Insecticide Resistance Monitoring in Field Populations of the Whitebacked Planthopper Sogatella furcifera (Horvath) in China, 2019–2020
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Populations
2.2. Insecticides
2.3. Bioassays
2.4. Enzyme Assays
2.5. Data Analyses
3. Results
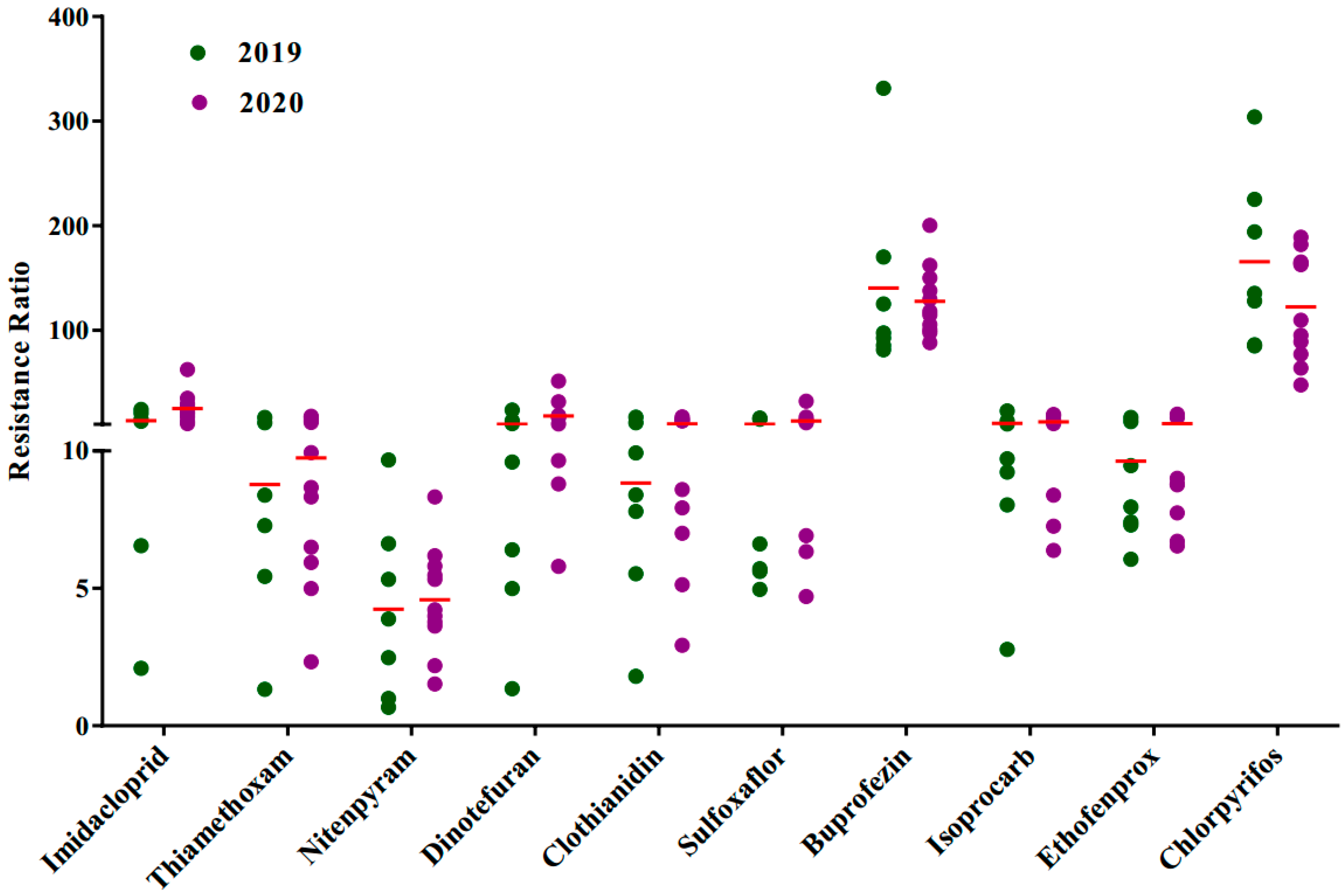
3.1. Insecticide Resistance
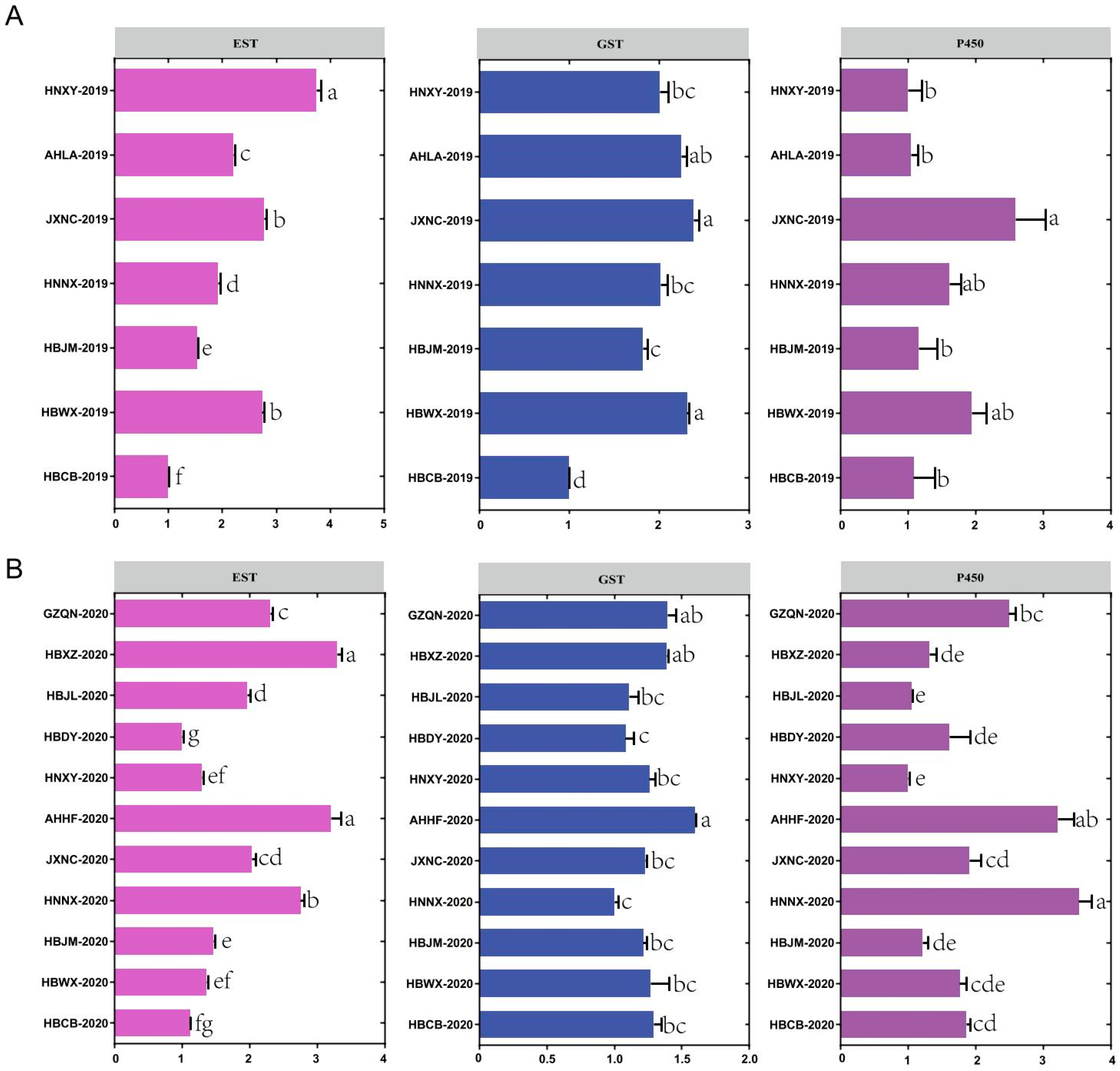
3.2. Enzyme Activity
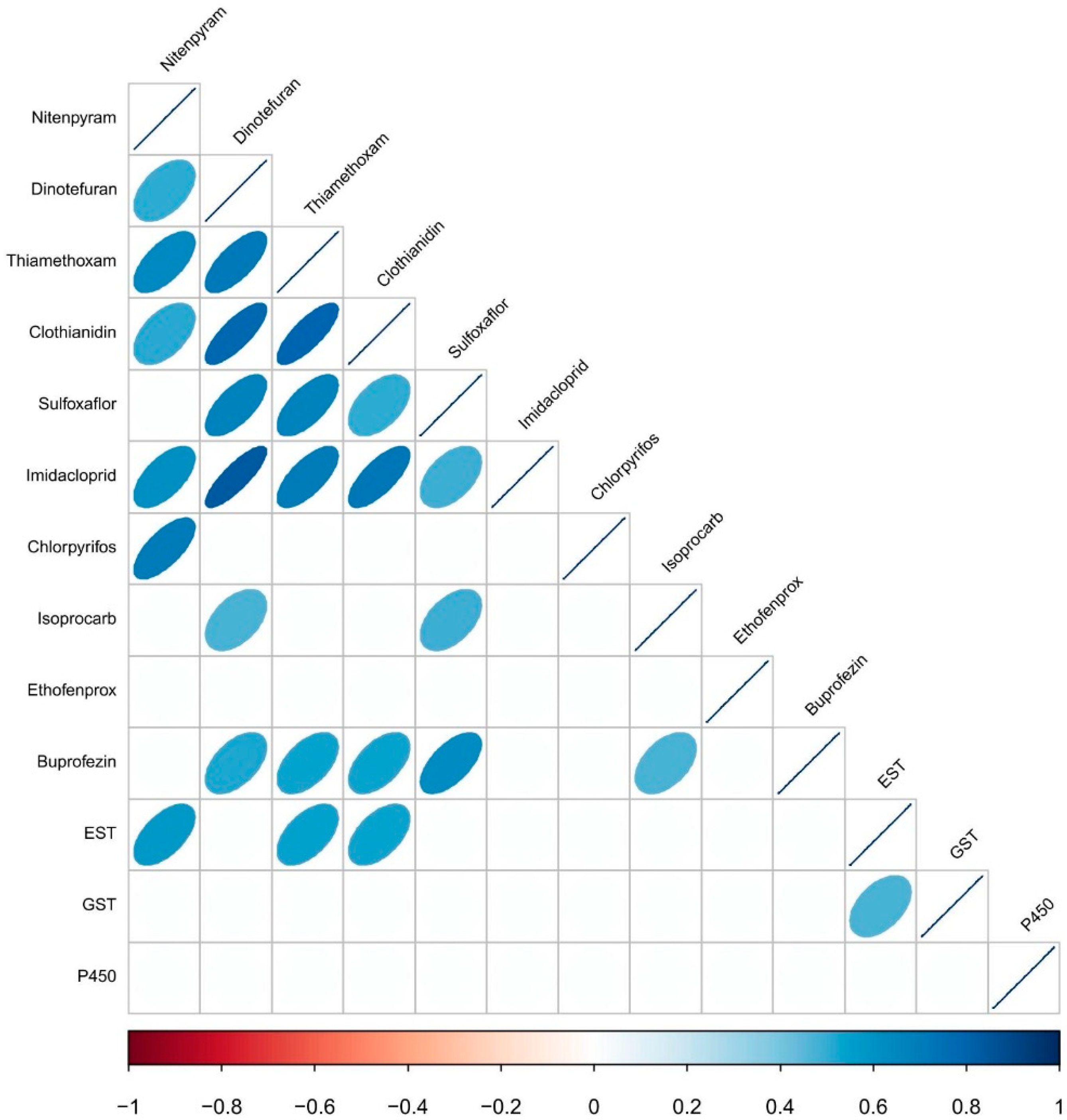
3.3. Pairwise Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Horgan, F.G.; Srinivasan, T.S.; Naik, B.S.; Ramal, A.F.; Bernal, C.C.; Almazan, M.L.P. Effects of nitrogen on egg-laying inhibition and ovicidal response in planthopper-resistant rice varieties. Crop Prot. 2016, 89, 223–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumura, M.; Sanada-Morimura, S.; Otuka, A.; Ohtsu, R.; Sakumoto, S.; Takeuchi, H.; Satoh, M. Insecticide susceptibilities in populations of two rice planthoppers, Nilaparvata lugens and Sogatella furcifera, immigrating into Japan in the period 2005–2012. Pest Manag. Sci. 2014, 70, 615–622. [Google Scholar] [CrossRef]
- Lei, W.; Liu, D.; Li, P.; Hou, M. Interactive effects of southern rice black-streaked dwarf virus infection of host plant and vector on performance of the vector, Sogatella furcifera (Homoptera: Delphacidae). J. Econ. Entomol. 2014, 107, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Xie, G.; Ji, C.; Ling, B.; Zhang, M.; Xu, D.; Zhou, G. Transmission characteristics of Southern rice black-streaked dwarf virus by rice planthoppers. Crop Prot. 2012, 41, 71–76. [Google Scholar] [CrossRef]
