Risk Assessment of Insecticides Used in Tomato to Control Whitefly on the Predator Macrolophus basicornis (Hemiptera: Miridae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Insecticides
2.3. Insecticide Exposure for Testing Acute Toxicity
2.4. Determination of LC50 of Harmful Insecticides
2.5. Statistical Analysis
2.6. Toxicity Classification
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassis, G.; Schuh, R. Systematics, biodiversity, biogeography, and host associations of the Miridae (Insecta: Hemiptera: Heteroptera: Cimicomorpha). Annu. Rev. Èntomol. 2012, 57, 377–404. [Google Scholar] [CrossRef]
- Van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. BioControl 2012, 57, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Bueno, V.H.P.; Montes, F.C.; Pereira, A.M.C.; Lins, J.C.; van Lenteren, J.C. Can recently found Brazilian hemipteran predatory bugs control Tuta absoluta? IOBC-WPRS Bull. 2012, 80, 63–67. [Google Scholar]
- Bueno, V.H.P.; van Lenteren, J.; Lins, J.J.C.; Calixto, A.M.; Montes, F.C.; Silva, D.B.; Santiago, L.D.; Pérez, L.M. New records of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) predation by Brazilian Hemipteran predatory bugs. J. Appl. Èntomol. 2013, 137, 29–34. [Google Scholar] [CrossRef]
- Bueno, V.H.P.; Calixto, A.M.; Montes, F.C.; van Lenteren, J.C. Population growth parameters of three Neotropical mirid predators (Hemiptera: Miridae) at five temperatures on tobacco with Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) eggs as food. Israel J. Entomol. 2018, 48, 1–22. [Google Scholar] [CrossRef]
- Bueno, V.H.P.; Montes, F.C.; Sampaio, M.V.; Calixto, A.M.; van Lenteren, J.C. Performance of immatures of three Neotropical Miridae at five different temperatures, reared on Ephestia kuehniella eggs on tobacco plants. Bull. Insectology 2018, 71, 77–87. [Google Scholar]
- Silva, W.M.; Berger, M.; Bass, C.; Williamson, M.; Moura, D.M.; Ribeiro, L.M.; Siqueira, H.A. Mutation (G275E) of the nicotinic acetylcholine receptor α6 subunit is associated with high levels of resistance to spinosyns in Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Pestic. Biochem. Physiol. 2016, 131, 1–8. [Google Scholar] [CrossRef] [Green Version]
- van Lenteren, J.C.; Hemerik, L.; Lins, J.C.; Bueno, V.H.P. Functional responses of three Neotropical mirid predators to eggs of Tuta absoluta on tomato. Insects 2016, 7, 34. [Google Scholar] [CrossRef]
- van Lenteren, J.C.; Bueno, V.H.P.; Smit, J.; Soares, M.A.; Calixto, A.M.; Montes, F.C.; De Jong, P. Predation of Tuta absoluta eggs during the nymphal stages of three Neotropical mirid predators on tomato. Bull. Insectology 2017, 70, 69–74. [Google Scholar]
- Castañé, C.; Arnó, J.; Gabarra, R.; Alomar, O. Plant damage to vegetable crops by zoophytophagous mirid predators. Biol. Control 2011, 59, 22–29. [Google Scholar] [CrossRef]
- Calvo, F.J.; Lorente, M.J.; Stansly, P.A.; Belda, J.E. Preplant release of Nesidiocoris tenuis and supplementary tactics for control of Tuta absoluta and Bemisa tabaci in greenhouse tomato. Èntomol. Exp. Appl. 2012, 143, 111–119. [Google Scholar] [CrossRef]
- Lins, J.J.C.; Van Loon, J.J.A.; Bueno, V.H.P.; Lucas-Barbosa, D.; Dicke, M.; van Lenteren, J. Response of the zoophytophagous predators Macrolophus pygmaeus and Nesidiocoris tenuis to volatiles of uninfested plants and to plants infested by prey or conspecifics. BioControl 2014, 59, 707–718. [Google Scholar] [CrossRef]
- De Barro, P.J.; Liu, S.-S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Èntomol. 2011, 56, 1–19. [Google Scholar] [CrossRef]
