The Impact of the Entomopathogenic Fungus Conidiobolus coronatus on the Free Fatty Acid Profile of the Flesh Fly Sarcophaga argyrostoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Fungus Conidiobolus coronatus
2.2. Insects
2.3. Infection of Insects
2.4. Extraction of Free Fatty Acids (FFAs)
2.5. Derivatization Method
2.6. GC–MS Analyses
2.7. Statistics
3. Results
3.1. Susceptibility of S. argyrostoma to Fungal Infection
3.2. Effectiveness of Extraction Process
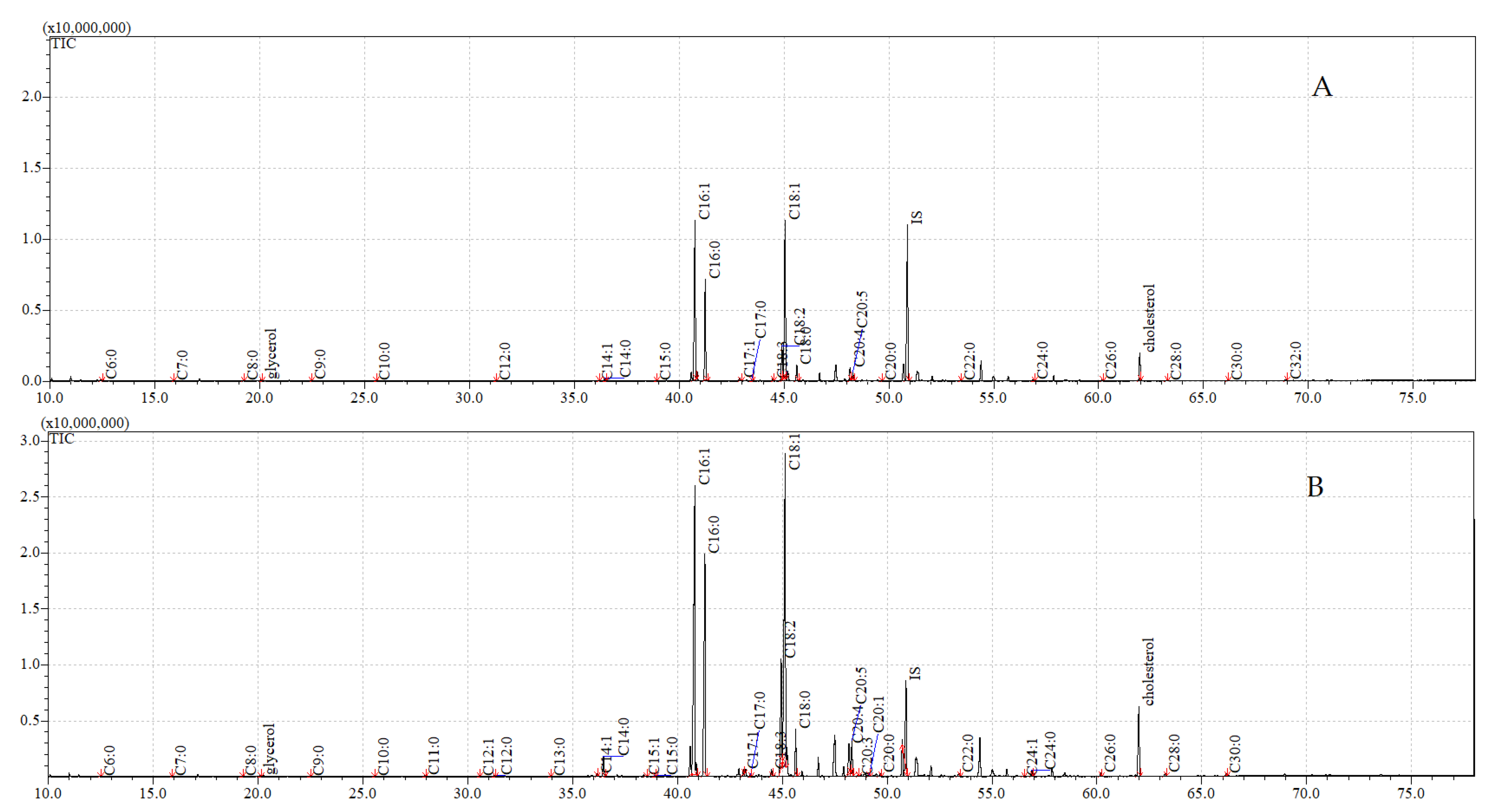
3.3. GC-MS Analyses
3.4. GC-MS Analyses of Compounds Extracted from Pupae
3.5. GC-MS Analyses of Compounds Extracted from Adults
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Mello-Patiu, C.A. Family Sarcophagidae. Zootaxa 2016, 4122, 884–903. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Shang, Y.; Chen, W.; Meng, F.; Cai, J.; Zhu, G.; Chen, L.; Wang, Y.; Deng, J.; Guo, Y. A brief review of forensically important flesh flies (Diptera: Sarcophagidae). Forensic Sci. Res. 2018, 3, 16–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pezzi, M.; Bonacci, T.; Leis, M.; Mamolini, E.; Marchetti, M.G.; Krčmar, S.; Chicca, M.; Del Zingaro, C.N.F.; Faucheux, M.J.; Scapoli, C. Myiasis in domestic cats: A global review. Parasites Vectors 2019, 12, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholl, P.J.; Colwell, D.D.; Cepeda-Palacios, R. Myiasis (Muscoidea, Oestroidea). In Medical and Veterinary Entomology; Mullen, G.R., Durden, L.A., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 383–419. ISBN 9780128140437. [Google Scholar]
- Ayalon, A.; Yehezkeli, V.; Paitan, Y.; Szpila, K.; Mumcuoglu, K.Y.; Moisseiev, E. Massive orbital myiasis caused by Sarcophaga argyrostoma complicating eyelid malignancy. Case Rep. Ophthalmol. Med. 2020, 2020, 1–5. [Google Scholar] [CrossRef]
- Thomann, T.; Jourdan, M.; Richet, R.; Sheppard, A.; Baker, G.H. Parasitism of the conical snail, Cochlicella acuta, by the fly, Sarcophaga villeneuveana, in south-western Europe. BioControl 2020, 65, 673–679. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Zhang, K.-L.; Shan, Q.-W. Bloody diarrhea caused by intestinal myiasis in an infant: A case report and review of pediatric literature. J. Trop. Pediatr. 2020, 67, fmaa037. [Google Scholar] [CrossRef]
- Vilte, R.; Gleiser, R.M.; Horenstein, M.B. Necrophagous fly assembly: Evaluation of species bait preference in field experiments. J. Med. Entomol. 2020, 57, 437–442. [Google Scholar] [CrossRef]
- Calvopina, M.; Ortiz-Prado, E.; Castañedaid, B.; Cueva, I.; Rodriguez-Hidalgo, R.; Cooperid, P.J. Human myiasis in Ecuador. PLoS Negl. Trop. Dis. 2020, 14, e0007858. [Google Scholar] [CrossRef] [Green Version]
- Kelehear, C.; Ibáñez, R.; Rodríguez, C.; Buitrago, S.; Durant-Archibold, A.A. Sarcophagid myiasis in the bufonid Rhinella alata in Panama. J. Wildl. Dis. 2020, 56, 667–672. [Google Scholar] [CrossRef]
- D’Bastiani, E.; Teixeira, C.P.; De La Torre, G.M.; Dudczak, A.C.; dos Santos, L.E.; Silva, A.L.F.; Oda, F.H.; Mello-Patiu, C.A.; Campião, K.M. How deadly sarcophagid fly larvae are for anurans? New interactions and review to Neotropical region. Parasitol. Res. 2020, 119, 1415–1422. [Google Scholar] [CrossRef]
