Vector Transmission of Tomato Yellow Leaf Curl Thailand Virus by the Whitefly Bemisia tabaci: Circulative or Propagative?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect, Virus, and Plants
2.2. Virus-Infected Tissues of B. tabaci
2.3. Virus Translocation in B. tabaci
2.4. Time-Series Quantification of the Virus in B. tabaci
2.5. Transovarial Passage and Transmission
3. Results
3.1. Virus-Infected Tissues of B. tabaci
3.2. Virus Translocation in B. tabaci
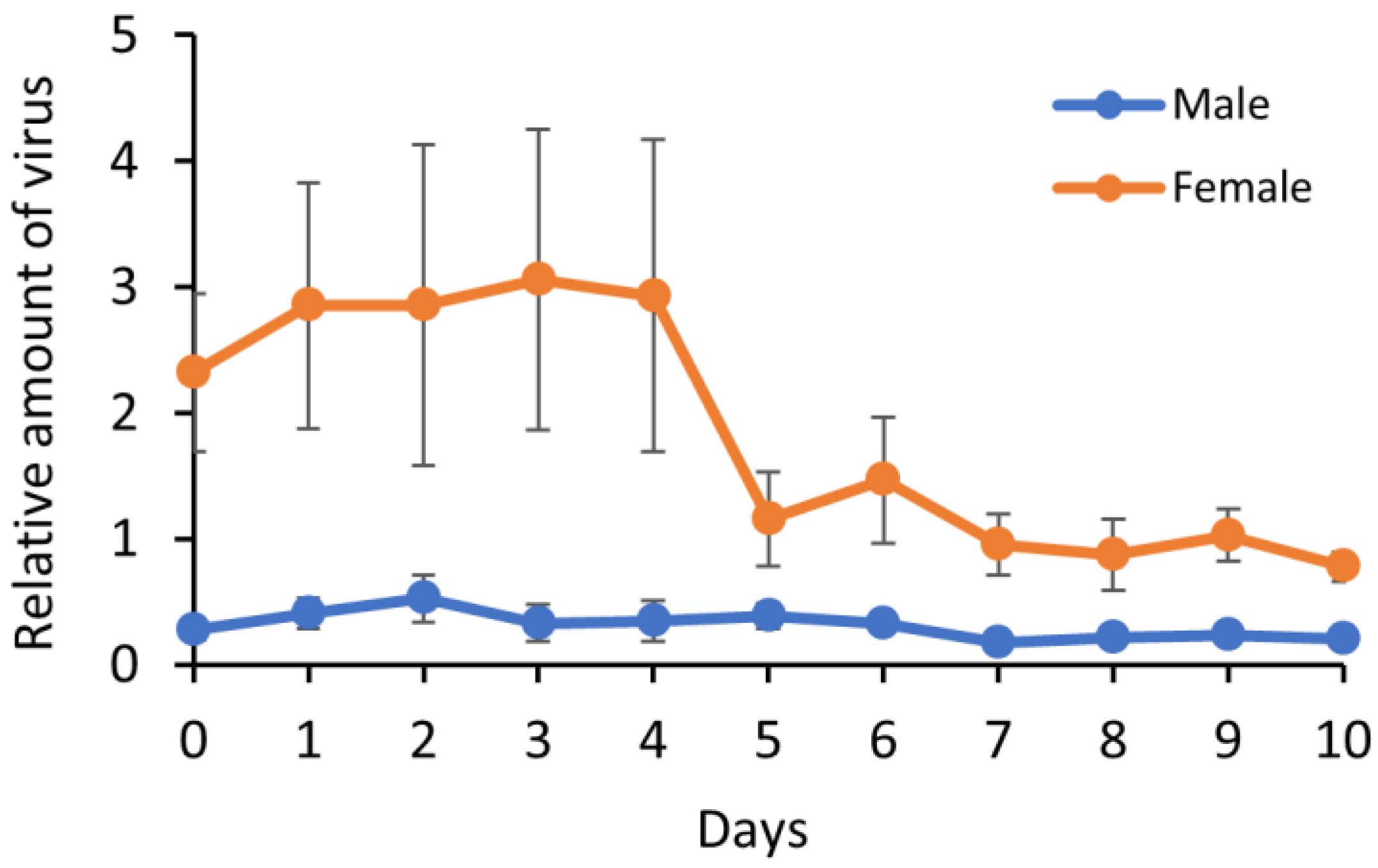
3.3. Virus Replication in B. tabaci
3.4. Transovarial Passage and Transmission
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitfield, A.E.; Falk, B.W.; Rotenberg, D. Insect vector-mediated transmission of plant viruses. Virology 2015, 479–480, 278–289. [Google Scholar] [CrossRef]
- Dáder, B.; Then, C.; Berthelot, E.; Ducousso, M.; Ng, J.C.K.; Drucker, M. Insect transmission of plant viruses: Multilayered interactions optimize viral propagation. Insect Sci. 2017, 24, 929–946. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Ammar, E.D.; Whitfield, A.E.; Redinbaugh, M.G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef]
- Persley, D.M.; Thomas, J.E.; Sharman, M. Tospoviruses—An Australian perspective. Australas. Plant Pathol. 2006, 35, 161–180. [Google Scholar] [CrossRef]
- Navas-Castillo, J.; Fiallo-Olivé, E.; Sánchez-Campos, S. Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar] [CrossRef]
- Rybicki, E.P. A Top Ten list for economically important plant viruses. Arch. Virol. 2015, 160, 17–20. [Google Scholar] [CrossRef]
- Ghanim, M. A review of the mechanisms and components that determine the transmission efficiency of Tomato yellow leaf curl virus (Geminiviridae; Begomovirus) by its whitefly vector. Virus Res. 2014, 186, 47–54. [Google Scholar] [CrossRef]
- Dietzgen, R.G.; Mann, K.S.; Johnson, K.N. Plant virus-insect vector interactions: Current and potential future research directions. Viruses 2016, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K.; Zerbini, F.M.; Navas-Castillo, J.; Moriones, E.; Ramos-Sobrinho, R.; Silva, J.C.F.; Fiallo-Olivé, E.; Briddon, R.W.; Hernández-Zepeda, C.; Idris, A.; et al. Revision of Begomovirus taxonomy based on pairwise sequence comparisons. Arch. Virol. 2015, 160, 1593–1619. [Google Scholar] [CrossRef] [PubMed]