- Perring, T.M.; Stansly, P.A.; Liu, T.; Smith, H.A.; Andreason, S.A. Whiteflies: Biology, ecology, and management. In Sustainable Management of Arthropod Pests of Tomato; Wakil, W., Brust, G.E., Perring, T.M., Eds.; Elsevier: London, UK, 2018; pp. 73–110. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Èntomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P.; et al. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Ellsworth, P.C.; Frisvold, G.B. Economic value of biological control in integrated pest management of managed plant systems. Annu. Rev. Èntomol. 2015, 60, 621–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanumen, A.C.; Carvalho, G.A.; Medina, P.; Viñuela, E.; Adán, Á. Residual acute toxicity of some modern insecticides toward two mirid predators of tomato pests. J. Econ. Èntomol. 2016, 109, 1079–1085. [Google Scholar] [CrossRef]
- Passos, L.C.; Soares, M.A.; Costa, M.A.; Michaud, J.; Freire, B.C.; Carvalho, G.A. Physiological susceptibility of the predator Macrolophus basicornis (Hemiptera: Miridae) to pesticides used to control of Tuta absoluta (Lepidoptera: Gelechiidae). Biocontrol Sci. Technol. 2017, 27, 1082–1095. [Google Scholar] [CrossRef]
- Passos, L.C.; Soares, M.A.; Collares, L.J.; Malagoli, I.; Desneux, N.; Carvalho, G.A. Lethal, sublethal and transgenerational effects of insecticides on Macrolophus basicornis, predator of Tuta absoluta. Èntomol. Gen. 2018, 38, 127–143. [Google Scholar] [CrossRef]
- Soares, M.A.; Passos, L.C.; de Campos, M.R.; Collares, L.J.; Desneux, N.; Carvalho, G.A. Side effects of insecticides commonly used against Tuta absoluta on the predator Macrolophus basicornis. J. Pest Sci. 2019, 92, 1447–1456. [Google Scholar] [CrossRef]
- Ellsworth, P.C.; Martinez-Carrillo, J.L. IPM for Bemisia tabaci: A case study from North America. Crop. Prot. 2001, 20, 853–869. [Google Scholar] [CrossRef]
- Naranjo, S.E. Conservation and evaluation of natural enemies in IPM systems for Bemisia tabaci. Crop. Prot. 2001, 20, 835–852. [Google Scholar] [CrossRef] [Green Version]
- Dale, A.G.; Borden, M.A. Evaluation of reduced-risk insecticides to control chilli thrips (Thysanoptera: Thripidae) and conserve natural enemies on ornamental plants. Fla. Èntomol. 2018, 101, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S. International Organization for Biological and Integrated Control of Noxious Animals and Plants/Working Group “Pesticides and Beneficial Organisms” 1992. Guidelines for testing the effects of pesticides on beneficial organisms: Description of test methods. IOBC-WPRS Bull. 1992, XV, 3. [Google Scholar]
- Preetha, G.; Stanley, J.; Suresh, S.; Samiyappan, R. Risk assessment of insecticides used in rice on miridbug, Cyrtorhinus lividipennis Reuter, the important predator of brown planthopper, Nilaparvata lugens (Stal.). Chemosphere 2010, 80, 498–503. [Google Scholar] [CrossRef]
- Cheng, S.; Lin, R.; Wang, L.; Qiu, Q.; Qu, M.; Ren, X.; Zong, F.; Jiang, H.; Yu, C. Comparative susceptibility of thirteen selected pesticides to three different insect egg parasitoid Trichogramma species. Ecotoxicol. Environ. Saf. 2018, 166, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dai, P.; Yang, X.; Ruan, C.-C.; Biondi, A.; Desneux, N.; Zang, L.-S. Selectivity of novel and traditional insecticides used for management of whiteflies on the parasitoid Encarsia formosa. Pest Manag. Sci. 2019, 75, 2716–2724. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, X.; Huang, X.; Yu, X.; Zhang, W.; Zhang, X.; Mu, W. Comparative ecotoxicity of neonicotinoid insecticides to three species of Trichogramma parasitoid wasps (Hymenoptera: Trichogrammatidae). Ecotoxicol. Environ. Saf. 2019, 183, 109587. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Zhou, C.; Liu, F.; Mu, W. Toxicity of nine insecticides on four natural enemies of Spodoptera exigua. Sci. Rep. 2016, 6, 39060. [Google Scholar] [CrossRef] [Green Version]
- Arnó, J.; Gabarra, R. Side effects of selected insecticides on the Tuta absoluta (Lepidoptera: Gelechiidae) predators Macrolophus pygmaeus and Nesidiocoris tenuis (Hemiptera: Miridae). J. Pest Sci. 2011, 84, 513–520. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University: London, UK, 1971; 333p. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. 2020. Available online: https://www.R-project.org/ (accessed on 24 October 2021).