- Endo, S.; Nagata, T.; Kawabe, S.; Kazano, H. Changes of insecticide susceptibility of the white backed planthopper Sogatella furcifera Horvath (Homoptera:Delphacidae) and the brown planthopper Nilaparvata lugens Stal (Homoptera:Delphacidae). Appl. Entomol. Zool. 1988, 23, 417–421. [Google Scholar] [CrossRef] [Green Version]
- Ping, X.; Endo, S.; Suzuki, K.; Ohtsu, K. The insecticide susceptibility of the brown planthopper, Nilaparvata lugens, and white-backed planthopper, Sogatella furcifera, collected from China and Japan. Kyushu Plant Prot. Res. 2001, 47, 54–57. [Google Scholar] [CrossRef]
- Li, W.; Mao, K.; Liu, C.; Gong, P.; Xu, P.; Wu, G.; Le, W.; Wan, H.; You, H.; Li, J. Resistance monitoring and assessment of the control failure likelihood of insecticides in field populations of the whitebacked planthopper Sogatella furcifera (Horváth). Crop Prot. 2020, 127, 104973. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, X.; Mao, K.; Yang, P.; Li, D.; Ali, E.; Wan, H.; Li, J. Neonicotinoid insecticide resistance in the field populations of Sogatella furcifera (Horváth) in Central China from 2011 to 2015. J. Asia. Pac. Entomol. 2017, 20, 955–958. [Google Scholar] [CrossRef]
- APRD. Arthropod Pesticide Resistance Database. 2021. Available online: http://www.pesticideresistance.org/search/Php/ (accessed on 12 August 2021).
