Evaluation of Resistance Development in Bemisia tabaci Genn. (Homoptera: Aleyrodidae) in Cotton against Different Insecticides
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Strains
2.2. Insecticides
2.3. Bioassays
2.3.1. Adult Bioassay
2.3.2. Nymphal Bioassay
2.4. Selection with Insecticides
2.5. Statistical Analysis
3. Results
3.1. Toxicity of the Insecticides against the Lab-PK and Field Population
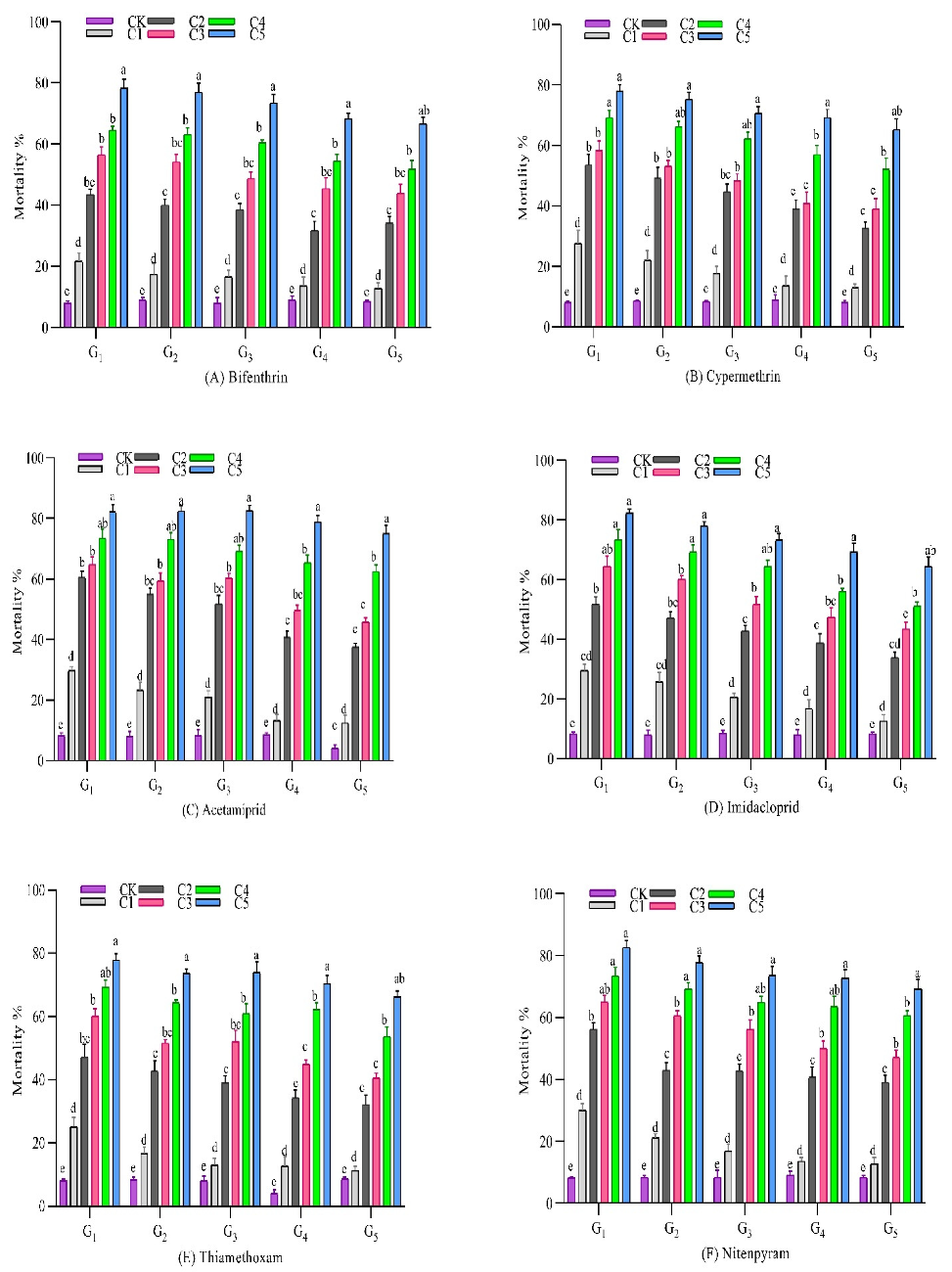
3.2. Response to Selection with Insecticides
3.2.1. Bifenthrin
3.2.2. Cypermethrin
3.2.3. Acetamiprid
3.2.4. Imidacloprid
3.2.5. Thiamethoxam
3.2.6. Nitenpyram
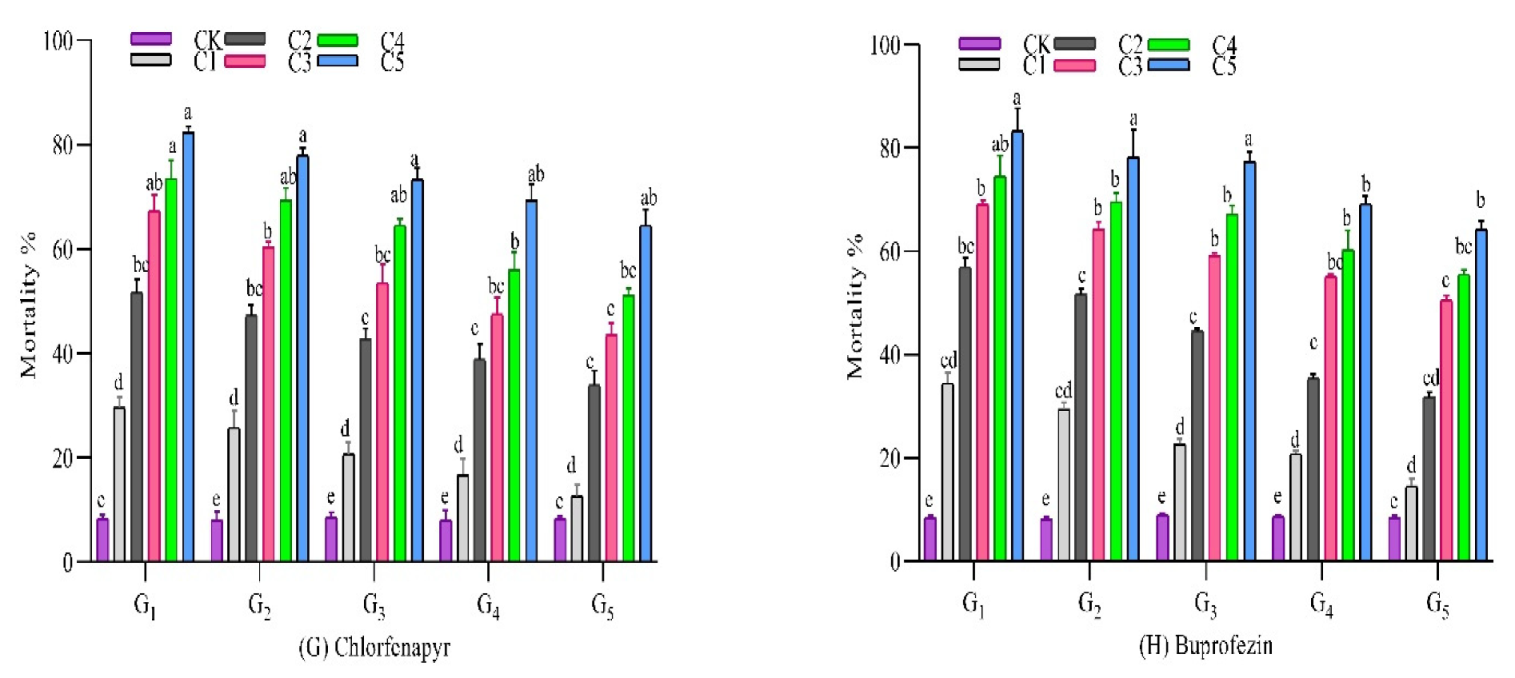
3.2.7. Chlorfenapyr
3.2.8. Buprofezin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malik, T.H.; Ahsan, M.Z. Review of the cotton market in Pakistan and its future prospects. OCL 2016, 23, D606. [Google Scholar] [CrossRef] [Green Version]
- Mansour, S.; Roff, M.M.; Khalid, A.; Ismail, A.; Idris, A. Population abundance of whitefly, Bemisia tabaci (Genn.), on chilli and other vegetable crops under glasshouse conditions. J. Trop. Agric. Fd. Sci. 2013, 41, 149–157. [Google Scholar]
- Hameed, A.; Aziz, M.A.; Aheer, G.M. Susceptibility of Bemisia tabaci Gen. (Homoptera: Aleyrodidae) to selected insecticides. Pak. J. Zool. 2010, 42, 295–300. [Google Scholar]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, C.; Jones, C.; Devine, G.; Zhang, F.; Denholm, I.; Gorman, K. Insecticide resistance in Bemisia tabaci biotype Q (Hemiptera: Aleyrodidae) from China. Crop Prot. 2010, 29, 429–434. [Google Scholar] [CrossRef]
- Sethi, A.; Dilawari, V. Spectrum of insecticide resistance in whitefly from upland cotton in Indian subcontinent. J. Entomol. 2008, 5, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Erdogan, C.; Moores, G.D.; Gurkan, M.O.; Gorman, K.J.; Denholm, I. Insecticide resistance and biotype status of populations of the tobacco whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) from Turkey. Crop Prot. 2008, 27, 600–605. [Google Scholar] [CrossRef]
- Fernández, E.; Grávalos, C.; Haro, P.J.; Cifuentes, D.; Bielza, P. Insecticide resistance status of Bemisia tabaci Q-biotype in south-eastern Spain. Pest Manag. Sci. 2009, 65, 885–891. [Google Scholar] [CrossRef]