- Kamut, M.; Jezierski, T. Ecological, behavioural and economic effects of insects on grazing farm animals—A review. Anim. Sci. Pap. Reports 2014, 32, 107–119. [Google Scholar]
- Souza, C.M.; Madeira-Ott, T.; Masiero, F.S.; Bunde, P.R.S.; Ribeiro, G.A.; Thyssen, P.J. Synanthropy of Sarcophaginae (Diptera: Sarcophagidae) from southern Brazil and its sanitary implications. J. Med. Entomol. 2020, 58, 913–920. [Google Scholar] [CrossRef]
- Jacques, B.J.; Bourret, T.J.; Shaffer, J.J. Role of fly cleaning behavior on carriage of Escherichia coli and Pseudomonas aeruginosa. J. Med. Entomol. 2017, 54, 1712–1717. [Google Scholar] [CrossRef]
- Hadi, A.M. Study of fly borne parasites (Brachycera): A review. Plant Arch. 2020, 20, 2419–2428. [Google Scholar]
- Maniania, N.K.; Ekesi, S. The use of entomopathogenic fungi in the control of tsetse flies. J. Invertebr. Pathol. 2013, 112, S83–S88. [Google Scholar] [CrossRef]
- de Oliveira, D.G.P.; Alves, L.F.A.; Sosa-Gómez, D.R. Advances and perspectives of the use of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae for the control of arthropod pests in poultry production. Rev. Bras. Cienc. Avic. 2014, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lacey, L.A.; Frutos, R.; Kaya, H.K.; Vail, P. Insect pathogens as biological control agents: Do they have a future? Biol. Control 2001, 21, 230–248. [Google Scholar] [CrossRef] [Green Version]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [Green Version]
- Lacey, L.A. Entomopathogens used as microbial control agents. In Microbial Control of Insect and Mite Pests: From Theory to Practice; Academic Press: Amsterdam, The Netherlands, 2016; pp. 1–10. ISBN 9780128035665. [Google Scholar]
- Kaczmarek, A.; Boguś, M.I. Fungi of entomopathogenic potential in Chytridiomycota and Blastocladiomycota, and in fungal allies of the Oomycota and Microsporidia. IMA Fungus 2021, 12, 29. [Google Scholar] [CrossRef]
- Goettel, M.S.; Eilenberg, J.; Glare, T. Entomopathogenic fungi and their role in regulation of insect populations. In Comprehensive Molecular Insect Science; Elsevier Pergamon: Oxford, UK, 2005; Volume 6, pp. 361–405. ISBN 9780444519245. [Google Scholar]
- Sandhu, S.S.; Sharma, A.K.; Beniwal, V.; Goel, G.; Batra, P.; Kumar, A.; Jaglan, S.; Sharma, A.K.; Malhotra, S. Myco-biocontrol of insect pests: Factors involved, mechanism, and regulation. J. Pathog. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Stone, L.B.L.; Bidochka, M.J. The multifunctional lifestyles of Metarhizium: Evolution and applications. Appl. Microbiol. Biotechnol. 2020, 104, 9935–9945. [Google Scholar] [CrossRef]
- Zhang, L.; Fasoyin, O.E.; Molnár, I.; Xu, Y. Secondary metabolites from hypocrealean entomopathogenic fungi: Novel bioactive compounds. Nat. Prod. Rep. 2020, 37, 1181–1206. [Google Scholar] [CrossRef]
- Bedding, R.A.; Molyneux, A.S. Penetration of insect cuticle by infective juveniles of heterorhabditis Spp. (Heterorhabditidae: Nematoda). Nematologica 1982, 28, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Urquiza, A.; Keyhani, N.O. Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects 2013, 4, 357. [Google Scholar] [CrossRef]