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Ali, E.; Mao, K.; Liao, X.; Jin, R.; Li, J. Cross-resistance and biochemical characterization of buprofezin resistance in the white-backed planthopper, Sogatella furcifera (Horvath). Pestic. Biochem. Physiol. 2019, 158, 47–53. [Google Scholar] [CrossRef]
- Ruan, Y.; Wang, X.; Xiang, X.; Xu, X.; Guo, Y.; Liu, Y.; Yin, Y.; Wu, Y.; Cheng, Q.; Gong, C.; et al. Status of insecticide resistance and biochemical characterization of chlorpyrifos resistance in Sogatella furcifera (Hemiptera:Delphacidae) in Sichuan Province, China. Pestic. Biochem. Physiol. 2021, 171, 104723. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.Q.; Huang, B.; Jiang, Y.P.; Gu, H.T.; Xia, T.; Yang, G.Q.; Liu, F.; Wu, J.C. Carboxylesterase precursor (EST-1) mediated the fungicide jinggangmycin-suppressed reproduction of Sogatella furcifera (Hemiptera: Delphacidae). J. Econ. Entomol. 2017, 110, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- IRAC. Insecticide Resistance Action Committee. 2021. Available online: https://www.irac-online.org/modes-of-action/ (accessed on 25 October 2019).
- Liao, X.; Mao, K.; Ali, E.; Zhang, X.; Wan, H.; Li, J. Temporal variability and resistance correlation of sulfoxaflor susceptibility among Chinese populations of the brown planthopper Nilaparvata lugens (Stål). Crop Prot. 2017, 102, 141–146. [Google Scholar] [CrossRef]
- Fouad, E.A.; Abou-Yousef, H.M.; Abdallah, I.S.; Kandil, M.A. Resistance monitoring and enzyme activity in three field populations of cowpea aphid (Aphis craccivora) from Egypt. Crop Prot. 2016, 81, 163–167. [Google Scholar] [CrossRef]
- Hangay, G.; Gayubo, S.F.; Hoy, M.A.; Goula, M.; Sanborn, A.; Morrill, W.L.; Gäde, G.; Marco, H.G.; Kabissa, J.C.B.; Ellis, J. Abbott’s Formula. Encyclopedia of Entomology; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Aitio, A. A simple and sensitive assay of 7-ethoxycoumarin deethylation. Anal. Biochem. 1978, 85, 488–491. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, G.; Zhong, L.; Zhang, F.; Bai, Q.; Zheng, X.; Lu, Z. Resistance monitoring of Chilo suppressalis (Walker) (Lepidoptera: Crambidae) to chlorantraniliprole in eight field populations from east and central China. Crop Prot. 2017, 100, 196–202. [Google Scholar] [CrossRef]
- Peng, Z.; Zheng, H.; Xie, W.; Wang, S.; Wu, Q.; Zhang, Y. Field resistance monitoring of the immature stages of the whitefly Bemisia tabaci to spirotetramat in China. Crop Prot. 2017, 98, 243–247. [Google Scholar] [CrossRef]
- Sparks, T.C.; Crossthwaite, A.J.; Nauen, R.; Banba, S.; Cordova, D.; Earley, F.; Ebbinghaus-Kintscher, U.; Fujioka, S.; Hirao, A.; Karmon, D.; et al. Insecticides, biologics and nematicides: Updates to IRAC’s mode of action classification—A tool for resistance management. Pestic. Biochem. Physiol. 2020, 167, 104587. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Nauen, R. Neonicotinoids-from zero to hero in insecticide chemistry. Pest Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef]
- Rao, Q.; Xu, Y.; Luo, C.; Zhang, H.; Jones, C.M.; Devine, G.J.; Gorman, K.; Denholm, I. Characterisation of neonicotinoid and pymetrozine resistance in strains of Bemisia tabaci (Hemiptera: Aleyrodidae) from China. J. Integr. Agric. 2012, 11, 321–326. [Google Scholar] [CrossRef]
- Matsumura, M.; Sanada-Morimura, S.; Otuka, A.; Sonoda, S.; Van Thanh, D.; Van Chien, H.; Van Tuong, P.; Loc, P.M.; Liu, Z.W.; Zhu, Z.R.; et al. Insecticide susceptibilities of the two rice planthoppers Nilaparvata lugens and Sogatella furcifera in East Asia, the Red River Delta, and the Mekong Delta. Pest Manag. Sci. 2018, 74, 456–464. [Google Scholar] [CrossRef]
- Mu, X.C.; Zhang, W.; Wang, L.X.; Zhang, S.; Zhang, K.; Gao, C.F.; Wu, S.F. Resistance monitoring and cross-resistance patterns of three rice planthoppers, Nilaparvata lugens, Sogatella furcifera and Laodelphax striatellus to dinotefuran in China. Pestic. Biochem. Physiol. 2016, 134, 8–13. [Google Scholar] [CrossRef]
- Endo, S.; Tsurumachi, M. Insecticide susceptibility of the brown planthopper and the white-backed planthopper collected from Southeast Asia. J. Pestic. Sci. 2001, 26, 82–86. [Google Scholar] [CrossRef] [Green Version]
- Nagata, T. Monitoring on insecticide resistance of the brown planthopper and the white backed planthopper in Asia. J. Asia-Pac. Entomol. 2002, 5, 103–111. [Google Scholar] [CrossRef]