- Ahmad, M.; Arif, M.I.; Ahmad, Z.; Denholm, I. Cotton whitefly (Bemisia tabaci) resistance to organophosphate and pyrethroid insecticides in Pakistan. Pest Manag. Sci. 2002, 58, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Karatolos, N.; Gorman, K.; Williamson, M.S.; Denholm, I. Mutations in the sodium channel associated with pyrethroid resistance in the greenhouse whitefly, Trialeurodes vaporariorum. Pest Manag. Sci. 2012, 68, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Gorman, K.; Devine, G.; Luo, W.; Denholm, I. The biotype and insecticide-resistance status of whiteflies, Bemisia tabaci (Hemiptera: Aleyrodidae), invading cropping systems in Xinjiang Uygur Autonomous Region, northwestern China. Crop Prot. 2007, 26, 612–617. [Google Scholar] [CrossRef]
- Basij, M.; Talebi, K.; Ghadamyari, M.; Hosseininaveh, V.; Salami, S. Status of resistance of Bemisia tabaci (Hemiptera: Aleyrodidae) to neonicotinoids in Iran and detoxification by cytochrome P450-dependent monooxygenases. Neotrop. Entomol. 2017, 46, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, H.; Yang, Y.; Wu, Y. Biotype and insecticide resistance status of the whitefly Bemisia tabaci from China. Pest Manag. Sci. 2010, 66, 1360–1366. [Google Scholar] [CrossRef]
- Naveen, N.; Chaubey, R.; Kumar, D.; Rebijith, K.; Rajagopal, R.; Subrahmanyam, B.; Subramanian, S. Insecticide resistance status in the whitefly, Bemisia tabaci genetic groups Asia-I, Asia-II-1 and Asia-II-7 on the Indian subcontinent. Sci. Rep. 2017, 7, 40634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, D.; Bhattacharjee, T.; Biswas, A.; Ghosh, A.; Sarkar, S.; Mondal, D.; Sarkar, P.K. Resistance monitoring for conventional and new chemistry insecticides on Bemisia tabaci genetic group Asia-I in major vegetable crops from India. Phytoparasitica 2019, 47, 55–66. [Google Scholar] [CrossRef]
- Yao, F.-L.; Zheng, Y.; Huang, X.-Y.; Ding, X.-L.; Zhao, J.-W.; Desneux, N.; He, Y.-X.; Weng, Q.-Y. Dynamics of Bemisia tabaci biotypes and insecticide resistance in Fujian province in China during 2005–2014. Sci. Rep. 2017, 7, 40803. [Google Scholar] [CrossRef] [Green Version]
- Basit, M.; Sayyed, A.H.; Saleem, M.A.; Saeed, S. Cross-resistance, inheritance and stability of resistance to acetamiprid in cotton whitefly, Bemisia tabaci Genn (Hemiptera: Aleyrodidae). Crop Prot. 2011, 30, 705–712. [Google Scholar] [CrossRef]
- Basit, M. Status of insecticide resistance in Bemisia tabaci: Resistance, cross-resistance, stability of resistance, genetics and fitness costs. Phytoparasitica 2019, 47, 207–225. [Google Scholar] [CrossRef]
- Ahmad, M.; Khan, R.A. Field-evolved resistance of Bemisia tabaci (Hemiptera: Aleyrodidae) to carbodiimide and neonicotinoids in Pakistan. J. Econ. Entomol. 2017, 110, 1235–1242. [Google Scholar] [CrossRef]
