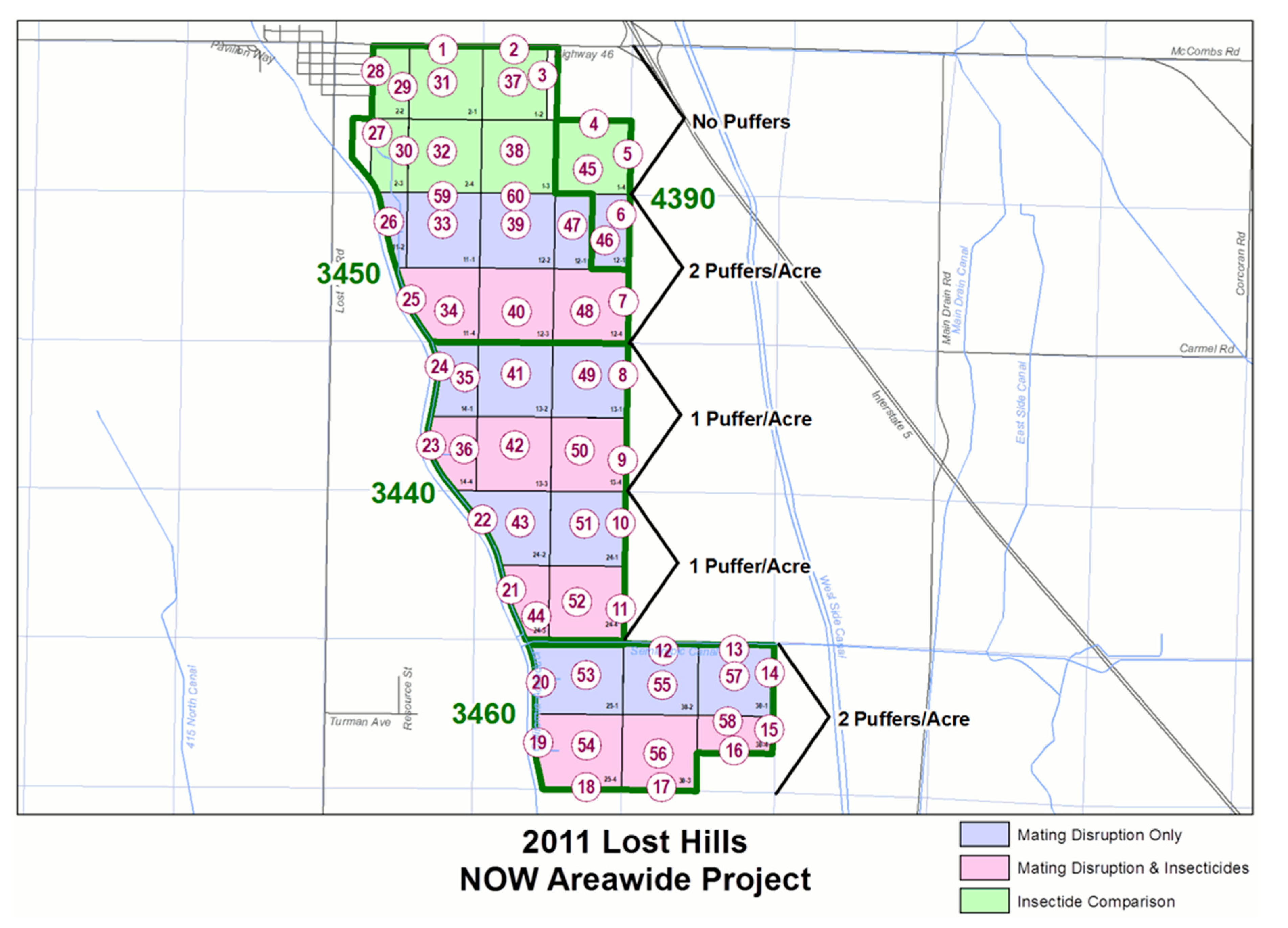
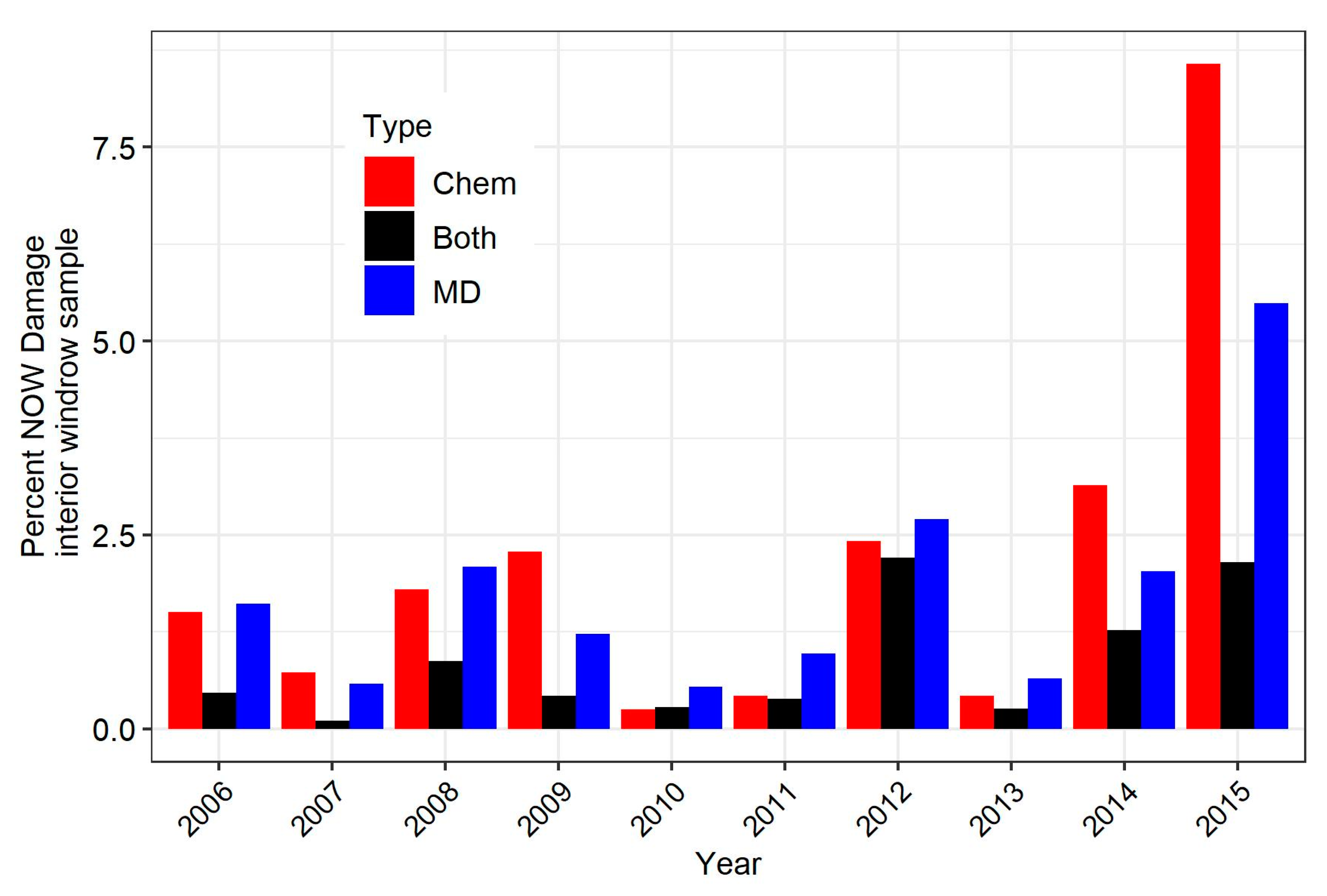
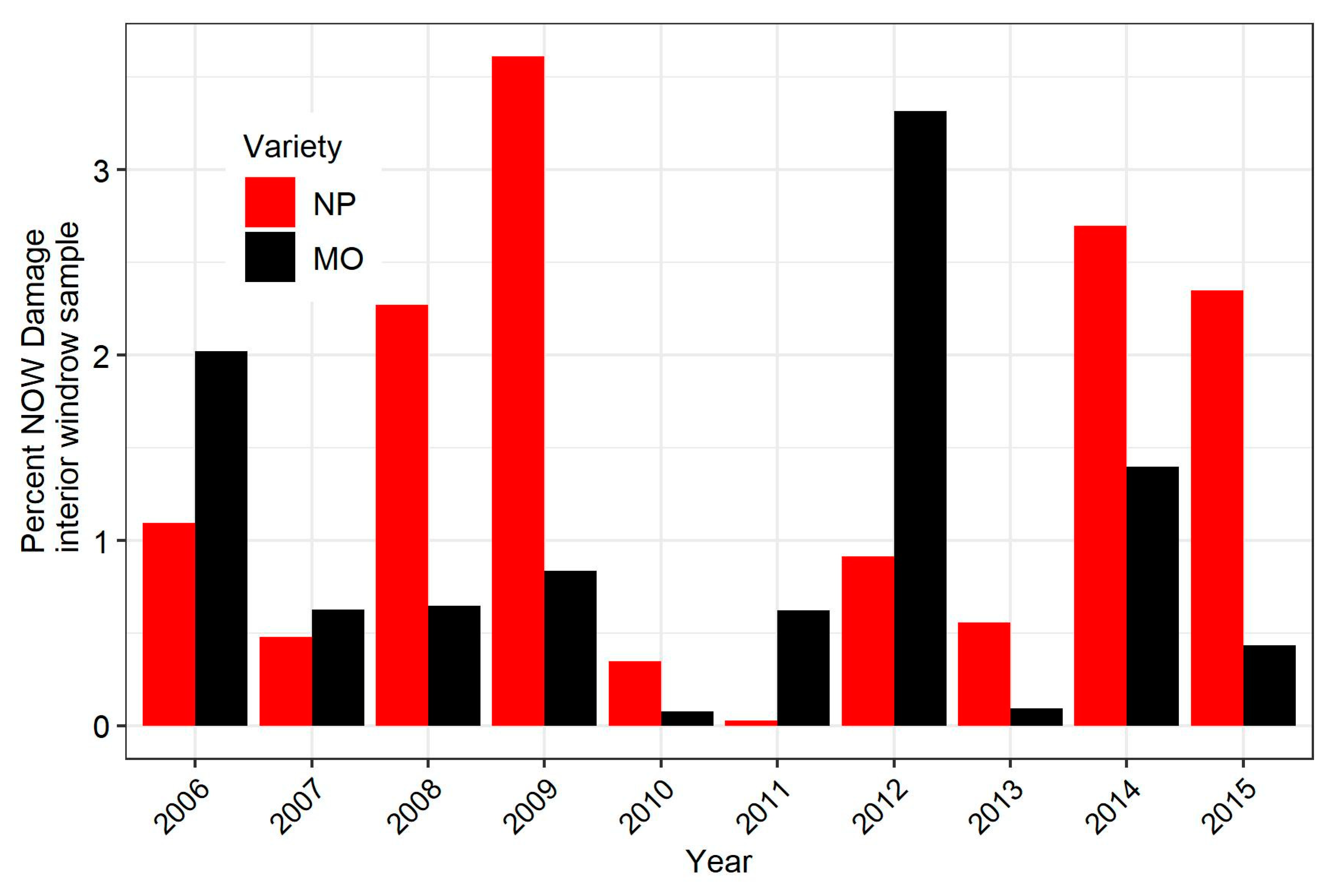
| Treatment Type | Huller | Windrow (Interior) | Windrow (Edge) |
|---|---|---|---|
| Mating disruption only | 0.61a | 0.91a | 2.22a |
| Insecticide only | 0.56a | 0.73a | 1.58ab |
| Both | 0.3b | 0.37b | 1.01b |
| Windrow (Interior) | Windrow (Edge) | |
|---|---|---|
| Intercept | −0.01 | 0.69 *** |
| Slope | 1.42 *** | 1.75 *** |
| Adjusted r2 | 0.69 | 0.59 |
| Treatment | Percent NOW Damage |
|---|---|
| Insecticide only | 1.9 ± 0.47a |
| Mating disruption only | 1.8 ± 0.49a |
| Both insecticide and mating disruption | 1.0 ± 0.18b |
