Ovary Structure and Oogenesis of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Scanning Electron Microscopy (SEM)
2.3. Transmission Electron Microscopy (TEM)
3. Results
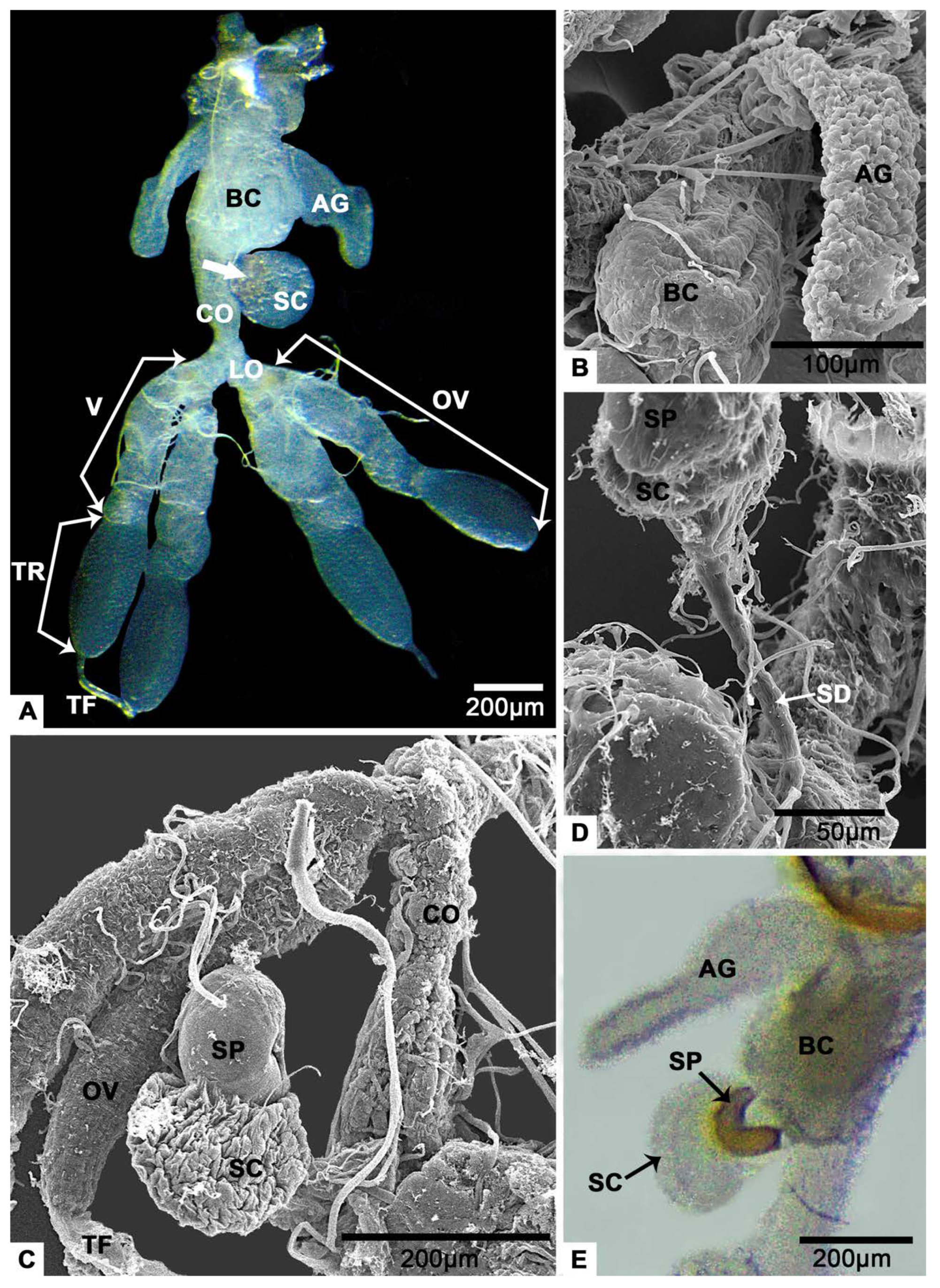
3.1. Gross Morphology of the Female Reproductive System
3.2. The Terminal Filament
3.3. The Tropharium
3.4. The Vitellarium
3.5. The Follicular Plug
3.6. The Spermathecal Sac and the Spermathecal Pump
3.7. The Accessory Glands
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Crowson, R.A. The phylogeny of Coleoptera. Ann. Rev. Entomol. 1960, 5, 111–134. [Google Scholar] [CrossRef]
- Stys, P.; Bilinski, S. Ovariole types and the phylogeny of hexapods. Biol. Rev. 1990, 65, 401–429. [Google Scholar] [CrossRef]
- Stringer, I.A.N. The female reproductive system of Costelytra zealandica (White) (Coleoptera: Scarabaeidae: Melolonthinae). N. Z. J. Zool. 1988, 15, 513–533. [Google Scholar] [CrossRef] [Green Version]
- Szklarzewicz, T.; Szlendak, E.; Boczek, J.; Biliński, S. Oogenesis in the lesser grain borer, Rhizopertha dominica (Fabricius) (Coleoptera: Bostrichidae). Int. J. Insect Morphol. Embryol. 1992, 21, 63–76. [Google Scholar] [CrossRef]
- Welch, C.R. Ovariole development in Staphylinidae (Coleoptera). Invertebr. Reprod. Dev. 1993, 23, 225–234. [Google Scholar] [CrossRef]
- Aslam, N.A. An assessment of some internal characters in the higher classification of the Curculionidae, S.L. (Coleoptera). Trans. R. Entomol. Soc. Lond. 1961, 113, 417–480. [Google Scholar] [CrossRef]
- López-López, A.; Vogler, A.P. The mitogenome phylogeny of Adephaga (Coleoptera). Mol. Phylogenet. Evol. 2017, 114, 166–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, T.; Vogler, A.P. A protocol for large-scale rRNA sequence analysis: Towards a detailed phylogeny of Coleoptera. Mol. Phylogen. Evol. 2008, 47, 289–301. [Google Scholar] [CrossRef]
- Cerezke, H.F. The morphology and functions of the reproductive systems of Dendroctonus monticolae Hopk. (Coleoptera: Scolytidae). Can. Entomol. 1964, 96, 477–500. [Google Scholar] [CrossRef]
- Ullmann, S.L. Oogenesis in Tenebrio molitor: Histological and autoradiographical observations on pupal and adult ovaries. J. Embryol. Exp. Morphol. 1973, 30, 179–217. [Google Scholar] [CrossRef]
- Goldson, S.; Emberson, R. Reproductive morphology of the argentine stem weevil, Hyperodes bonariensis (Coleoptera: Curculionidae). N. Z. J. Zool. 1981, 8, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Barker, G.M. Functional anatomy of the reproductive system of Listronotus bonariensis (Kuschel). N. Z. Entomol. 1989, 12, 34–42. [Google Scholar] [CrossRef]
- Calder, A.A. Gross morphology of the soft parts of the male and female reproductive systems of Curculionoidea (Coleoptera). J. Nat. Hist. 1990, 24, 453–505. [Google Scholar] [CrossRef]