- Ahmed, M.Z.; Ren, S.-X.; Mandour, N.S.; Maruthi, M.; Naveed, M.; Qiu, B.-L. Phylogenetic analysis of Bemisia tabaci (Hemiptera: Aleyrodidae) populations from cotton plants in Pakistan, China, and Egypt. J. Pest Sci. 2010, 83, 135–141. [Google Scholar] [CrossRef]
- Basit, M.; Saleem, M.A.; Saeed, S.; Sayyed, A.H. Cross resistance, genetic analysis and stability of resistance to buprofezin in cotton whitefly, Bemisia tabaci (Homoptera: Aleyrodidae). Crop Prot. 2012, 40, 16–21. [Google Scholar] [CrossRef]
- Dara, S.K. The new integrated pest management paradigm for the modern age. J. Integr. Pest Manag. 2019, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; He, C.; Xie, W.; Liu, Y.; Xia, J.; Yang, Z.; Guo, L.; Wen, Y.; Wang, S.; Wu, Q. Glutathione S-transferases are involved in thiamethoxam resistance in the field whitefly Bemisia tabaci Q (Hemiptera: Aleyrodidae). Pestic. Biochem. Physiol. 2016, 134, 73–78. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, M.; Wu, Y. Cross-resistance, inheritance and biochemical mechanisms of imidacloprid resistance in B-biotype Bemisia tabaci. Pest Manag. Sci. 2009, 65, 1189–1194. [Google Scholar] [CrossRef]
- Marasinghe, J.; Hemachandra, K.; Nugaliyadde, L.; Karunaratne, S. Control failure of Sri Lankan whitefly (Bemisia tabaci Genn.) is due to high resistance development against recommended insecticides. J. Natl. Sci. Found. Sri Lanka 2017, 45, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Basit, M.; Saeed, S.; Saleem, M.A.; Denholm, I.; Shah, M. Detection of resistance, cross-resistance, and stability of resistance to new chemistry insecticides in Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol. 2013, 106, 1414–1422. [Google Scholar] [CrossRef]
- Prabhaker, N.; Castle, S.; Henneberry, T.; Toscano, N. Assessment of cross-resistance potential to neonicotinoid insecticides in Bemisia tabaci (Hemiptera: Aleyrodidae). Bull. Entomol. Res. 2005, 95, 535–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horowitz, A.R.; Kontsedalov, S.; Khasdan, V.; Ishaaya, I. Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance. Arch. Insect Biochem. Physiol. 2005, 58, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Akhtar, K.P. Susceptibility of cotton whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) to diverse pesticides in Pakistan. J. Econ. Entomol. 2018, 111, 1834–1841. [Google Scholar] [CrossRef]