- Picó, B.; Díez, M.J.; Nuez, F. Viral diseases causing the greatest economic losses to the tomato crop. II. The Tomato yellow leaf curl virus—A review. Sci. Hortic. 1996, 67, 151–196. [Google Scholar] [CrossRef]
- Czosnek, H.; Laterrot, H. A worldwide survey of tomato yellow leaf curl viruses. Arch. Virol. 1997, 142, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Harpaz, I. Periodic, rather than continual acquisition of a new tomato virus by its vector, the tobacco whitefly (Bemisia tabaci Gennadius). Entomol. Exp. Appl. 1964, 7, 155–166. [Google Scholar] [CrossRef]
- Tsai, W.S.; Shih, S.L.; Kenyon, L.; Green, S.K.; Jan, F.J. Temporal distribution and pathogenicity of the predominant tomato-infecting begomoviruses in Taiwan. Plant. Pathol. 2011, 60, 787–799. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhou, X.P.; Zhang, X.; Xie, Y. Molecular characterization of tomato-infecting begomoviruses in Yunnan, China. Arch. Virol. 2004, 149, 1721–1732. [Google Scholar] [CrossRef]
- Sawangjit, S.; Chatchawankanphanich, O.; Chiemsombat, P.; Attathom, T.; Dale, J.; Attathom, S. Molecular characterization of tomato-infecting begomoviruses in Thailand. Virus Res. 2005, 109, 1–8. [Google Scholar] [CrossRef]
- Yule, S.; Chiemsombat, P.; Srinivasan, R. Detection of Tomato yellow leaf curl Thailand virus transmitted by Bemisia tabaci Asia I in tomato and pepper. Phytoparasitica 2019, 47, 143–153. [Google Scholar] [CrossRef]
- Czosnek, H.; Hariton-Shalev, A.; Sobol, I.; Gorovits, R.; Ghanim, M. The incredible journey of begomoviruses in their whitefly vector. Viruses 2017, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Rimbaud, L.; Chiroleu, F.; Reynaud, B.; Thebaud, G.; Lett, J.M. Rapid accumulation and low degradation: Key parameters of Tomato yellow leaf curl virus persistence in its insect vector Bemisia tabaci. Sci. Rep. 2015, 5, 17696. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Campos, S.; Rodriguez-Negrete, E.A.; Cruzado, L.; Grande-Pérez, A.; Bejarano, E.R.; Navas-Castillo, J.; Moriones, E. Tomato yellow leaf curl virus: No evidence for replication in the insect vector Bemisia tabaci. Sci. Rep. 2016, 6, 30942. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Wyman, J.A.; Nakhla, M.K.; Maxwell, D.P. Transmission of tomato yellow leaf curl geminivirus by Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol. 1994, 87, 1291–1297. [Google Scholar] [CrossRef]
- Czosnek, H.; Ghanim, M.; Morin, S.; Rubinstein, G.; Fridman, V.; Zeidan, M. Whiteflies: Vectors, and victims (?), of geminiviruses. Adv. Virus Res. 2001, 57, 291–322. [Google Scholar] [PubMed]
- Pakkianathan, B.C.; Kontsedalov, S.; Lebedev, G.; Mahadav, A.; Zeidan, M.; Czosnek, H.; Ghanim, M. Replication of Tomato yellow leaf curl virus in its whitefly vector, Bemisia tabaci. J. Virol. 2015, 89, 9791–9803. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Wang, X.R.; Wei, X.M.; Huang, H.; Wu, J.X.; Chen, X.X.; Liu, S.S.; Wang, X.W. The autophagy pathway participates in resistance to tomato yellow leaf curl virus infection in whiteflies. Autophagy 2016, 12, 1560–1574. [Google Scholar] [CrossRef]
- Ghanim, M.; Morin, S.; Zeidan, M.; Czosnek, H. Evidence for transovarial transmission of tomato yellow leaf curl virus by its vector, the whitefly Bemisia tabaci. Virology 1998, 240, 295–303. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Liu, J.; Jiu, M.; Qian, Y.J.; Liu, S.S. Low frequency of horizontal and vertical transmission of two begomoviruses through whiteflies exhibits little relevance to the vector infectivity. Ann. Appl. Biol. 2010, 157, 125–133. [Google Scholar] [CrossRef]
- Pan, H.; Chu, D.; Yan, W.; Su, Q.; Liu, B.; Wang, S.; Wu, Q.; Xie, W.; Jiao, X.; Li, R.; et al. Rapid spread of Tomato yellow leaf curl virus in China is aided differentially by two invasive whiteflies. PLoS ONE 2012, 7, e34817. [Google Scholar] [CrossRef]
- Wei, J.; He, Y.Z.; Guo, Q.; Guo, T.; Liu, Y.Q.; Zhou, X.P.; Liu, S.S.; Wang, X.W. Vector development and vitellogenin determine the transovarial transmission of begomoviruses. Proc. Natl. Acad. Sci. USA 2017, 114, 6746–6751. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.H.; Tsai, W.S.; Kenyon, L.; Tsai, C.W. Different transmission efficiencies may drive displacement of tomato begomoviruses in the fields in Taiwan. Ann. Appl. Biol. 2015, 166, 321–330. [Google Scholar] [CrossRef]