- Nagata, T.; Masuda, T. Insecticide susceptibility and wing-form ratio of the brown planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae) and the white backed planthopper, Sogatella furcifera (Horvath) (Hemiptera: Delphacidae) of Southeast Asia. Appl. Entomol. Zool. 1980, 15, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Wang, Z.; Zhang, K.; Tian, X.; Yin, Y.; Zhao, X.; Shen, A.; Gao, C.F. Status of insecticide resistance of the whitebacked planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Florida Entomol. 2013, 96, 948–956. [Google Scholar] [CrossRef]
- Kisimoto, R. Synoptic weather conditions inducing long-distance immigration of planthoppers, Sogatella furcifera Horvath and Nilaparvata lugens Stal. Ecol. Entomol. 1976, 1, 95–109. [Google Scholar] [CrossRef]
- Nagata, T.; Kamimuro, T.; Wang, Y.C.; Han, S.G.; Noor, N.M. Recent status of insecticide resistance of long-distance migrating rice planthoppers monitored in Japan, China and Malaysia. J. Asia-Pac. Entomol. 2002, 5, 113–116. [Google Scholar] [CrossRef]
- Cordova, D.; Benner, E.A.; Schroeder, M.E.; Holyoke, C.W.; Zhang, W.; Pahutski, T.F.; Leighty, R.M.; Vincent, D.R.; Hamm, J.C. Mode of action of triflumezopyrim: A novel mesoionic insecticide which inhibits the nicotinic acetylcholine receptor. Insect Biochem. Mol. Biol. 2016, 74, 32–41. [Google Scholar] [CrossRef]
- Sparks, T.C.; Watson, G.B.; Loso, M.R.; Geng, C.; Babcock, J.M.; Thomas, J.D. Sulfoxaflor and the sulfoximine insecticides: Chemistry, mode of action and basis for efficacy on resistant insects. Pestic. Biochem. Physiol. 2013, 107, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Loso, M.R.; Watson, G.B.; Sparks, T.C.; Rogers, R.B.; Huang, J.X.; Gerwick, B.C.; Babcock, J.M.; Kelley, D.; Hegde, V.B.; et al. Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap-feeding pests. J. Agric. Food Chem. 2011, 59, 2950–2957. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Jin, R.; Zhang, X.; Ali, E.; Mao, K.; Xu, P.; Li, J.; Wan, H. Characterization of sulfoxaflor resistance in the brown planthopper, Nilaparvata lugens (Stål). Pest Manag. Sci. 2019, 75, 1646–1654. [Google Scholar] [CrossRef]
- Hu, Z.; Feng, X.; Lin, Q.; Chen, H.; Li, Z.; Yin, F.; Liang, P.; Gao, X. Biochemical mechanism of chlorantraniliprole resistance in the diamondback moth, Plutella xylostella Linnaeus. J. Integr. Agric. 2014, 13, 2452–2459. [Google Scholar] [CrossRef] [Green Version]
- Mao, K.; Ren, Z.; Li, W.; Cai, T.; Qin, X.; Wan, H.; Jin, B.R.; He, S.; Li, J. Carboxylesterase genes in nitenpyram-resistant brown planthoppers, Nilaparvata lugens. Insect Sci. 2021, 28, 1049–1060. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Gao, X.; Song, D. Functional analysis of cytochrome P450 genes linked with acetamiprid resistance in melon aphid, Aphis gossypii. Pestic. Biochem. Physiol. 2020, 170, 104687. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Lin, Q.; Wang, X.; Li, Z.; Feng, X.; Shabbir, M.Z. The glutathione S-transferase (PxGST2L) may contribute to the detoxification metabolism of chlorantraniliprole in Plutella xylostella (L.). Ecotoxicology 2021, 30, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Bergé, J.B.; Feyereisen, R.; Amichot, M. Cytochrome P450 monooxygenases and insecticide resistance in insects. Philos. Trans. R. Soc. B Biol. Sci. 1998, 353, 1701–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Chai, P.; Zheng, C.; Xu, H.; Wu, Y.; Gao, X.; Xi, J.; Shang, Q. Contribution of cytochrome P450 monooxygenase CYP380C6 to spirotetramat resistance in Aphis gossypii Glover. Pestic. Biochem. Physiol. 2018, 148, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Ma, Z.; Zhai, D.; Gao, X.; Shi, X. Cytochrome P450 monooxygenases-mediated sex-differential spinosad resistance in house flies Musca domestica (Diptera: Muscidae). Pestic. Biochem. Physiol. 2019, 157, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Roush, R.T. Designing resistance management programs: How can you choose? Pestic. Sci. 1989, 26, 423–441. [Google Scholar] [CrossRef]
- Elzaki, M.E.A.; Zhang, W.; Han, Z. Cytochrome P450 CYP4DE1 and CYP6CW3v2 contribute to ethiprole resistance in Laodelphax striatellus (Fallén). Insect Mol. Biol. 2015, 24, 368–376. [Google Scholar] [CrossRef]
- Kalsi, M.; Palli, S.R. Transcription factor cap n collar C regulates multiple cytochrome P450 genes conferring adaptation to potato plant allelochemicals and resistance to imidacloprid in Leptinotarsa decemlineata (Say). Insect Biochem. Mol. Biol. 2017, 83, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.; Tang, Q.; Zhang, B.; Liang, P.; Wang, B.; Gao, X. Overexpression of multiple cytochrome P450 genes associated with sulfoxaflor resistance in Aphis gossypii Glover. Pestic. Biochem. Physiol. 2019, 157, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Zhang, X.; Ali, E.; Liao, X.; Jin, R.; Ren, Z.; Wan, H.; Li, J. Characterization of nitenpyram resistance in Nilaparvata lugens (Stål). Pestic. Biochem. Physiol. 2019, 157, 26–32. [Google Scholar] [CrossRef] [PubMed]