- Rubio, G.J.D.; Bustillo, P.A.E.; Vallejo, E.L.F.; Acuña, Z.J.R.; Benavides, M.P. Alimentary canal and reproductive tract of Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae, Scolytinae). Neotrop. Entomol. 2008, 37, 143–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J. Morphology of Female Reproductive Organ and Ovarian Development of Dendroctonus armandi (Coleoptera: Curculionidae: Scolytinae). Master’s Thesis, Northwest A&F University, Yangling, China, 2010. [Google Scholar]
- Salazar, K.; Boucher, S.; Serrão, J.E. Structure and ultrastructure of the ovary in the South American Veturius sinuatus (Eschscholtz) (Coleoptera, Passalidae). Arthropod Struct. Dev. 2017, 46, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Kheirallah, D.A.; El-Samad, L.M. Oogenesis anomalies induced by heavy metal contamination in two tenebrionid beetles (Blaps polycresta and Trachyderma hispida). Folia Biol. Krakow 2019, 67, 9–23. [Google Scholar] [CrossRef]
- Perez-Mendoza, J.; Throne, J.E.; Baker, J.E. Ovarian physiology and age-grading in the rice weevil, Sitophilus oryzae (Coleoptera: Curculionidae). J. Stored Prod. Res. 2004, 40, 179–196. [Google Scholar] [CrossRef]
- Aggarwal, S.K. Morphological and histochemical studies on oogenesis in Callosobruchus analis Fabr. (Bruchidae-Coleoptera). J. Morphol. 1967, 122, 19–33. [Google Scholar] [CrossRef]
- Büning, J. The trophic tissue of telotrophic ovarioles in polyphage coleoptera. Zoomorphologie 1979, 93, 33–50. [Google Scholar] [CrossRef]
- Büning, J. Ovariole structure supports sistergroup relationship of Neuropterida and Coleoptera. Arthropod Syst. Phylo. 2006, 64, 115–126. [Google Scholar]
- Gottanka, J.; Büning, J. Mayflies (Ephemeroptera), the most “primitive” winged insects, have telotrophic meroistic ovaries. Roux’s Arch. Dev. Biol. 1993, 203, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Büning, J. The Insect Ovary: Ultrastructure, Previtellogenic Growth and Evolution; Chapman and Hall: London, UK, 1994. [Google Scholar]
- Huebner, E.; Diehl-Jones, W. Developmental biology of insect ovaries: Germ cells and nurse cell oocyte polarity. In Microscopic Anatomy of Invertebrates; Harrison, F.W., Ed.; Wiley-Liss: New York, NY, USA, 1998; Volume 11, pp. 957–993. [Google Scholar]
- Kugler, J.-M.; Rübsam, R.; Trauner, J.; Büning, J. The larval development of the telotrophic meroistic ovary in the bug Dysdercus intermedius (Heteroptera, Pyrrhocoridae). Arthropod Struct. Dev. 2006, 35, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Büning, J. The telotrophic ovary known from Neuropterida exists also in the myxophagan beetle Hydroscapha natans. Dev. Genes Evol. 2005, 215, 597–607. [Google Scholar] [CrossRef]
- Matusewski, B.; Ciechomski, K.; Nurkowska, J.; Kloc, M. The linear clusters of oogonial cells in the development of telotrophic ovarioles in polyphage Coleoptera. Roux’s Arch. Dev. Biol. 1985, 194, 462–469. [Google Scholar] [CrossRef]