- Püntener, W. Manual for Field Trials in Plant Protection, 2nd ed.; Ciba-Geigy, Ltd.: Basel, Switzerland, 1981. [Google Scholar]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.B.; Bueno, V.H.P.; Van Loon, J.J.A.; Peñaflor, M.F.G.V.; Bento, J.M.S.; Van Lenteren, J.C. Attraction of three mirid predators to tomato infested by both the tomato leaf mining moth Tuta absoluta and the whitefly Bemisia tabaci. J. Chem. Ecol. 2017, 44, 29–39. [Google Scholar] [CrossRef]
- van Lenteren, J.C.; Bueno, V.H.P.; Montes, F.C.; Hemerik, L.; De Jong, P.W. Adult lifetime predation of Tuta absoluta eggs by three Neotropical mirid predators on tomato. Bull. Insectology 2018, 71, 179–188. [Google Scholar]
- Stansly, P.; Sánchez, P.; Rodríguez, J.; Cañizares, F.; Nieto, A.; Leyva, M.; Fajardo, M.; Suárez, V.; Urbaneja, A. Prospects for biological control of Bemisia tabaci (Homoptera, Aleyrodidae) in greenhouse tomatoes of southern Spain. Crop. Prot. 2004, 23, 701–712. [Google Scholar] [CrossRef]
- Koss, A.M.; Jensen, A.S.; Schreiber, A.; Pike, K.S.; Snyder, W.E. Comparison of predator and pest communities in Washington potato fields treated with broad-spectrum, selective, or organic insecticides. Environ. Èntomol. 2005, 34, 87–95. [Google Scholar] [CrossRef]
- Togni, P.H.B.; Venzon, M.; de Souza, L.M.; Santos, J.P.C.R.; Sujii, E.R. Biodiversity provides whitefly biological control based on farm management. J. Pest Sci. 2018, 92, 393–403. [Google Scholar] [CrossRef]
- Sohrabi, F.; Shishehbor, P.; Saber, M.; Mosaddegh, M. Lethal and sublethal effects of buprofezin and imidacloprid on Bemisia tabaci (Hemiptera: Aleyrodidae). Crop. Prot. 2011, 30, 1190–1195. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Yousaf, H.K.; Xiu, W.; Qian, D.; Gao, X.; Tariq, K.; Han, P.; Desneux, N.; Song, D. Impact of low lethal concentrations of buprofezin on biological traits and expression profile of chitin synthase 1 gene (CHS1) in melon aphid, Aphis gossypii. Sci. Rep. 2019, 9, 12291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bretschneider, T.; Benet-Buchholz, J.; Fischer, R.; Nauen, R. Spirodiclofen and spiromesifen—novel acaricidal and insecticidal tetronic acid derivatives with a new mode of action. Chim. Int. J. Chem. 2003, 57, 697–701. [Google Scholar] [CrossRef]