| Populations | Sampling Sites | Collection Date | Site | Insect Stage | Number |
|---|---|---|---|---|---|
| HBCB-2019 | Chibi, Hubei | 15 July 2019 | 29°31′ N, 113°42′ E | Nymph and adult | 1206 |
| HBWX-2019 | Wuxue, Hubei | 6 September 2019 | 29°57′ N, 115°36′ E | Nymph and adult | 1374 |
| HBJM-2019 | Jingmen, Hubei | 5 September 2019 | 30°32′ N, 112°18′ E | Nymph and adult | 1453 |
| HNNX-2019 | Ningxiang, Hunan | 20 June 2019 | 28°10′ N, 112°33′ E | Nymph and adult | 1069 |
| JXNC-2019 | Nanchang, Jiangxi | 12 June 2019 | 28°32′ N, 115°58′ E | Nymph and adult | 1146 |
| AHLA-2019 | Luan, Anhui | 10 July 2019 | 31°32′ N, 116°18′ E | Nymph and adult | 1073 |
| HNXY-2019 | Xinyang, Henan | 14 July 2019 | 31°58′ N, 115°24′ E | Nymph and adult | 1421 |
| HBDY-2020 | Dangyang, Hubei | 19 July 2020 | 30°59′ N, 111°52′ E | Nymph and adult | 1306 |
| HBCB-2020 | Chibi, Hubei | 21 July 2020 | 29°31′ N, 113°42′ E | Nymph and adult | 1154 |
| HBWX-2020 | Wuxue, Hubei | 8 August 2020 | 30°07′ N, 115°36′ E | Nymph and adult | 1073 |
| HBJL-2020 | Jianli, Hubei | 25 July 2020 | 29°49′ N, 112°54′ E | Nymph and adult | 1209 |
| HBJM-2020 | Jingmen, Hubei | 14 July 2020 | 30°54′ N, 112°14′ E | Nymph and adult | 1043 |
| HBXZ-2020 | Xinzhou, Hubei | 14 July 2020 | 30°51′ N, 114°37′ E | Nymph and adult | 1194 |
| HNNX-2020 | Ningxiang, Hunan | 3 August 2020 | 28°10′ N, 112°33′ E | Nymph and adult | 1206 |
| JXNC-2020 | Nanchang, Jiangxi | 25 July 2020 | 28°32′ N, 115°58′ E | Nymph and adult | 1311 |
| AHHF-2020 | Luan, Anhui | 12 August 2020 | 31°31′ N, 116°37′ E | Nymph and adult | 1248 |
| HNXY-2020 | Xinyang, Henan | 15 August 2020 | 31°58′ N, 115°24′ E | Nymph and adult | 1076 |
| GZQN-2020 | Qiannan, Guizhou | 15 August 2020 | 25°59′ N, 106°35′ E | Nymph and adult | 1108 |
| Group | Insecticide | LC50 (95%CI a) (mg/L) | Reference |
|---|---|---|---|
| neonicotinoids | imidacloprid | 0.11 (0.06–0.17) | Su et al., 2013 |
| thiamethoxam | 0.18 (0.11–0.23) | Su et al., 2013 | |
| nitenpyram | 0.27 (0.19–0.36) | Zhang et al., 2017 | |
| dinotefuran | 0.20 (0.16–0.25) | Li et al., 2020 | |
| clothianidin | 0.15 (0.09–0.21) | Zhang et al., 2017 | |
| Sulfoximines | sulfoxaflor | 0.50 (0.33–0.66) | Li et al., 2020 |
| Insect growth regulators | buprofezin | 0.04 (0.03–0.06) | Li et al., 2020 |
| Carbamates | isoprocarb | 9.42 (6.97–11.80) | Li et al., 2020 |
| Pyrethroids | ethofenprox | 34.61 (20.28–52.76) | Li et al., 2020 |
| Organophosphates | chlorpyrifos | 0.24 (0.17–0.31) | Li et al., 2020 |
| A | ||||||||||||
| Population | Imidacloprid | Thiamethoxam | ||||||||||
| No. | Slope (SE a) | LC50 (95% CI b; mg/L) | χ2 (df) | p Value | RR c | No. | Slope (SE a) | LC50 (95% CI b; mg/L) | χ2 (df) | p Value | RR c | |
| HBCB-2019 | 270 | 2.00 (0.27) | 0.72 (0.49–0.94) | 0.67 (3) | 0.88 | 6.55 | 270 | 1.53 (0.23) | 1.31 (0.99–1.90) | 5.62 (3) | 0.13 | 7.28 |
| HBWX-2019 | 270 | 1.88 (0.25) | 0.23 (0.17–0.30) | 0.89 (3) | 0.83 | 2.09 | 270 | 1.64 (0.23) | 0.24 (0.17–0.32) | 3.08 (3) | 0.38 | 1.33 |
| HBJM-2019 | 270 | 2.32 (0.27) | 1.43 (1.14–1.74) | 1.11 (3) | 0.77 | 13.00 | 270 | 1.67 (0.23) | 0.98 (0.77–1.30) | 0.69 (3) | 0.88 | 5.44 |
| HNNX-2019 | 270 | 1.72 (0.24) | 1.63 (1.11–2.14) | 0.82 (3) | 0.84 | 14.82 | 270 | 2.02 (0.25) | 1.51 (1.21–1.87) | 4.37 (3) | 0.22 | 8.39 |
| JXNC-2019 | 270 | 1.90 (0.28) | 2.28 (1.66–3.82) | 1.63 (3) | 0.65 | 20.73 | 270 | 1.62 (0.25) | 2.08 (1.50–3.50) | 1.84 (3) | 0.61 | 11.56 |
| AHLA-2019 | 270 | 2.48 (0.28) | 2.67 (2.19–3.21) | 2.16 (3) | 0.54 | 24.27 | 270 | 1.70 (0.24) | 2.99 (2.29–4.35) | 4.08 (3) | 0.25 | 16.61 |
| HNXY-2019 | 270 | 1.65 (0.23) | 1.46 (1.08–1.88) | 2.04 (3) | 0.56 | 13.27 | 270 | 1.54 (0.24) | 1.96 (1.41–3.29) | 1.17 (3) | 0.11 | 10.88 |
| HBDY-2020 | 270 | 1.68 (0.23) | 1.19 (0.92–1.63) | 1.78 (3) | 0.58 | 10.82 | 270 | 1.81 (0.24) | 1.07 (0.79–1.35) | 0.95 (3) | 0.81 | 5.94 |
| HBCB-2020 | 270 | 1.30 (0.22) | 1.88 (1.37–2.66) | 2.28 (3) | 0.19 | 17.09 | 270 | 1.30 (0.22) | 1.17 (0.86–1.75) | 3.61 (3) | 0.31 | 6.50 |
| HBWX-2020 | 270 | 0.82 (0.21) | 1.40 (0.77–6.04) | 3.20 (3) | 0.86 | 12.73 | 270 | 1.17 (0.21) | 0.42 (0.30–0.61) | 2.29 (3) | 0.51 | 2.33 |
| HBJL-2020 | 270 | 1.17 (0.21) | 2.94 (2.09–4.61) | 3.72 (3) | 0.84 | 26.73 | 270 | 1.84 (0.26) | 1.56 (1.17–2.33) | 3.54 (2) | 0.17 | 8.67 |