- Pedrini, N.; Crespo, R.; Juárez, M.P. Biochemistry of insect epicuticle degradation by entomopathogenic fungi. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 124–137. [Google Scholar] [CrossRef]
- Crespo, R.; Patricia Juárez, M.; Cafferata, L.F.R. Biochemical interaction between entomopathogenous fungi and their insect-host-like hydrocarbons. Mycologia 2000, 92, 528–536. [Google Scholar] [CrossRef]
- Pedrini, N.; Ortiz-Urquiza, A.; Huarte-Bonnet, C.; Zhang, S.; Keyhani, N.O. Targeting of insect epicuticular lipids by the entomopathogenic fungus Beauveria bassiana: Hydrocarbon oxidation within the context of a host-pathogen interaction. Front. Microbiol. 2013, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Gołębiowski, M.; Maliński, E.; Boguś, M.I.; Kumirska, J.; Stepnowski, P. The cuticular fatty acids of Calliphora vicina, Dendrolimus pini and Galleria mellonella larvae and their role in resistance to fungal infection. Insect Biochem. Mol. Biol. 2008, 38, 619–627. [Google Scholar] [CrossRef]
- Gołębiowski, M.; Boguś, M.I.; Paszkiewicz, M.; Wieloch, W.; Włóka, E.; Stepnowski, P. The composition of the cuticular and internal free fatty acids and alcohols from Lucilia sericata males and females. Lipids 2012, 47, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Gołębiowski, M.; Boguś, M.I.; Paszkiewicz, M.; Stepnowski, P. The composition of the free fatty acids from Dendrolimus pini exuviae. J. Insect Physiol. 2010, 56, 391–397. [Google Scholar] [CrossRef]
- Gołębiowski, M.; Cerkowniak, M.; Boguś, M.I.; Włóka, E.; Dawgul, M.; Kamysz, W.; Stepnowski, P. Free fatty acids in the cuticular and internal lipids of Calliphora vomitoria and their antimicrobial activity. J. Insect Physiol. 2013, 59, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Gołębiowski, M.; Cerkowniak, M.; Urbanek, A.; Dawgul, M.; Kamysz, W.; Boguś, M.I.; Stepnowski, P. Identification and antifungal activity of novel organic compounds found in cuticular and internal lipids of medically important flies. Microbiol. Res. 2015, 170, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Gołębiowski, M.; Cerkowniak, M.; Urbanek, A.; Dawgul, M.; Kamysz, W.; Boguś, M.I.; Sosnowska, D.; Stepnowski, P. Antimicrobial activity of untypical lipid compounds in the cuticular and internal lipids of four fly species. J. Appl. Microbiol. 2014, 116, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.; Boguś, M.I.; Włóka, E.; Wrońska, A.K.; Krawiel, A.; Kazek, M.; Zalewska, K.; Kłocinska-Biały, K.; Sobocinska, M.; Gliniewicz, A.; et al. The interaction between cuticle free fatty acids (FFAs) of the cockroaches Blattella germanica and Blatta orientalis and hydrolases produced by the entomopathogenic fungus Conidiobolus coronatus. PLoS ONE 2020, 15, e0235785. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.; Wrońska, A.K.; Kazek, M.; Boguś, M.I. Metamorphosis-related changes in the free fatty acid profiles of Sarcophaga (Liopygia) argyrostoma (Robineau-Desvoidy, 1830). Sci. Rep. 2020, 10, 17337. [Google Scholar] [CrossRef] [PubMed]