- Lahm, G.P.; Selby, T.P.; Freudenberger, J.H.; Stevenson, T.M.; Myers, B.J.; Seburyamo, G.; Smith, B.K.; Flexner, L.; Clark, C.E.; Cordova, D. Insecticidal anthranilic diamides: A new class of potent ryanodine receptor activators. Bioorganic Med. Chem. Lett. 2005, 15, 4898–4906. [Google Scholar] [CrossRef]
- Sattelle, D.B.; Cordova, D.; Cheek, T.R. Insect ryanodine receptors: Molecular targets for novel pest control chemicals. Invertebr. Neurosci. 2008, 8, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, K.; Kashitani, R.; Tomoda, M.; Kodama, R.; Ito, K.; Yamanaka, S.; Momoshita, M.; Arakawa, R. Side effects of vegetable pesticides on a predatory mirid bug, Pilophorus typicus Distant (Heteroptera: Miridae). Appl. Èntomol. Zool. 2010, 45, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Rasdi, M.Z.; Che Salmah, M.R.; Abu Hassan, A.; Hamady, D.; Hamaseh, A.; Fauziah, I. Field evaluation of some insecticides on whitefly (Trialeurodes vaporariorum) and predator (Macrolophus caliginosus) on brinjal and tomato plants. Asian J. Agric. Rural Dev. 2012, 2, 302–311. [Google Scholar]
- Amarasekare, K.G.; Shearer, P.W. Laboratory bioassays to estimate the lethal and sublethal effects of various insecticides and fungicides on Deraeocoris brevis (Hemiptera: Miridae). J. Econ. Èntomol. 2013, 106, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Müller, C. Impacts of sublethal insecticide exposure on insects—Facts and knowledge gaps. Basic Appl. Ecol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Daam, M.A.; Chelinho, S.; Niemeyer, J.; Owojori, O.; De Silva, P.M.C.; Sousa, J.P.; van Gestel, C.A.; Römbke, J. Environmental risk assessment of pesticides in tropical terrestrial ecosystems: Test procedures, current status and future perspectives. Ecotoxicol. Environ. Saf. 2019, 181, 534–547. [Google Scholar] [CrossRef]
- Casida, J.E.; Durkin, K.A. Neuroactive insecticides: Targets, selectivity, resistance, and secondary effects. Annu. Rev. Èntomol. 2013, 58, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Simon-Delso, N.; Amaralrogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- Saha, S.; Mondal, R.; Mukherjee, S.; Sarkar, M.; Kole, R.K. Persistence of acetamiprid in paddy and soil under West Bengal agro-climatic conditions. Environ. Monit. Assess. 2017, 189, 150. [Google Scholar] [CrossRef]