- Hu, F.Y.; Mou, D.F.; Tsai, C.W. Evaluation of barrier plants for the cultural control of tomato yellow leaf curl disease. J. Asia Pac. Entomol. 2020, 23, 132–137. [Google Scholar] [CrossRef]
- Guo, J.Y.; Dong, S.Z.; Yang, X.L.; Cheng, L.; Wan, F.H.; Liu, S.S.; Zhou, X.P.; Ye, G.Y. Enhanced vitellogenesis in a whitefly via feeding on a begomovirus-infected plant. PLoS ONE 2012, 7, e43567. [Google Scholar] [CrossRef]
- Li, R.; Xie, W.; Wang, S.; Wu, Q.; Yang, N.; Yang, X.; Pan, H.; Zhou, X.; Bai, L.; Xu, B.; et al. Reference gene selection for qRT-PCR analysis in the sweetpotato whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). PLoS ONE 2013, 8, e53006. [Google Scholar] [CrossRef]
- Czosnek, H.; Ghanim, M.; Ghanim, M. The circulative pathway of begomoviruses in the whitefly vector Bemisia tabaci—Insights from studies with Tomato yellow leaf curl virus. Ann. Appl. Biol. 2002, 140, 215–231. [Google Scholar] [CrossRef]
- Medina, V.; Pinner, M.S.; Bedford, I.D.; Achon, M.A.; Gemeno, C.; Markham, P.G. Immunolocalization of Tomato yellow leaf curl Sardinia virus in natural host plants and its vector Bemisia tabaci. J. Plant Pathol. 2006, 88, 299–308. [Google Scholar]
- Ohnishi, J.; Kitamura, T.; Terami, F.; Honda, K. A selective barrier in the midgut epithelial cell membrane of the nonvector whitefly Trialeurodes vaporariorum to Tomato yellow leaf curl virus uptake. J. Gen. Plant Pathol. 2009, 75, 131–139. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, J.J.; Zhang, T.; Li, F.F.; Ghanim, M.; Zhou, X.P.; Ye, G.Y.; Liu, S.S.; Wang, X.W. Specific cells in the primary salivary glands of the whitefly Bemisia tabaci control retention and transmission of begomoviruses. J. Virol. 2014, 88, 13460–13468. [Google Scholar] [CrossRef]