- Mannino, M.C.; Huarte-Bonnet, C.; Davyt-Colo, B.; Pedrini, N. Is the insect cuticle the only entry gate for fungal infection? Insights into alternative modes of action of entomopathogenic fungi. J. Fungi 2019, 5, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora, M.A.E.; Castilho, A.M.C.; Fraga, M.E. Classification and infection mechanism of entomopathogenic fungi. Arq. Inst. Biol. 2018, 84, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Boguś, M.I.; Włóka, E.; Wrońska, A.; Kaczmarek, A.; Kazek, M.; Zalewska, K.; Ligęza-Żuber, M.; Gołębiowski, M. Cuticle hydrolysis in four medically important fly species by enzymes of the entomopathogenic fungus Conidiobolus coronatus. Med. Vet. Entomol. 2017, 31, 23–35. [Google Scholar] [CrossRef]
- Wrońska, A.K.; Boguś, M.I.; Włóka, E.; Kazek, M.; Kaczmarek, A.; Zalewska, K. Cuticular fatty acids of Galleria mellonella (Lepidoptera) inhibit fungal enzymatic activities of pathogenic Conidiobolus coronatus. PLoS ONE 2018, 13, e0192715. [Google Scholar] [CrossRef] [Green Version]
- Boguś, M.I.; Czygier, M.; Gołębiowski, M.; Kędra, E.; Kucińska, J.; Mazgajska, J.; Samborski, J.; Wieloch, W.; Włóka, E. Effects of insect cuticular fatty acids on in vitro growth and pathogenicity of the entomopathogenic fungus Conidiobolus coronatus. Exp. Parasitol. 2010, 125, 400–408. [Google Scholar] [CrossRef]
- Urbanek, A.; Szadziewski, R.; Stepnowski, P.; Boros-Majewska, J.; Gabriel, I.; Dawgul, M.; Kamysz, W.; Sosnowska, D.; Gołębiowski, M. Composition and antimicrobial activity of fatty acids detected in the hygroscopic secretion collected from the secretory setae of larvae of the biting midge Forcipomyia nigra (Diptera: Ceratopogonidae). J. Insect Physiol. 2012, 58, 1265–1276. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [Green Version]
- Gołębiowski, M.; Urbanek, A.; Oleszczak, A.; Dawgul, M.; Kamysz, W.; Boguś, M.I.; Stepnowski, P. The antifungal activity of fatty acids of all stages of Sarcophaga carnaria L. (Diptera: Sarcophagidae). Microbiol. Res. 2014, 169, 279–286. [Google Scholar] [CrossRef]
- Babiarz, E.; Boguś, M.I.; Czygier, M.; Kucińska, J.; Samborski, J.; Szafranek, J. Influence of free fatty acids on growth, sporulation and virulence of the parasitic fungus Conidiobolus coronatus. Wiadomości Parazytol. 2001, 47, 763–768. [Google Scholar]
- Smith, R.J.; Grula, E.A. Toxic components on the larval surface of the corn earworm (Heliothis zea) and their effects on germination and growth of Beauveria bassiana. J. Invertebr. Pathol. 1982, 39, 15–22. [Google Scholar] [CrossRef]
- Koidsumi, K. Antifungal action of cuticular lipids in insects. J. Insect Physiol. 1957, 1, 40–51. [Google Scholar] [CrossRef]
- Barnes, S.E.; Moore, D. The effect of fatty, organic or phenolic acids on the germination of conidia of Metarhizium flavoviride. Mycol. Res. 1997, 101, 662–666. [Google Scholar] [CrossRef]
- Kerwin, J.L. Fatty acid regulation of the germination of Erynia variabilis conidia on adults and puparia of the lesser housefly, Fannia canicularis. Can. J. Microbiol. 1984, 30, 158–161. [Google Scholar] [CrossRef]