- Mostafiz, M.; Hassan, E.; Shim, J.-K.; Lee, K.-Y. Lethal and sublethal effects of methyl benzoate on the predatory bug Nesidiocoris tenuis. Insects 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Takahashi, H.; Hatano, R. A Novel insecticide, acetamiprid. In Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor; Yamamoto, I., Casida, J.E., Eds.; Springer: Tokyo, Japan, 1999; pp. 149–176. [Google Scholar] [CrossRef]
- Kiriyama, K.; Itazu, Y.; Kagabu, S.; Nishimura, K. Insecticidal and neuroblocking activities of acetamiprid and related compounds. J. Pestic. Sci. 2003, 28, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Van de Veire, M.; Tirry, L. Side effects of pesticides on four species of beneficials used in IPM in glasshouse vegetable crops:” worst case” laboratory tests. IOBC-WPRS Bull. 2003, 26, 41–50. [Google Scholar]
- Coll, M.; Guershon, M. Omnivory in terrestrial arthropods: Mixing plant and prey diets. Annu. Rev. Èntomol. 2002, 47, 267–297. [Google Scholar] [CrossRef] [Green Version]
- Gammon, D.W.; Liu, Z.; Chandrasekaran, A.; El-Naggar, S.F.; Kuryshev, Y.A.; Jackson, S. Pyrethroid neurotoxicity studies with bifenthrin indicate a mixed Type I/II mode of action. Pest Manag. Sci. 2019, 75, 1190–1197. [Google Scholar] [CrossRef]
- Rabelo, M.M.; Paula-Moraes, S.V.; Pereira, E.J.G.; Siegfried, B.D. Contrasting susceptibility of lepidopteran pests to diamide and pyrethroid insecticides in a region of overwintering and migratory intersection. Pest Manag. Sci. 2020, 76, 4240–4247. [Google Scholar] [CrossRef]
- Soares, M.A.; Carvalho, G.A.; Campos, M.R.; Passos, L.C.; Haro, M.M.; Lavoir, A.; Biondi, A.; Zappalà, L.; Desneux, N. Detrimental sublethal effects hamper the effective use of natural and chemical pesticides in combination with a key natural enemy of Bemisia tabacion tomato. Pest Manag. Sci. 2020, 76, 3551–3559. [Google Scholar] [CrossRef]
- Perdikis, D.; Psaroudaki, S.; Papadoulis, G. Compatibility of Nesidiocoris tenuis and Iphiseius degenerans with insecticides, miticides and fungicides used in tomato crops. Bull. Insectology 2020, 73, 181–192. [Google Scholar]
- Prabhaker, N.; Naranjo, S.; Perring, T.; Castle, S. Comparative toxicities of newer and conventional insecticides: Against four generalist predator species. J. Econ. Èntomol. 2017, 110, 2630–2636. [Google Scholar] [CrossRef]