| Treatment | n (Replicate Block by Year) | Percent NOW Damage |
|---|---|---|
| Insecticide only | 9 | 1.0 ± 0.24a |
| Mating disruption only | 4 | 1.1 ± 0.32a |
| Both insecticide and mating disruption | 3 | 0.4 ± 0.13b |
| Mating Disruption Dispensers per ha | Without Insecticide | With Insecticide |
|---|---|---|
| 2.5 | 1.67 ± 0.64 | 0.64 ± 0.20 |
| 5 | 0.93 ± 0.22 | 0.33 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higbee, B.S.; Burks, C.S. Individual and Additive Effects of Insecticide and Mating Disruption in Integrated Management of Navel Orangeworm in Almonds. Insects 2021, 12, 188. https://doi.org/10.3390/insects12020188
Higbee BS, Burks CS. Individual and Additive Effects of Insecticide and Mating Disruption in Integrated Management of Navel Orangeworm in Almonds. Insects. 2021; 12(2):188. https://doi.org/10.3390/insects12020188
Chicago/Turabian StyleHigbee, Bradley S., and Charles S. Burks. 2021. "Individual and Additive Effects of Insecticide and Mating Disruption in Integrated Management of Navel Orangeworm in Almonds" Insects 12, no. 2: 188. https://doi.org/10.3390/insects12020188
APA StyleHigbee, B. S., & Burks, C. S. (2021). Individual and Additive Effects of Insecticide and Mating Disruption in Integrated Management of Navel Orangeworm in Almonds. Insects, 12(2), 188. https://doi.org/10.3390/insects12020188