- Kerwin, J.L. Chemical control of the germination of asexual spores of Entomophthora culicis, a fungus parasitic on dipterans. J. Gen. Microbiol. 1982, 128, 2179–2186. [Google Scholar] [CrossRef] [Green Version]
- Boguś, M.I.; Scheller, K. Extraction of an insecticidal protein fraction from the parasitic fungus Conidiobolus coronatus (Entomophthorales). Acta Parasitol. 2002, 47, 66–72. [Google Scholar]
- Sehnal, F. A critical study of the biome and biometry of the wax moth Galleria mellonella raised in varying conditions. Zeitschrift für wissenschaftliche Zool. 1966, 174, 53–82. [Google Scholar]
- Wieloch, W. Exploring pathogenicity potential of Conidiobolus coronatus against insect larvae in various infection conditions. Pestycydy 2005, 4, 133–137. [Google Scholar]
- Gołębiowski, M.; Boguś, M.I.; Paszkiewicz, M.; Stepnowski, P. Cuticular lipids of insects as potential biofungicides: Methods of lipid composition analysis. Anal. Bioanal. Chem. 2011, 399, 3177–3191. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Wrońska, A.K.; Boguś, M.I.; Kazek, M.; Gliniewicz, A.; Mikulak, E.; Matławska, M. The type of blood used to feed Aedes aegypti females affects their cuticular and internal free fatty acid (FFA) profiles. PLoS ONE 2021, 16, e0251100. [Google Scholar] [CrossRef]
- Cerkowniak, M.; Puckowski, A.; Stepnowski, P.; Gołębiowski, M. The use of chromatographic techniques for the separation and the identification of insect lipids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 937, 67–78. [Google Scholar] [CrossRef]
- Kazek, M.; Kaczmarek, A.; Wrońska, A.K.; Boguś, M.I. Diet influences the bacterial and free fatty acid profiles of the cuticle of Galleria mellonella larvae. PLoS ONE 2019, 14, e0211697. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Gołębiowski, M.; Dawgul, M.; Kamysz, W.; Boguś, M.I.; Wieloch, W.; Włóka, E.; Paszkiewicz, M.; Przybysz, E.; Stepnowski, P. Antimicrobial activity of alcohols from Musca domestica. J. Exp. Biol. 2012, 215, 3419–3428. [Google Scholar] [CrossRef] [Green Version]
- Boguś, M.I.; Kedra, E.; Bania, J.; Szczepanik, M.; Czygier, M.; Jabłoński, P.; Pasztaleniec, A.; Samborski, J.; Mazgajska, J.; Polanowski, A. Different defense strategies of Dendrolimus pini, Galleria mellonella, and Calliphora vicina against fungal infection. J. Insect Physiol. 2007, 53, 909–922. [Google Scholar] [CrossRef]
- Wieloch, W.; Boguś, M.I.; Ligeza, M.; Koszela-Piotrowska, I.; Szewczyk, A. Coronatin-1 isolated from entomopathogenic fungus Conidiobolus coronatus kills Galleria mellonella hemocytes in vitro and forms potassium channels in planar lipid membrane. Toxicon 2011, 58, 369–379. [Google Scholar] [CrossRef]