- Stecca, C.D.S.; Da Silva, D.M.; Bueno, A.D.F.; Pasini, A.; Denez, M.D.; Andrade, K. Selectivity of insecticide use in soybean crop to the predator Podisus nigrispinus (Hemiptera: Pentatomidae). Semin. Ciências Agrárias 2017, 38, 3469–3480. [Google Scholar] [CrossRef]
- Collier, R.; Jukes, A.; Daniel, C.; Hommes, M. Ecological selectivity of pesticides and application methods. IOBC-WPRS Bull. 2016, 118, 94–98. [Google Scholar]
- Duso, C.; Van Leeuwen, T.; Pozzebon, A. Improving the compatibility of pesticides and predatory mites: Recent findings on physiological and ecological selectivity. Curr. Opin. Insect Sci. 2020, 39, 63–68. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, C.; Wang, Y.; Cang, T.; Chen, L.; Yu, R.; Wang, Q. Assessment of toxicity risk of insecticides used in rice ecosystem on Trichogramma japonicum, an egg parasitoid of rice lepidopterans. J. Econ. Èntomol. 2012, 105, 92–101. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.; Cang, T.; Yang, L.; Yu, W.; Zhao, X.; Wang, Q.; Cai, L. Toxicity risk of insecticides to the insect egg parasitoid Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae). Pest Manag. Sci. 2014, 70, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Pazini, J.D.B.; Padilha, A.C.; Cagliari, D.; Bueno, F.A.; Rakes, M.; Zotti, M.J.; Martins, J.F.D.S.; Grützmacher, A.D. Differential impacts of pesticides on Euschistus heros (Hem.: Pentatomidae) and its parasitoid Telenomus podisi (Hym.: Platygastridae). Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bueno, A.D.F.; Batistela, M.J.; Bueno, R.C.O.D.F.; França-Neto, J.D.B.; Nishikawa, M.A.N.; Filho, A.L. Effects of integrated pest management, biological control and prophylactic use of insecticides on the management and sustainability of soybean. Crop. Prot. 2011, 30, 937–945. [Google Scholar] [CrossRef]
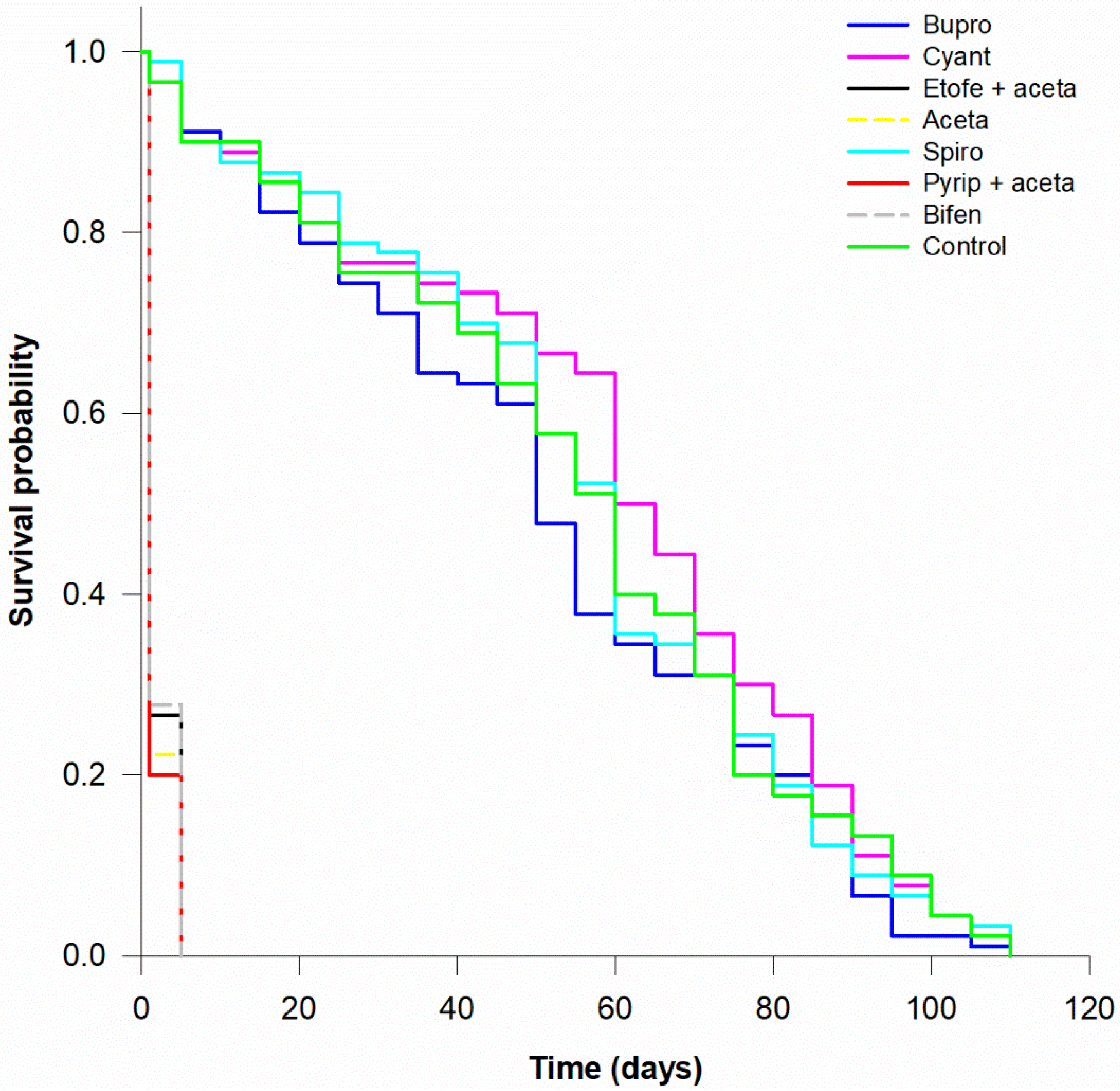
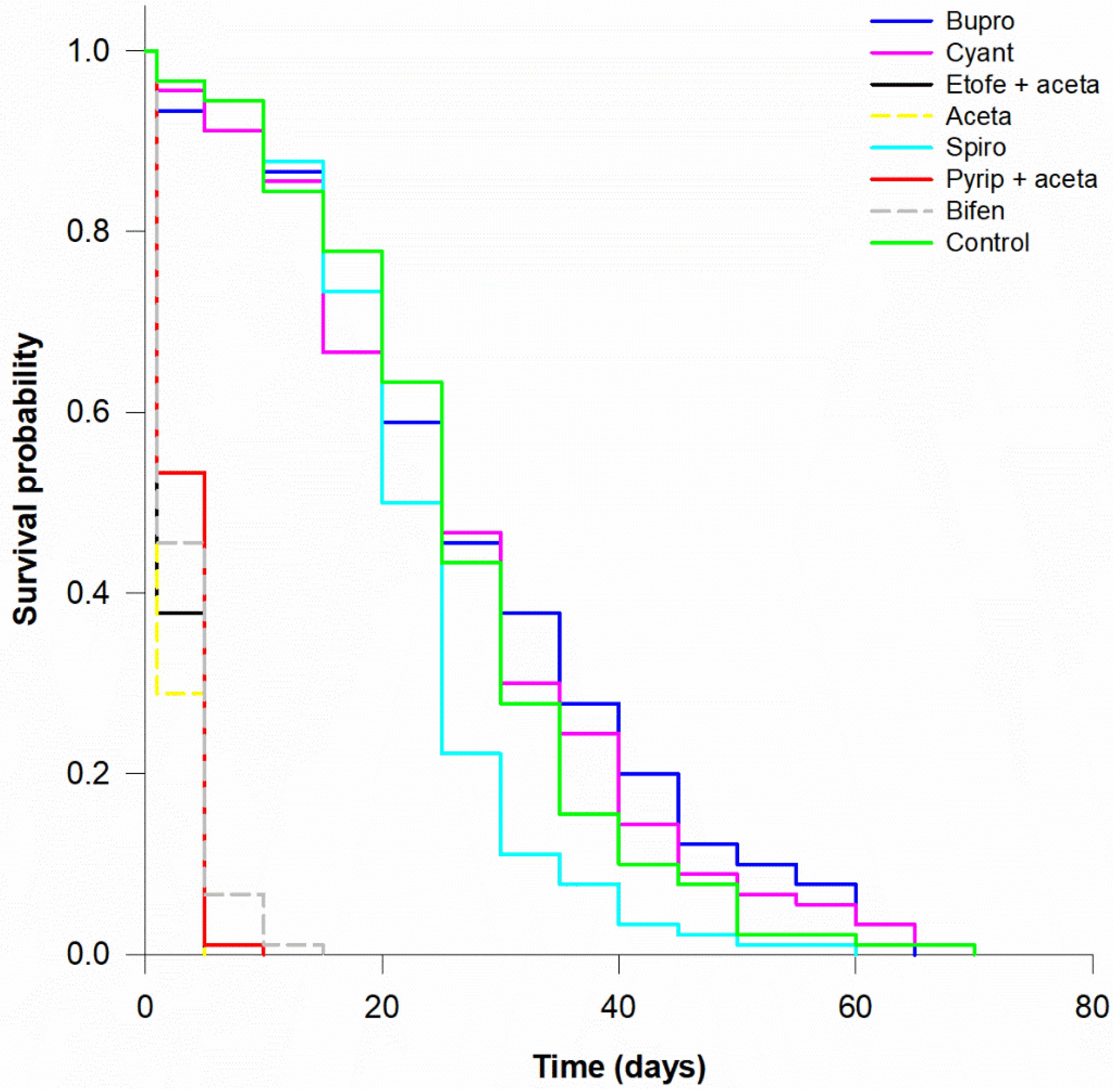
| Active Ingredient | Trade Name | Chemical Group | Exposure Route | Mode of Action | Field Rate (g or mL 100 L−1) | Field Rate (g a.i. ha−1) | |
|---|---|---|---|---|---|---|---|
| a.i. | c.p. | ||||||
| Acetamiprid | Mospilan WG | Neonicotinoid | Systemic | Competitive modulator of nicotinic acetylcholine receptors | 21.8 | 30 | 87 |
| Bifenthrin | Seizer® 10 EC | Pyrethroid | Contact and ingestion | Sodium channel modulator | 1.5 | 15 | 15 |
| Buprofezin | Applaud® 25 WP | Thiadiazinone | Contact | Chitin synthesis inhibitors | 50 | 200 | 500 |