- Kranthi, K.; Jadhav, D.; Kranthi, S.; Wanjari, R.; Ali, S.; Russell, D. Insecticide resistance in five major insect pests of cotton in India. Crop Prot. 2002, 21, 449–460. [Google Scholar] [CrossRef]
- Gutiérrez-Moreno, R.; Mota-Sanchez, D.; Blanco, C.A.; Whalon, M.E.; Terán-Santofimio, H.; Rodriguez-Maciel, J.C.; DiFonzo, C. Field-evolved resistance of the fall armyworm (Lepidoptera: Noctuidae) to synthetic insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 2019, 112, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Huseth, A.S.; D’Ambrosio, D.A.; Kennedy, G.G. Responses of neonicotinoid resistant and susceptible Frankliniella fusca life stages to multiple insecticide groups in cotton. Pest Manag. Sci. 2017, 73, 2118–2130. [Google Scholar] [CrossRef] [PubMed]
- Shaurub, E.-S.H.; Abdel Aal, A.E.; Emara, S.A. Suppressive effects of insect growth regulators on development, reproduction and nutritional indices of the Egyptian cotton leafworm, Spodoptera littoralis (Lepidoptera: Noctuidae). Invertebr. Reprod. Dev. 2020, 64, 178–187. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Yousaf, H.K.; Xiu, W.; Qian, D.; Gao, X.; Tariq, K.; Han, P.; Desneux, N.; Song, D. Impact of low lethal concentrations of buprofezin on biological traits and expression profile of chitin synthase 1 gene (CHS1) in melon aphid, Aphis gossypii. Sci. Rep. 2019, 9, 12291. [Google Scholar] [CrossRef] [Green Version]
- Shadmany, M.; Omar, D.; Muhamad, R. Biotype and insecticide resistance status of Bemisia tabaci populations from Peninsular Malaysia. J. Appl. Entomol. 2015, 139, 67–75. [Google Scholar] [CrossRef]
- Xie, W.; Liu, Y.; Wang, S.; Wu, Q.; Pan, H.; Yang, X.; Guo, L.; Zhang, Y. Sensitivity of Bemisia tabaci (Hemiptera: Aleyrodidae) to several new insecticides in China: Effects of insecticide type and whitefly species, strain, and stage. J. Insect Sci. 2014, 14, 261. [Google Scholar] [CrossRef]
- Zafar, J.; Freed, S.; Khan, B.A.; Farooq, M. Effectiveness of Beauveria bassiana against cotton whitefly, Bemisia tabaci (Gennadius) (Aleyrodidae: Homoptera) on different host plants. Pak. J. Zool. 2016, 48, 91–99. [Google Scholar]
- Schuster, D.J.; Mann, R.S.; Toapanta, M.; Cordero, R.; Thompson, S.; Cyman, S.; Shurtleff, A.; Morris II, R.F. Monitoring neonicotinoid resistance in biotype B of Bemisia tabaci in Florida. Pest Manag. Sci. 2010, 66, 186–195. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, S.; Zhou, J.; Du, Y.; Zhang, Y.; Wang, J. Status of insecticide resistance and associated mutations in Q-biotype of whitefly, Bemisia tabaci, from eastern China. Crop Prot. 2012, 31, 67–71. [Google Scholar] [CrossRef]