- Boguś, M.I.; Wieloch, W.; Ligȩza-Żuber, M. Coronatin-2 from the entomopathogenic fungus Conidiobolus coronatus kills Galleria mellonella larvae and incapacitates hemocytes. Bull. Entomol. Res. 2017, 107, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Paszkiewicz, M.; Tyma, M.; Ligęza-Żuber, M.; Włóka, E.; Boguś, M. Trichothecenes production by entomopathogenic fungus Conidiobolus coronatus. Adv. Toxicol. Toxic Eff. 2016, 1, 007–014. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.; Khanam, Z.; Khan, A.U. Isolation and characterization of n-octacosanoic acid from Viburnum foetens: A novel antibiofilm agent against Streptococcus mutans. Med. Chem. Res. 2012, 21, 1411–1417. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Chu, A.J.; Remaley, S. Biosynthesis of wax in the honeybee, Apis mellifera L. Insect Biochem. 1980, 10, 313–321. [Google Scholar] [CrossRef]
- Gołębiowski, M. Comparison of free fatty acids composition of cuticular lipids of Calliphora vicina larvae and pupae. Lipids 2012, 47, 1001–1009. [Google Scholar] [CrossRef]
- Cerkowniak, M.; Boguś, M.I.; Włóka, E.; Stepnowski, P.; Gołębiowski, M. The composition of lipid profiles in different developmental stages of Dermestes ater and Dermestes maculatus and their susceptibility to the entomopathogenic fungus Conidiobolus coronatus. Phytoparasitica 2020, 48, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Elmastaş, M.; Gülmez, Y. Fatty acid composition of sexes and body parts in a solitary wasp, Sphex flavipennis (Insecta: Hymenoptera). J. Inst. Sci. Technol. 2017, 7, 73–78. [Google Scholar] [CrossRef]
- Messina, C.M.; Gaglio, R.; Morghese, M.; Tolone, M.; Arena, R.; Moschetti, G.; Santulli, A.; Francesca, N.; Settanni, L. Microbiological profile and bioactive properties of insect powders used in food and feed formulations. Foods 2019, 8, 400. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Chen, J.; Yu, X.; Gao, J.M. A mini review of nervonic acid: Source, production, and biological functions. Food Chem. 2019, 301, 125286. [Google Scholar] [CrossRef]
- Fan, Y.; Meng, H.M.; Hu, G.R.; Li, F.L. Biosynthesis of nervonic acid and perspectives for its production by microalgae and other microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 3027–3035. [Google Scholar] [CrossRef]
- Ghosh, S.; Lee, S.M.; Jung, C.; Meyer-Rochow, V.B. Nutritional composition of five commercial edible insects in South Korea. J. Asia. Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- Youn, K.; Kim, J.Y.; Yeo, H.; Yun, E.Y.; Hwang, J.S.; Jun, M. Fatty acid and volatile oil compositions of Allomyrina dichotoma larvae. Prev. Nutr. Food Sci. 2012, 17, 310–314. [Google Scholar] [CrossRef] [Green Version]
- Yeo, H.; Youn, K.; Kim, M.; Yun, E.Y.; Hwang, J.S.; Jeong, W.S.; Jun, M. Fatty acid composition and volatile constituents of Protaetia brevitarsis larvae. Prev. Nutr. Food Sci. 2013, 18, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Akinnawo, O.; Ketiku, A.O. Chemical composition and fatty acid profile of edible larva of Cirina forda (Westwood). African J. Biomed. Res. 2000, 3, 93–96. [Google Scholar]
- Batalha, M.D.M.C.; Goulart, H.F.; Santana, A.E.G.; Barbosa, L.A.O.; Nascimento, T.G.; da Silva, M.K.H.; Dornelas, C.B.; Grillo, L.A.M. Chemical composition and antimicrobial activity of cuticular and internal lipids of the insect Rhynchophorus palmarum. Arch. Insect Biochem. Physiol. 2020, 105, e21723. [Google Scholar] [CrossRef]