- Bosco, D.; Mason, G.; Accotto, G.P. TYLCSV DNA, but not infectivity, can be transovarially inherited by the progeny of the whitefly vector Bemisia tabaci (Gennadius). Virology 2004, 323, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Shu, Y.N.; Liu, C.; Chi, Y.; Liu, Y.Q.; Wang, X.W. Transovarial transmission of tomato yellow leaf curl virus by seven species of the Bemisia tabaci complex indigenous to China: Not all whiteflies are the same. Virology 2019, 531, 240–247. [Google Scholar] [CrossRef]
- Caciagli, P.; Bosco, D. Quantitation over time of tomato yellow leaf curl geminivirus DNA in its whitefly vector. Phytopathology 1997, 87, 610–613. [Google Scholar] [CrossRef]
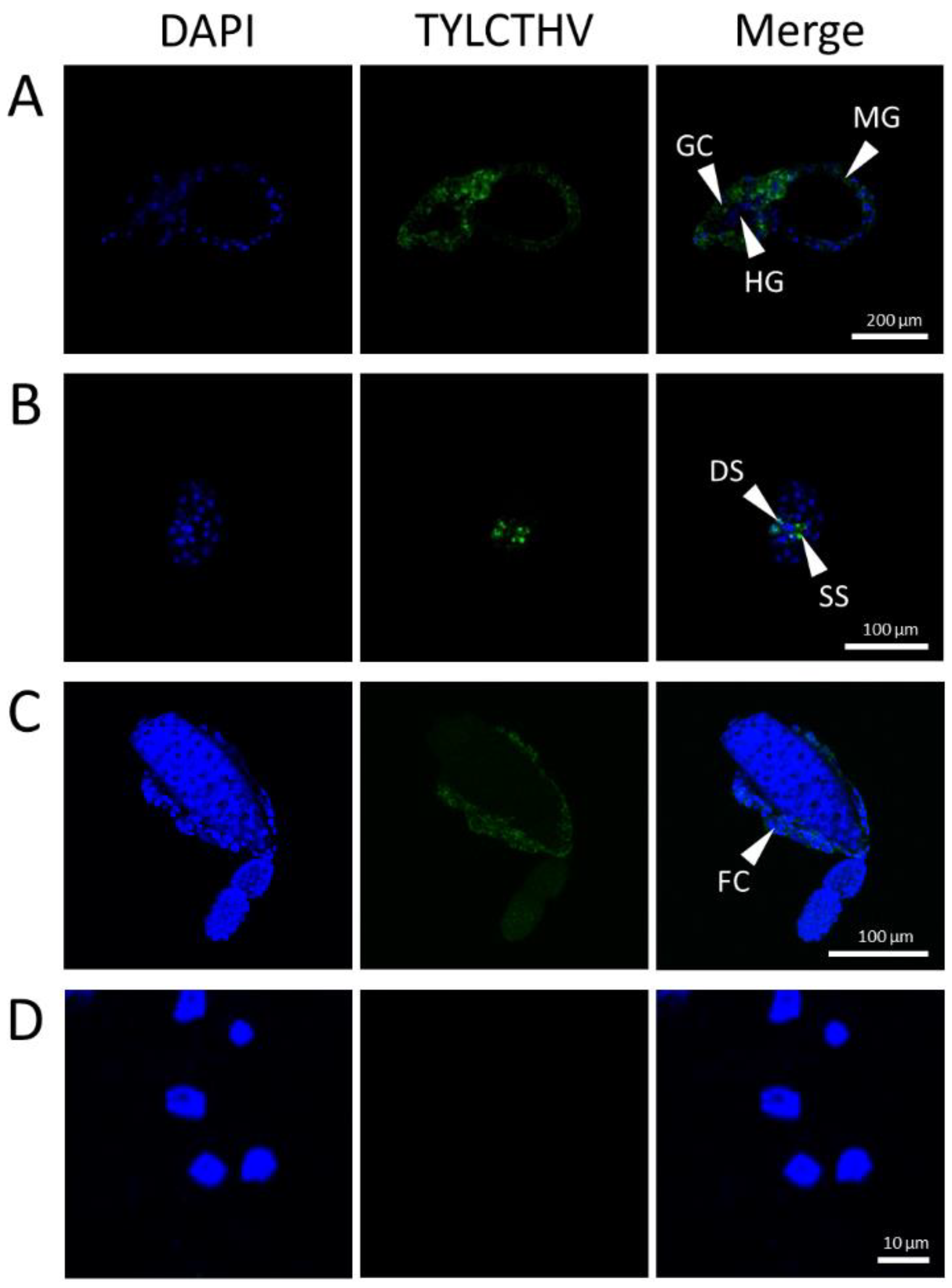
| Organ | n | Infection Rate (%) |
|---|---|---|
| Midgut | 12 | 100 |
| Gastric caecum | 12 | 100 |
| Filter chamber | 12 | 100 |
| Hindgut | 12 | 0 |
| Primary salivary gland | 12 | 100 |
| Ovariole | 12 | 100 |
| Hemocyte | 12 | 0 |
| AAP | n | Infection Rate (%) | ||
|---|---|---|---|---|
| Midgut | Hemolymph | Primary Salivary Gland | ||
| 2 h | 10 | 0 | 0 | 0 |
| 3 h | 10 | 20 | 10 | 0 |
| 4 h | 10 | 50 | 10 | 0 |
| 14 h | 10 | 100 | 70 | 80 |
| 24 h | 10 | 100 | 80 | 70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.-H.; Mou, D.-F.; Hsieh, C.-K.; Weng, S.-H.; Tsai, W.-S.; Tsai, C.-W. Vector Transmission of Tomato Yellow Leaf Curl Thailand Virus by the Whitefly Bemisia tabaci: Circulative or Propagative? Insects 2021, 12, 181. https://doi.org/10.3390/insects12020181
Li W-H, Mou D-F, Hsieh C-K, Weng S-H, Tsai W-S, Tsai C-W. Vector Transmission of Tomato Yellow Leaf Curl Thailand Virus by the Whitefly Bemisia tabaci: Circulative or Propagative? Insects. 2021; 12(2):181. https://doi.org/10.3390/insects12020181
Chicago/Turabian StyleLi, Wei-Hua, De-Fen Mou, Chien-Kuei Hsieh, Sung-Hsia Weng, Wen-Shi Tsai, and Chi-Wei Tsai. 2021. "Vector Transmission of Tomato Yellow Leaf Curl Thailand Virus by the Whitefly Bemisia tabaci: Circulative or Propagative?" Insects 12, no. 2: 181. https://doi.org/10.3390/insects12020181
APA StyleLi, W.-H., Mou, D.-F., Hsieh, C.-K., Weng, S.-H., Tsai, W.-S., & Tsai, C.-W. (2021). Vector Transmission of Tomato Yellow Leaf Curl Thailand Virus by the Whitefly Bemisia tabaci: Circulative or Propagative? Insects, 12(2), 181. https://doi.org/10.3390/insects12020181