- Khalid, M.Z.; Ahmad, S.; Ngegba, P.M.; Zhong, G. Role of Endocrine System in the Regulation of Female Insect Reproduction. Biology 2021, 10, 614. [Google Scholar] [CrossRef]
- Shabbir, R.; Javed, T.; Afzal, I.; Sabagh, A.E.; Ali, A.; Vicente, O.; Chen, P. Modern biotechnologies: Innovative and sustainable approaches for the improvement of sugarcane tolerance to environmental stresses. Agronomy 2021, 11, 1042. [Google Scholar] [CrossRef]
- Javed, T.; Shabbir, R.; Ali, A.; Afzal, I.; Zaheer, U.; Gao, S.J. Transcription factors in plant stress responses: Challenges and potential for sugarcane improvement. Plants 2020, 9, 491. [Google Scholar] [CrossRef] [PubMed]
| Trade Name | Active Ingredient | Formulation | Field Rate Acre−1 | IRAC Main Group |
|---|---|---|---|---|
| Rapid | Acetamiprid | 20 SL | 250 mL | 4A |
| Kalorfen | Chlorfenapyr | 36 SC | 225 mL | 13 |
| Imidacloprid | Imidacloprid | 20 SL | 250 mL | 4A |
| Contest | Thiamethoxam | 25 SC | 200 mL | 4A |
| Seradix | Nitenpyram | 10 SL | 125 mL | 4A |
| Bifenthrin | Bifenthrin | 10 EC | 250 mL | 3A |
| Cypermethrin | Cypermethrin | 10 EC | 250 mL | 3A |
| Buprofezin | Buprofezin | 25 EC | 450 mL | 16 |
| Population | Insecticides | LC50 (95% FL)(ug a.i. mL−1) | Slope (±SE) | Probit Fit Line | RRa | ||
|---|---|---|---|---|---|---|---|
| χ2 | df | p | |||||
| Lab-PK | Bifenthrin | 3.19 (1.05–7.01) | 0.17 ± 0.02 | 8.43 | 5 | 0.038 | - |
| Lab-PK | Cypermethrin | 1.91 (0.54–4.44) | 0.16 ± 0.02 | 12.63 | 5 | 0.006 | - |
| Lab-PK | Acetamiprid | 1.08 (0.41–2.18) | 0.22 ± 0.02 | 17.20 | 5 | 0.001 | - |
| Lab-PK | Imidacloprid | 0.65 (0.15–1.66) | 0.17 ± 0.02 | 8.54 | 5 | 0.036 | - |
| Lab-PK | Thiamethoxam | 1.47 (0.57–2.95) | 0.21 ± 0.02 | 11.72 | 5 | 0.008 | - |
| Lab-PK | Nitenpyram | 0.87 (0.33–1.75) | 0.22 ± 0.02 | 9.99 | 5 | 0.019 | - |
| Lab-PK | Chlorfenapyr | 0.52 (0.12–1.34) | 0.17 ± 0.02 | 8.12 | 5 | 0.044 | - |
| Lab-PK | Buprofezin | 0.35 (0.08–0.93) | 0.17 ± 0.02 | 8.13 | 5 | 0.043 | - |
| Field (G1) | Bifenthrin | 9.19 (4.32–17.16) | 0.20 ± 0.03 | 10.54 | 5 | 0.014 | 3 |
| Field (G1) | Cypermethrin | 4.66 (1.80–9.50) | 0.18 ± 0.02 | 11.13 | 5 | 0.011 | 2 |
| Field (G1) | Acetamiprid | 1.48 (0.43–3.40) | 0.17 ± 0.02 | 16.08 | 5 | 0.001 | 1 |
| Field (G1) | Imidacloprid | 2.49 (0.91–5.12) | 0.19 ± 0.02 | 9.07 | 5 | 0.028 | 4 |