- Trauner, J.; Büning, J. Germ-cell cluster formation in the telotrophic meroistic ovary of Tribolium castaneum (Coleoptera, Polyphaga, Tenebrionidae) and its implication on insect phylogeny. Dev. Genes Evol. 2007, 217, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.S. Weevils and plants: Phylogenetic versus ecological mediation of evolution of host plant associations in Curculioninae (Coleoptera: Curculionidae). Can. Entomol. 1993, 125, 197–232. [Google Scholar] [CrossRef]
- Swiatek, P. Structure and development of ovaries in the weevil, Anthonomus pomorum (Coleoptera Polyphaga). II. Germ cells of the trophic chamber. Folia Biol. Krakow 2002, 50, 153–163. [Google Scholar] [PubMed]
- Korman, A.K.; Oseto, C.Y. Structure of the female reproductive system and maturation of oöcytes in Smicronyx fulvus (Coleoptera: Curculionidae). Ann. Entomol. Soc. Am. 1989, 82, 94–100. [Google Scholar] [CrossRef]
- Eggers, V.O.H. Trypophloeus klimeschi nov. spec. Entomol. Bl. 1915, 25, 7–9. [Google Scholar]
- Cao, Y.; Luo, Z.; Wang, S.; Zhang, P. Trypophloeus klimeschi Eggers—A new insect pest to Xinjiang poplar. J. Tarim Univ. 2003, 15, 9–11. [Google Scholar]
- Cao, Y.; Luo, Z.; Wang, S.; Zhang, P. Bionomics and control of Trypophloeus klimeschi. Entomol. Knowl. 2004, 41, 36–38. [Google Scholar]
- Gao, G. Occurrence and Host Selection Mechanism of Trypophloeus klimeschi Eggers. Ph.D. Thesis, Northwest A&F University, Yangling, China, 2018. [Google Scholar]
- Gao, G.; Dai, L.; Gao, J.; Wang, J.; Chen, H. Volatile organic compound analysis of host and non-host poplars for Trypophloeus klimeschi (Coleoptera: Curculionidae: Ipinae). Russ. J. Plant Physl. 2018, 65, 916–925. [Google Scholar] [CrossRef]
- Gao, G.; Gao, J.; Hao, C.; Dai, L.; Chen, H. Biodiversity and activity of gut fungal communities across the life history of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae). Int. J. Mol. Sci. 2018, 19, 2010. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.; Dai, L.; Gao, J.; Wang, J.; Chen, H. Electroantennogram, behavioural responses, and field trapping of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae) to eight host volatiles. Can. Entomol. 2019, 151, 236–250. [Google Scholar] [CrossRef]
- Wigglesworth, V.B. The Principles of Insect Physiology; Chapman and Hall: London, UK, 1972. [Google Scholar]
- Lococo, D.; Huebner, E. The ultrastructure of the female accessory gland, the cement gland, in the insect Rhodnius prolixus. Tissue Cell 1980, 12, 557–580. [Google Scholar] [CrossRef]