| Cyantraniliprole | Benevia® 10 OD | Diamide | Systemic and contact | Ryanodine receptor modulator | 12.5 | 125 | 50 |
| Etofenprox + acetamiprid | Eleitto® 30 + 16.7 OD | Pyrethroid + Neonicotinoid | Systemic and contact | Sodium channel modulator + competitive modulator of nicotinic acetylcholine receptors | 12 + 6.8 | 40 | 120 + 66.8 |
| Pyriproxyfen + acetamiprid | Privilege® 10 + 20 OD | Pyridyloxypropyl ether + Neonicotinoid | Contact, ingestion, translaminar and systemic | Juvenile hormone mimics + Nicotinic acetylcholine receptor (NACHR) competitive modulators | 3 + 6 | 30 | 30 + 60 |
| Spiromesifen | Oberon® 24 SC | Cetoenol | Contact and ingestion | Inhibitors of acetyl CoA carboxylase | 14.4 | 60 | 144 |
| Treatment | Number of Live Nymphs (n = 15) | Ma (%) * | Class 1 | ||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | |||
| Control | 14.5 ± 0.3 a | 14.0 ± 0.3 a | 13.8 ± 0.3 a | - | - |
| Acetamiprid | 3.3 ± 1.7 b | 1.0 ± 0.7 b | 0.2 ± 0.2 b | 99.9 | 4 |
| Bifenthrin | 4.2 ± 1.3 b | 0.7 ± 0.5 b | 0.0 ± 0.0 b | 100.0 | 4 |
| Buprofezin | 14.5 ± 0.2 a | 14.0 ± 0.2 a | 13.8 ± 0.3 a | 0.0 | 1 |
| Cyantraniliprole | 14.5 ± 0.2 a | 14.0 ± 0.5 a | 13.6 ± 0.5 a | 0.8 | 1 |
| Etofenprox + acetamiprid | 4.0 ± 0.9 b | 0.7 ± 0.3 b | 0.2 ± 0.2 b | 99.9 | 4 |
| Pyriproxyfen + acetamiprid | 3.0 ± 1.1 b | 1.7 ± 0.9 b | 1.2 ± 0.9 b | 87.4 | 4 |
| Spiromesifen | 14.8 ± 0.2 a | 14.6 ± 0.2 a | 14.0 ± 0.4 a | 0.0 | 1 |
| χ2 | 37.3 | 37.6 | 39.0 | - | - |
| df | 7 | 7 | 7 | - | - |
| p | <0.001 | <0.001 | <0.001 | - | - |
| Treatment | Number of Live Adults (n = 15) | Ma (%) * | Class 1 | ||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | |||
| Control | 14.5 ± 0.2 a | 14.5 ± 0.2 a | 14.3 ± 0.3 a | - | - |
| Acetamiprid | 4.1 ± 1.1 c | 2.2 ± 0.9 c | 0.2 ± 0.2 c | 98.6 | 4 |
| Bifenthrin | 6.8 ± 0.8 b | 5.2 ± 0.8 b | 2.8 ± 0.3 b | 80.3 | 4 |
| Buprofezin | 14.0 ± 0.4 a | 14.0 ± 0.4 a | 14.0 ± 0.4 a | 2.1 | 1 |
| Cyantraniliprole | 14.3 ± 0.3 a | 14.0 ± 0.4 a | 13.8 ± 0.5 a | 3.5 | 1 |
| Etofenprox + acetamiprid | 6.2 ± 0.9 b | 2.6 ± 1.0 c | 0.5 ± 0.3 c | 96.5 | 4 |
| Pyriproxyfen + acetamiprid | 7.8 ± 0.9 b | 5.2 ± 0.9 b | 1.8 ± 0.5 b | 87.4 | 4 |
| Spiromesifen | 14.5 ± 0.2 a | 14.3 ± 0.2 a | 14.3 ± 0.2 a | 0.0 | 1 |
| CV (%) | 17.9 | 19.8 | 12.9 | - | - |
| F | 34.9 | 60.9 | 288.2 | - | - |
| df | 7 | 7 | 7 | - | - |