| HBJM-2020 | 270 | 2.33 (0.27) | 2.38 (1.97–2.94) | 2.45 (3) | 0.87 | 21.64 | 270 | 1.86 (0.25) | 2.53 (2.00–3.43) | 1.31 (3) | 0.73 | 14.06 |
| HBXZ-2020 | 270 | 1.32 (0.22) | 2.28 (1.68–3.20) | 0.79 (3) | 0.42 | 20.73 | 270 | 1.27 (0.22) | 3.22 (2.29–5.46) | 3.73 (3) | 0.29 | 17.89 |
| HNNX-2020 | 270 | 1.43 (0.22) | 3.25 (2.43–4.82) | 2.44 (3) | 0.40 | 29.55 | 270 | 2.08 (0.25) | 1.50 (1.21–1.85) | 3.06 (3) | 0.38 | 8.33 |
| JXNC-2020 | 270 | 2.13 (0.25) | 2.02 (1.64–2.49) | 0.57 (3) | 0.61 | 18.36 | 270 | 2.10 (0.25) | 1.79 (1.45–2.22) | 3.09 (3) | 0.38 | 9.94 |
| AHHF-2020 | 270 | 1.58 (0.26) | 6.88 (4.64–13.67) | 2.18 (3) | 0.80 | 62.55 | 270 | 2.20 (0.26) | 2.13 (1.74–2.69) | 0.31 (3) | 0.96 | 11.83 |
| HNXY-2020 | 270 | 2.58 (0.31) | 2.21 (1.68–2.71) | 1.59 (3) | 0.29 | 20.09 | 270 | 1.84 (0.26) | 0.90 (0.60–1.19) | 2.09 (3) | 0.55 | 5.00 |
| GZQN-2020 | 270 | 2.35 (0.29) | 3.86 (3.09–5.24) | 3.83 (3) | 0.21 | 35.09 | 270 | 1.70 (0.28) | 3.01 (2.01–6.19) | 1.88 (3) | 0.60 | 16.72 |
| B | ||||||||||||
| Population | Nitenpyram | Dinotefuran | ||||||||||
| No. | Slope (SE a) | LC50 (95% CI b; mg/L) | χ2 (df) | p Value | RR c | No. | Slope (SE a) | LC50 (95% CI b; mg/L) | χ2 (df) | p Value | RR c | |
| HBCB-2019 | 270 | 1.56 (0.23) | 0.67 (0.51–0.96) | 3.34 (3) | 0.34 | 2.48 | 270 | 2.12 (0.25) | 1.92 (1.55–2.36) | 0.57 (3) | 0.90 | 9.60 |
| HBWX-2019 | 270 | 1.88 (0.24) | 0.27 (0.20–0.34) | 3.51 (3) | 0.32 | 1.00 | 270 | 2.25 (0.27) | 0.27 (0.21–0.33) | 1.31 (3) | 0.73 | 1.35 |
| HBJM-2019 | 270 | 1.56 (0.26) | 0.12 (0.06–0.17) | 2.47 (3) | 0.48 | 0.67 | 270 | 2.14 (0.28) | 1.00 (0.71–1.27) | 0.32 (3) | 0.96 | 5.00 |
| HNNX-2019 | 270 | 2.79 (0.30) | 1.44 (1.20–1.70) | 2.02 (3) | 0.57 | 5.33 | 270 | 2.76 (0.31) | 2.70 (2.27–3.30) | 0.31 (3) | 0.96 | 13.50 |
| JXNC-2019 | 270 | 2.35 (0.27) | 1.05 (0.87–1.32) | 0.04 (3) | 0.99 | 3.89 | 270 | 1.69 (0.25) | 4.75 (3.50–7.56) | 1.14 (3) | 0.77 | 23.75 |
| AHLA-2019 | 270 | 1.85 (0.24) | 1.79 (1.42–2.28) | 2.00 (3) | 0.57 | 6.63 | 270 | 2.03 (0.25) | 2.13 (1.62–2.65) | 1.05 (3) | 0.79 | 10.65 |
| HNXY-2019 | 270 | 1.70 (0.24) | 2.61 (2.02–3.64) | 4.10 (3) | 0.25 | 9.67 | 270 | 2.72 (0.37) | 1.28 (0.87–1.65) | 1.59 (3) | 0.66 | 6.40 |
| HBDY-2020 | 270 | 2.00 (0.33) | 0.41 (0.20–0.61) | 1.78 (3) | 0.62 | 1.52 | 270 | 2.10 (0.25) | 1.93 (1.56–2.38) | 3.33 (3) | 0.34 | 9.65 |
| HBCB-2020 | 270 | 2.03 (0.25) | 1.08 (0.83–1.35) | 2.28 (3) | 0.52 | 4.00 | 270 | 2.12 (0.28) | 1.76 (1.20–2.29) | 5.24 (3) | 0.16 | 8.80 |
| HBWX-2020 | 270 | 2.75 (0.31) | 0.59 (0.49–0.74) | 3.20 (3) | 0.36 | 2.19 | 270 | 1.58 (0.25) | 1.16 (0.81–2.09) | 2.42 (3) | 0.49 | 5.80 |
| HBJL-2020 | 270 | 1.73 (0.24) | 1.48 (1.14–2.13) | 3.72 (3) | 0.29 | 5.48 | 270 | 2.06 (0.26) | 6.30 (5.06–8.32) | 3.82 (3) | 0.28 | 31.50 |
| HBJM-2020 | 270 | 1.91 (0.24) | 1.44 (1.13–1.80) | 2.45 (3) | 0.48 | 5.33 | 270 | 1.77 (0.24) | 3.25 (2.54–4.49) | 0.62 (3) | 0.89 | 16.25 |
| HBXZ-2020 | 270 | 1.73 (0.23) | 2.25 (1.76–3.02) | 0.79 (3) | 0.85 | 8.33 | 270 | 2.61 (0.32) | 3.72 (3.02–4.92) | 2.99 (3) | 0.39 | 18.60 |
| HNNX-2020 | 270 | 2.16 (0.25) | 1.67 (1.36–2.06) | 2.44 (3) | 0.49 | 6.19 | 270 | 1.44 (0.23) | 3.52 (2.62–5.33) | 2.10 (3) | 0.55 | 17.60 |
| JXNC-2020 | 270 | 2.03 (0.26) | 0.98 (0.73–1.23) | 0.57 (3) | 0.90 | 3.63 | 270 | 1.62 (0.22) | 2.10 (1.62–2.74) | 1.18 (3) | 0.76 | 10.50 |
| AHHF-2020 | 270 | 2.34 (0.27) | 1.57 (1.29–1.91) | 2.18 (3) | 0.53 | 5.81 | 270 | 1.57 (0.24) | 3.28 (2.48–4.78) | 0.90 (3) | 0.83 | 16.40 |
| HNXY-2020 | 270 | 2.02 (0.25) | 1.14 (0.88–1.42) | 1.59 (3) | 0.67 | 4.22 | 270 | 1.77 (0.25) | 2.12 (1.47–2.76) | 2.56 (3) | 0.47 | 10.60 |
| GZQN-2020 | 270 | 2.33 (0.27) | 1.02 (0.84–1.27) | 3.83 (3) | 0.28 | 3.78 | 270 | 2.15 (0.30) | 10.29 (7.76–15.90) | 1.80 (3) | 0.62 | 51.45 |
| C | ||||||||||||
| Population | Clothianidin | Sulfoxaflor | ||||||||||
| No. | Slope (SE a) | LC50 (95% CI b; mg/L) | χ2 (df) | p Value | RR c | No. | Slope (SE a) | LC50 (95% CI b; mg/L) | χ2 (df) | p Value | RR c | |
| HBCB-2019 | 270 | 2.10 (0.26) | 1.17 (0.92–1.45) | 0.91 (3) | 0.82 | 7.80 | 270 | 2.49 (0.32) | 3.31 (2.32–4.22) | 1.36 (3) | 0.82 | 6.62 |
| HBWX-2019 | 270 | 2.42 (0.28) | 0.27 (0.21–0.33) | 0.63 (3) | 0.89 | 1.80 | 270 | 2.31 (0.28) | 2.47 (1.90–3.03) | 3.10 (3) | 0.38 | 4.94 |
| HBJM-2019 | 270 | 2.64 (0.32) | 0.83 (0.62–1.03) | 1.80 (3) | 0.61 | 5.53 | 270 | 2.86 (0.32) | 2.86(2.33–3.39) | 2.50 (3) | 0.48 | 5.72 |
| HNNX-2019 | 270 | 2.32 (0.27) | 1.26 (1.01–1.52) | 0.99 (3) | 0.80 | 8.40 | 270 | 2.22 (0.26) | 8.08 (6.61–10.21) | 0.73 (3) | 0.86 | 16.16 |