- Courrent, A.; Quennedey, A.; Nalepa, C.A.; Robert, A.; Lenz, M.; Bordereau, C. The fine structure of colleterial glands in two cockroaches and three termites, including a detailed study of Cryptocercus punctulatus (Blattaria, Cryptocercidae) and Mastotermes darwiniensis (Isoptera, Mastotermitidae). Arthropod Struct. Dev. 2008, 37, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.J. The fine structure of the colleterial glands of Hyalophora cecropia (Lepidoptera). J. Morphol. 1968, 125, 259–279. [Google Scholar] [CrossRef]
- Sturm, R. Morphology and ultrastructure of the female accessory sex glands in various crickets (Orthoptera, Saltatoria, Gryllidae). Dtsch. Entomol. Z. 2002, 49, 185–195. [Google Scholar] [CrossRef]
- Gillott, C. Accessory sex glands in arthropoda-insecta. In Reproductive Biology of Invertebrates; Adiyodi, K.G., Adiyodi, R.G., Eds.; Wiley: New York, NY, USA, 1988; pp. 319–471. [Google Scholar]
- Quennedey, A.; Peppuy, A.; Courrent, A.; Robert, A.; Everaerts, C.; Bordereau, C. Ultrastructure of posterior sternal glands of Macrotermes annandalei (Silvestri): New members of the sexual glandular set found in termites (Insecta). J. Morphol. 2004, 262, 683–691. [Google Scholar] [CrossRef]
- Krause, J.B. The structure of the gonads of the woodeating beetle, Passalus cornutus Fabricius. Ann. Entomol. Soc. Am. 1946, 39, 193–206. [Google Scholar] [CrossRef]
- Pascini, T.V.; Martins, G.F. The insect spermatheca: An overview. Zoology 2017, 121, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Tombes, A.S.; Roppel, R.M. Ultrastructure of the spermatheca of the granary weevil, Sitophilus granarius (L.) (Coleoptera: Curculionidae). Int. J. Insect Morphol. Embryol. 1972, 1, 141–152. [Google Scholar] [CrossRef]
- Schoeters, E.; Billen, J. The importance of the spermathecal duct in bumblebees. J. Insect Physiol. 2000, 46, 1303–1312. [Google Scholar] [CrossRef]
- Dallai, R.; Mercati, D.; Gottardo, M.; Machida, R.; Mashimo, Y.; Beutel, R. The fine structure of the female reproductive system of Zorotypus caudelli Karny (Zoraptera). Arthropod Struct. Dev. 2012, 41, 51–63. [Google Scholar] [CrossRef]
- Pascini, T.; Ramalho-Ortigão, J.; Martins, G. The fine structure of the spermatheca in Anopheles aquasalis (Diptera: Culicidae). Ann. Entomol. Soc. Am. 2013, 106, 857–867. [Google Scholar] [CrossRef]
- Bleiker, K.P.; Heron, R.J.; Braithwaite, E.C.; Smith, G.D. Preemergence mating in the mass-attacking bark beetle, Dendroctonus ponderosae (Coleoptera: Curculionidae). Can. Entomol. 2013, 145, 12–19. [Google Scholar] [CrossRef]
- Akster, H.; Smit, W. The structure of the terminal filament, the ovariole sheath and the oviduct musculature of the Colorado Beetle (Leptinotarsa decemlineata Say, 1824). Bijdr. Tot De Dierkd. 1977, 46, 136–150. [Google Scholar] [CrossRef] [Green Version]
- Büning, J. Development of telotrophic-meroistic ovarioles of polyphage beetles with special reference to the formation of nutritive cords. J. Morphol. 1978, 156, 237–255. [Google Scholar] [CrossRef]