| p | <0.001 | <0.001 | <0.001 | - | - |
| Treatment | LT50 (95% CI) | |||
|---|---|---|---|---|
| Third-Instar Nymphs | Adults | |||
| Control | 55.0 (48.8–61.1) | a | 26.4 (23.7–29.1) | a |
| Acetamiprid | 1.9 (1.5–2.2) | b | 2.2 (1.7–2.5) | b |
| Bifenthrin | 2.1 (1.7–2.5) | b | 3.2 (2.6–3.8) | b |
| Buprofezin | 51.1 (45.1–57.1) | a | 28.5 (25.1–31.8) | a |
| Cyantraniliprole | 58.9 (52.7–65.2) | a | 26.5 (23.3–29.8) | a |
| Etofenprox + acetamiprid | 2.1 (1.7–2.4) | b | 2.5 (2.1–2.9) | b |
| Pyriproxyfen + acetamiprid | 1.8 (1.5–2.1) | b | 3.2 (2.8–3.6) | b |
| Spiromesifen | 55.4 (49.5–61.3) | a | 22.6 (20.5–24.7) | a |
| χ2 | 686.96 | 661.1 | ||
| df | 7 | 7 | ||
| p | <0.001 | <0.001 | ||
| Insecticides | LC50 (95% CI) (mg a.i. L−1) | χ2 | df | RQ | Category * |
|---|---|---|---|---|---|
| Acetamiprid | 0.26 (0.16–0.35) | 11.58 | 4 | 334.6 | 2 |
| Bifenthrin | 0.38 (0.29–0.48) | 30.34 | 7 | 3.95 | 1 |
| Etofenprox + acetamiprid | 4.80 (3.28–6.31) | 32.07 | 5 | 38.91 | 1 |
| Pyriproxyfen + acetamiprid | 8.71 (6.18–11.25) | 65.86 | 4 | 10.33 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matioli, T.F.; da Silva, M.R.; de Bastos Pazini, J.; Barroso, G.; Vieira, J.G.A.; Yamamoto, P.T. Risk Assessment of Insecticides Used in Tomato to Control Whitefly on the Predator Macrolophus basicornis (Hemiptera: Miridae). Insects 2021, 12, 1092. https://doi.org/10.3390/insects12121092
Matioli TF, da Silva MR, de Bastos Pazini J, Barroso G, Vieira JGA, Yamamoto PT. Risk Assessment of Insecticides Used in Tomato to Control Whitefly on the Predator Macrolophus basicornis (Hemiptera: Miridae). Insects. 2021; 12(12):1092. https://doi.org/10.3390/insects12121092
Chicago/Turabian StyleMatioli, Thaís Fagundes, Mariana Rosa da Silva, Juliano de Bastos Pazini, Geovanny Barroso, Júlia Gabriela Aleixo Vieira, and Pedro Takao Yamamoto. 2021. "Risk Assessment of Insecticides Used in Tomato to Control Whitefly on the Predator Macrolophus basicornis (Hemiptera: Miridae)" Insects 12, no. 12: 1092. https://doi.org/10.3390/insects12121092
APA StyleMatioli, T. F., da Silva, M. R., de Bastos Pazini, J., Barroso, G., Vieira, J. G. A., & Yamamoto, P. T. (2021). Risk Assessment of Insecticides Used in Tomato to Control Whitefly on the Predator Macrolophus basicornis (Hemiptera: Miridae). Insects, 12(12), 1092. https://doi.org/10.3390/insects12121092