| JXNC-2019 | 270 | 1.65 (0.26) | 2.53 (1.75–4.69) | 1.76 (3) | 0.62 | 16.87 | 270 | 1.73 (0.23) | 7.51 (5.91–9.89) | 8.31 (3) | 0.04 | 15.02 |
| AHLA-2019 | 270 | 1.66 (0.23) | 1.49 (1.15–1.92) | 2.39 (3) | 0.50 | 9.93 | 270 | 1.88 (0.24) | 8.14 (6.50–10.54) | 0.67 (3) | 0.88 | 16.28 |
| HNXY-2019 | 270 | 1.52 (0.24) | 1.72 (1.26–2.75) | 5.95 (3) | 0.11 | 11.47 | 270 | 2.51 (0.29) | 2.81 (2.33–3.50) | 0.21 (3) | 0.98 | 5.62 |
| HBDY-2020 | 270 | 2.06 (0.27) | 0.77 (0.54–0.99) | 3.70 (3) | 0.30 | 5.13 | 270 | 3.28 (0.23) | 3.46 (2.45–4.33) | 1.45 (3) | 0.70 | 6.92 |
| HBCB-2020 | 270 | 2.25 (0.27) | 1.05 (0.82–1.29) | 4.99 (3) | 0.17 | 7.00 | 270 | 1.89 (0.24) | 3.17 (2.46–3.96) | 2.19 (3) | 0.53 | 6.34 |
| HBWX-2020 | 270 | 2.53 (0.28) | 0.44 (0.37–0.54) | 4.47 (3) | 0.22 | 2.93 | 270 | 1.55 (0.23) | 7.08 (5.34–10.45) | 1.32 (3) | 0.73 | 14.16 |
| HBJL-2020 | 270 | 1.69 (0.25) | 1.89 (1.40–3.01) | 2.92 (3) | 0.40 | 12.60 | 270 | 1.88 (0.25) | 7.01 (5.50–9.71) | 3.16 (3) | 0.37 | 14.02 |
| HBJM-2020 | 270 | 2.51 (0.28) | 1.29 (1.06–1.55) | 1.90 (3) | 0.59 | 8.60 | 270 | 4.08 (0.46) | 6.39 (5.44–7.34) | 3.09 (3) | 0.38 | 12.78 |
| HBXZ-2020 | 270 | 1.96 (0.25) | 2.28 (1.83–2.99) | 3.46 (3) | 0.33 | 15.20 | 270 | 2.09 (0.25) | 8.44 (6.84–10.47) | 2.36 (3) | 0.50 | 16.88 |
| HNNX-2020 | 270 | 2.02 (0.25) | 1.19 (0.92–1.47) | 2.37 (3) | 0.50 | 7.93 | 270 | 2.32 (0.27) | 5.92 (4.72–7.19) | 2.83 (3) | 0.42 | 11.84 |
| JXNC-2020 | 270 | 2.12 (0.25) | 1.97 (1.60–2.47) | 2.17 (3) | 0.54 | 13.13 | 270 | 1.81 (0.24) | 6.52 (5.02–8.22) | 1.72 (3) | 0.63 | 13.04 |
| AHHF-2020 | 270 | 1.94 (0.25) | 2.07 (1.66–2.66) | 1.65 (3) | 0.65 | 13.80 | 270 | 2.25 (0.27) | 5.79 (4.57–7.06) | 2.03 (3) | 0.57 | 11.58 |
| HNXY-2020 | 270 | 1.74 (0.23) | 1.79 (1.38–2.28) | 2.64 (3) | 0.45 | 11.93 | 270 | 2.47 (0.29) | 2.35 (1.80–2.87) | 3.13 (3) | 0.37 | 4.70 |
| GZQN-2020 | 270 | 1.39 (0.24) | 2.60 (1.73–5.35) | 1.63 (3) | 0.65 | 17.33 | 270 | 1.77 (0.25) | 16.13 (12.30–23.74) | 0.77 (3) | 0.86 | 32.26 |
| D | ||||||||||||
| Population | Buprofezin | Chlorpyrifos | ||||||||||
| No. | Slope (SE a) | LC50 (95% CI b; mg/L) | χ2 (df) | p Value | RR c | No. | Slope (SE a) | LC50 (95% CI b; mg/L) | χ2 (df) | p Value | RR c | |
| HBCB-2019 | 270 | 1.89 (0.24) | 3.90 (3.09–4.90) | 0.86 (3) | 0.84 | 97.50 | 270 | 2.44 (0.28) | 30.72 (25.28–39.14) | 1.80 (3) | 0.83 | 128.00 |
| HBWX-2019 | 270 | 1.72 (0.23) | 3.71 (2.87–4.74) | 4.39 (3) | 0.22 | 92.75 | 270 | 1.74 (0.23) | 20.40 (15.96–26.16) | 3.27 (3) | 0.35 | 85.00 |
| HBJM-2019 | 270 | 1.93 (0.24) | 3.42 (2.69–4.26) | 1.01 (3) | 0.80 | 85.50 | 270 | 3.09 (0.33) | 20.67 (17.60–24.35) | 7.13 (3) | 0.07 | 86.13 |
| HNNX-2019 | 270 | 1.95 (0.26) | 3.25 (2.33–4.14) | 1.01 (3) | 0.80 | 81.25 | 270 | 3.57 (0.40) | 54.07 (46.52–64.75) | 5.00 (3) | 0.17 | 225.29 |
| JXNC-2019 | 270 | 1.43 (0.23) | 13.26 (9.55–21.97) | 4.70 (3) | 0.20 | 331.50 | 270 | 2.39 (0.27) | 32.51 (26.36–39.23) | 4.72 (3) | 0.19 | 135.46 |
| AHLA-2019 | 270 | 1.57 (0.23) | 6.80 (5.22–9.03) | 1.19 (3) | 0.75 | 170.00 | 270 | 2.02 (0.26) | 73.00 (57.61–100.50) | 0.81 (3) | 0.85 | 304.17 |
| HNXY-2019 | 270 | 1.99 (0.25) | 5.01 (4.04–6.39) | 6.49 (3) | 0.09 | 125.25 | 270 | 1.96 (0.24) | 46.58 (37.47–59.03) | 6.34 (3) | 0.10 | 194.08 |
| HBDY-2020 | 270 | 1.89 (0.24) | 4.64 (3.58–5.80) | 1.71 (3) | 0.64 | 105.45 | 270 | 2.92 (0.32) | 39.04 (32.97–46.13) | 0.38 (3) | 0.94 | 162.67 |
| HBCB-2020 | 270 | 1.65 (0.23) | 5.20 (4.05–6.96) | 1.30 (3) | 0.73 | 118.18 | 270 | 2.68 (0.29) | 15.33 (12.81–18.27) | 2.37 (3) | 0.50 | 63.88 |
| HBWX-2020 | 270 | 1.48 (0.22) | 5.70 (4.32–8.04) | 2.25 (3) | 0.52 | 129.55 | 270 | 2.14 (0.27) | 11.46 (8.53–14.32) | 1.50 (3) | 0.68 | 47.75 |
| HBJL-2020 | 270 | 2.33 (0.28) | 7.13 (5.75–9.48) | 1.45 (3) | 0.69 | 162.05 | 270 | 3.07 (0.34) | 26.34 (21.29–31.22) | 2.14 (3) | 0.54 | 109.75 |
| HBJM-2020 | 270 | 1.70 (0.23) | 5.05 (3.96–6.68) | 3.74 (3) | 0.29 | 114.77 | 270 | 3.00 (0.32) | 21.34 (18.14–25.25) | 4.24 (3) | 0.24 | 88.92 |
| HBXZ-2020 | 270 | 1.45 (0.23) | 6.60 (4.94–9.77) | 0.34 (3) | 0.95 | 150.00 | 270 | 2.36 (0.31) | 43.69 (34.31–61.90) | 1.74 (3) | 0.63 | 182.04 |
| HNNX-2020 | 270 | 1.57 (0.23) | 4.43 (3.23–5.76) | 3.57 (3) | 0.31 | 100.68 | 270 | 3.20 (0.34) | 39.65 (33.81–46.46) | 2.47 (3) | 0.48 | 165.21 |
| JXNC-2020 | 270 | 1.59 (0.23) | 5.51 (4.18–7.16) | 0.69 (3) | 0.87 | 137.75 | 270 | 2.06 (0.25) | 22.88 (18.55–28.69) | 1.34 (3) | 0.72 | 95.33 |
| AHHF-2020 | 270 | 1.58 (0.23) | 3.88 (2.76–5.07) | 2.49 (3) | 0.48 | 88.18 | 270 | 2.27 (0.26) | 45.34 (37.32–55.83) | 0.30 (3) | 0.96 | 188.92 |