- Swiatek, P. Structure and development of ovaries in the weevil, Anthonomus pomorum (Coleoptera, Polyphaga). I. Somatic tissues of the trophic chamber. Folia Biol. Krakow 2001, 49, 215–224. [Google Scholar] [PubMed]
- Sperandio, S.; De Belle, I.; Bredesen, D.E. An alternative, nonapoptotic form of programmed cell death. Proc. Natl. Acad. Sci. USA 2000, 97, 14376–14381. [Google Scholar] [CrossRef] [Green Version]
- Bröker, L.E.; Kruyt, F.A.; Giaccone, G. Cell death independent of caspases: A review. Clin. Cancer Res. 2005, 11, 3155–3162. [Google Scholar] [CrossRef] [Green Version]
- Mpakou, V.E.; Velentzas, A.D.; Velentzas, P.D.; Margaritis, L.H.; Stravopodis, D.J.; Papassideri, I.S. Programmed cell death of the ovarian nurse cells during oogenesis of the ladybird beetle Adalia bipunctata (Coleoptera: Coccinellidae). Dev. Growth Differ. 2011, 53, 804–815. [Google Scholar] [CrossRef]
- McCall, K. Eggs over easy: Cell death in the Drosophila ovary. Dev. Biol. 2004, 274, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Baum, J.S.; George, J.P.S.; McCall, K. Programmed cell death in the germline. Semin. Cell Dev. Biol. 2005, 16, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Nezis, I.P.; Lamark, T.; Velentzas, A.D.; Rusten, T.E.; Bjørkøy, G.; Johansen, T.; Papassideri, I.S.; Stravopodis, D.J.; Margaritis, L.H.; Stenmark, H.; et al. Cell death during Drosophila melanogaster early oogenesis is mediated through autophagy. Autophagy 2009, 5, 298–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mpakou, V.E.; Nezis, I.P.; Stravopodis, D.J.; Margaritis, L.H.; Papassideri, I.S. Programmed cell death of the ovarian nurse cells during oogenesis of the silkmoth Bombyx mori. Dev. Growth Differ. 2006, 48, 419–428. [Google Scholar] [CrossRef]
- Reginato, R.D.; Da Cruz-Landim, C. Morphological characterization of cell death during the ovary differentiation in worker honey bee. Cell Biol. Int. 2002, 26, 243–251. [Google Scholar] [CrossRef] [PubMed]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Wang, J.; Chen, H. Ovary Structure and Oogenesis of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae). Insects 2021, 12, 1099. https://doi.org/10.3390/insects12121099
Gao J, Wang J, Chen H. Ovary Structure and Oogenesis of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae). Insects. 2021; 12(12):1099. https://doi.org/10.3390/insects12121099
Chicago/Turabian StyleGao, Jing, Jiaxing Wang, and Hui Chen. 2021. "Ovary Structure and Oogenesis of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae)" Insects 12, no. 12: 1099. https://doi.org/10.3390/insects12121099
APA StyleGao, J., Wang, J., & Chen, H. (2021). Ovary Structure and Oogenesis of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae). Insects, 12(12), 1099. https://doi.org/10.3390/insects12121099