| Field (G1) | Thiamethoxam | 4.86 (1.96- 9.68) | 0.19 ± 0.02 | 9.42 | 5 | 0.024 | 3 |
| Field (G1) | Nitenpyram | 1.89 (0.61- 4.14) | 0.18 ± 0.02 | 12.74 | 5 | 0.005 | 3 |
| Field (G1) | Chlorfenapyr | 1.36 (0.37–3.24) | 0.16 ± 0.02 | 8.25 | 5 | 0.041 | 3 |
| Field (G1) | Buprofezin | 1.10 (0.27–2.70) | 0.16 ± 0.02 | 10.48 | 5 | 0.015 | 3 |
| Population | Insecticides | LC50 (95% FL) (ug a.i. mL−1) | Slope (±SE) | Probit Fit Line | RRa | ||
|---|---|---|---|---|---|---|---|
| χ2 | df | p | |||||
| Bifenthrin-SEL | Bifenthrin | 55.86 (28.48–121.03) | 0.21 ± 0.03 | 12.29 | 5 | 0.006 | 18 |
| Cypermethrin-SEL | Cypermethrin | 64.27 (33.67–136.34) | 0.22 ± 0.03 | 9.77 | 5 | 0.021 | 34 |
| Acetamiprid-SEL | Acetamiprid | 22.74 (12.76–39.06) | 0.25 ± 0.03 | 13.33 | 5 | 0.004 | 21 |
| Imidacloprid-SEL | Imidacloprid | 55.86 (28.48–121.03) | 0.21 ± 0.03 | 12.29 | 5 | 0.006 | 86 |
| Thiamethoxam-SEL | Thiamethoxam | 50.29 (27.04–99.44) | 0.22 ± 0.03 | 10.01 | 5 | 0.018 | 34 |
| Nitenpyram-SEL | Nitenpyram | 26.04 (13.86–48.04) | 0.22 ± 0.03 | 14.69 | 5 | 0.002 | 30 |
| Chlorfenapyr-SEL | Chlorfenapyr | 15.32 (6.83–31.61) | 0.18 ± 0.03 | 19.04 | 5 | 0.000 | 29 |
| Buprofezin-SEL | Buprofezin | 44.42 (22.85–91.60) | 0.21 ± 0.03 | 11.76 | 5 | 0.008 | 127 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, M.Z.; Ahmed, S.; Al-Ashkar, I.; EL Sabagh, A.; Liu, L.; Zhong, G. Evaluation of Resistance Development in Bemisia tabaci Genn. (Homoptera: Aleyrodidae) in Cotton against Different Insecticides. Insects 2021, 12, 996. https://doi.org/10.3390/insects12110996
Khalid MZ, Ahmed S, Al-Ashkar I, EL Sabagh A, Liu L, Zhong G. Evaluation of Resistance Development in Bemisia tabaci Genn. (Homoptera: Aleyrodidae) in Cotton against Different Insecticides. Insects. 2021; 12(11):996. https://doi.org/10.3390/insects12110996
Chicago/Turabian StyleKhalid, Muhammad Zaryab, Sohail Ahmed, Ibrahim Al-Ashkar, Ayman EL Sabagh, Liyun Liu, and Guohua Zhong. 2021. "Evaluation of Resistance Development in Bemisia tabaci Genn. (Homoptera: Aleyrodidae) in Cotton against Different Insecticides" Insects 12, no. 11: 996. https://doi.org/10.3390/insects12110996
APA StyleKhalid, M. Z., Ahmed, S., Al-Ashkar, I., EL Sabagh, A., Liu, L., & Zhong, G. (2021). Evaluation of Resistance Development in Bemisia tabaci Genn. (Homoptera: Aleyrodidae) in Cotton against Different Insecticides. Insects, 12(11), 996. https://doi.org/10.3390/insects12110996