| HNXY-2020 | 270 | 1.51 (0.22) | 4.30 (3.27–5.73) | 1.37 (3) | 0.71 | 97.73 | 270 | 1.68 (0.24) | 39.41 (29.88–58.46) | 1.49 (3) | 0.69 | 164.21 |
| GZQN-2020 | 270 | 1.98 (0.27) | 8.82 (6.76–13.01) | 3.18 (3) | 0.36 | 200.45 | 270 | 2.45 (0.28) | 18.54 (15.289–22.31) | 1.46 (3) | 0.69 | 77.25 |
| E | ||||||||||||
| Population | Isoprocarb | Ethofenprox | ||||||||||
| No. | Slope (SE a) | LC50 (95% CI b; mg/L) | χ2 (df) | p Value | RR c | No. | Slope (SE a) | LC50 (95% CI b; mg/L) | χ2 (df) | p Value | RR c | |
| HBCB-2019 | 270 | 2.63 (0.29) | 97.84 (81.69–116.95) | 0.88 (3) | 0.83 | 10.39 | 270 | 1.25 (0.22) | 435.50 (304.77–770.96) | 0.53 (3) | 0.91 | 12.58 |
| HBWX-2019 | 270 | 2.16 (0.26) | 91.57 (73.98–112.21) | 2.52 (3) | 0.47 | 9.72 | 270 | 1.86 (0.24) | 252.99 (201.67–327.75) | 5.73 (3) | 0.13 | 7.31 |
| HBJM-2019 | 270 | 2.53 (0.28) | 124.26 (103.72–152.18) | 2.79 (3) | 0.43 | 13.20 | 270 | 2.13 (0.26) | 327.97(263.69–434.08) | 1.99 (3) | 0.57 | 9.47 |
| HNNX-2019 | 270 | 2.64 (0.30) | 86.96 (73.33–104.62) | 4.89 (3) | 0.18 | 9.23 | 270 | 1.62 (0.25) | 575.55 (420.53–933.75) | 1.91 (3) | 0.59 | 16.63 |
| JXNC-2019 | 270 | 1.43 (0.24) | 215.22 (150.02–390.68) | 0.04 (3) | 0.99 | 22.85 | 270 | 1.38(0.22) | 209.86 (154.59–322.82) | 2.46 (3) | 0.48 | 6.06 |
| AHLA-2019 | 270 | 1.65 (0.23) | 75.78 (58.24–97.76) | 1.52 (3) | 0.68 | 8.04 | 270 | 1.61 (0.24) | 275.60 (202.47–440.60) | 0.53 (3) | 0.91 | 7.96 |
| HNXY-2019 | 270 | 1.88 (0.24) | 26.14 (20.86–33.95) | 4.80 (3) | 0.19 | 2.78 | 270 | 2.14 (0.26) | 256.60 (209.20–324.06) | 0.63 (3) | 0.89 | 7.41 |
| HBDY-2020 | 270 | 1.70 (0.24) | 134.86 (104.16–190.17) | 2.07 (3) | 0.56 | 14.32 | 270 | 1.27 (0.22) | 301.41 (219.77–458.33) | 0.73 (3) | 0.87 | 8.71 |
| HBCB-2020 | 270 | 2.23 (0.26) | 68.42 (55.22–83.31) | 0.64 (3) | 0.89 | 7.26 | 270 | 2.26 (0.26) | 232.67 (191.46–287.45) | 2.46 (3) | 0.48 | 6.72 |
| HBWX-2020 | 270 | 2.32 (0.27) | 116.69 (96.33–143.72) | 2.00 (3) | 0.57 | 12.39 | 270 | 1.33 (0.22) | 311.79 (229.35–470.59) | 2.85 (3) | 0.41 | 9.01 |
| HBJL-2020 | 270 | 2.04 (0.25) | 140.85 (113.65–182.22) | 2.57 (3) | 0.46 | 14.95 | 270 | 1.60 (0.26) | 679.15 (459.97–1333.14) | 3.75 (3) | 0.29 | 19.62 |
| HBJM-2020 | 270 | 2.61 (0.29) | 101.95 (85.21–122.22) | 6.32 (3) | 0.10 | 10.82 | 270 | 2.08 (0.26) | 303.41 (244.62–396.48) | 4.52 (3) | 0.21 | 8.77 |
| HBXZ-2020 | 270 | 2.25 (0.28) | 171.83 (138.58–227.68) | 6.35 (3) | 0.10 | 18.24 | 270 | 1.17 (0.21) | 268.36 (191.17–410.57) | 5.27 (3) | 0.15 | 7.75 |
| HNNX-2020 | 270 | 1.89 (0.25) | 120.27 (95.57–160.13) | 1.06 (3) | 0.79 | 12.77 | 270 | 1.10 (0.22) | 561.88 (362.20–1273.63) | 0.51 (3) | 0.92 | 16.23 |
| JXNC-2020 | 270 | 1.97 (0.25) | 101.17 (81.44–129.51) | 3.98 (3) | 0.26 | 10.74 | 270 | 1.54 (0.23) | 232.41 (173.47–353.93) | 2.45 (3) | 0.49 | 6.72 |
| AHHF-2020 | 270 | 2.13 (0.26) | 60.14 (47.36–73.85) | 1.34 (3) | 0.72 | 6.38 | 270 | 1.34 (0.22) | 226.23 (167.47–330.85) | 0.64 (3) | 0.89 | 6.54 |
| HNXY-2020 | 270 | 2.00 (0.26) | 79.05 (61.69–111.69) | 4.31 (3) | 0.23 | 8.39 | 270 | 1.59 (0.23) | 304.42 (233.94–426.32) | 3.59 (3) | 0.31 | 8.80 |
| GZQN-2020 | 270 | 1.98 (0.26) | 183.31 (144.14–253.85) | 1.42 (3) | 0.70 | 19.46 | 270 | 1.71 (0.27) | 665.53 (458.41–1255.03) | 2.62 (3) | 0.45 | 19.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Qin, Y.; Jin, R.; Zhang, Y.; Ren, Z.; Cai, T.; Yu, C.; Liu, Y.; Cai, Y.; Zeng, Q.; et al. Insecticide Resistance Monitoring in Field Populations of the Whitebacked Planthopper Sogatella furcifera (Horvath) in China, 2019–2020. Insects 2021, 12, 1078. https://doi.org/10.3390/insects12121078
Li Z, Qin Y, Jin R, Zhang Y, Ren Z, Cai T, Yu C, Liu Y, Cai Y, Zeng Q, et al. Insecticide Resistance Monitoring in Field Populations of the Whitebacked Planthopper Sogatella furcifera (Horvath) in China, 2019–2020. Insects. 2021; 12(12):1078. https://doi.org/10.3390/insects12121078
Chicago/Turabian StyleLi, Zhao, Yao Qin, Ruoheng Jin, Yunhua Zhang, Zhijie Ren, Tingwei Cai, Chang Yu, Yu Liu, Yongfeng Cai, Qinghong Zeng, and et al. 2021. "Insecticide Resistance Monitoring in Field Populations of the Whitebacked Planthopper Sogatella furcifera (Horvath) in China, 2019–2020" Insects 12, no. 12: 1078. https://doi.org/10.3390/insects12121078
APA StyleLi, Z., Qin, Y., Jin, R., Zhang, Y., Ren, Z., Cai, T., Yu, C., Liu, Y., Cai, Y., Zeng, Q., Wan, H., & Li, J. (2021). Insecticide Resistance Monitoring in Field Populations of the Whitebacked Planthopper Sogatella furcifera (Horvath) in China, 2019–2020. Insects, 12(12), 1078. https://doi.org/10.3390/insects12121078