- Gołębiowski, M.; Bojke, A.; Tkaczuk, C.; Majchrowska-Safaryan, A.; Stepnowski, P. Comparison of the organic compounds composition of Hylobius abietis males and females before and after exposure to Beauveria bassiana infection. Physiol. Entomol. 2020, 45, 81–88. [Google Scholar] [CrossRef]
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect cuticle: A critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef]
- Keyhani, N.O. Lipid biology in fungal stress and virulence: Entomopathogenic fungi. Fungal Biol. 2018, 122, 420–429. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A.; Fan, Y.; Garrett, T.; Keyhani, N.O. Growth substrates and caleosin-mediated functions affect conidial virulence in the insect pathogenic fungus Beauveria bassiana. Microbiology 2016, 162, 1913–1921. [Google Scholar] [CrossRef]
- Zhang, S.; Widemann, E.; Bernard, G.; Lesot, A.; Pinot, F.; Pedrini, N.; Keyhani, N.O. CYP52X1, representing new cytochrome P450 subfamily, displays fatty acid hydroxylase activity and contributes to virulence and growth on insect cuticular substrates in entomopathogenic fungus Beauveria bassiana. J. Biol. Chem. 2012, 287, 13477–13486. [Google Scholar] [CrossRef] [Green Version]
- Kazek, M.; Kaczmarek, A.; Wrońska, A.K.; Boguś, M.I. Dodecanol, metabolite of entomopathogenic fungus Conidiobolus coronatus, affects fatty acid composition and cellular immunity of Galleria mellonella and Calliphora vicina. Sci. Rep. 2021, 11, 15963. [Google Scholar] [CrossRef]
- Pohl, C.H.; Kock, J.L.F.; Thibane, V.S. Antifungal free fatty acids: A review. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Formatex Research Center: Badajoz, Spain, 2011; Volume 1, pp. 61–71. [Google Scholar]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.J. Antibacterial free fatty acids and monoglycerides: Biological activities, experimental testing, and therapeutic applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef] [Green Version]
- Paszkiewicz, M.; Sikora, A.; Boguś, M.I.; Włóka, E.; Stepnowski, P.; Gołębiowski, M. Effect of exposure to chlorpyrifos on the cuticular and internal lipid composition of Blattella germanica males. Insect Sci. 2016, 23, 94–104. [Google Scholar] [CrossRef]
- Everatt, M.J.; Convey, P.; Bale, J.S.; Worland, M.R.; Hayward, S.A.L. Responses of invertebrates to temperature and water stress: A polar perspective. J. Therm. Biol. 2015, 54, 118–132. [Google Scholar] [CrossRef] [Green Version]
- Yi, S.X.; Gantz, J.D.; Lee, R.E. Desiccation enhances rapid cold-hardening in the flesh fly Sarcophaga bullata: Evidence for cross tolerance between rapid physiological responses. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2017, 187, 79–86. [Google Scholar] [CrossRef]
- Davis, D.J.; Lee, R.E. Intracellular freezing, viability, and composition of fat body cells from freeze-intolerant larvae of Sarcophaga crassipalpis. Arch. Insect Biochem. Physiol. 2001, 48, 199–205. [Google Scholar] [CrossRef]
- Yoder, J.A.; Benoit, J.B.; Denlinger, D.L.; Rivers, D.B. Stress-induced accumulation of glycerol in the flesh fly, Sarcophaga bullata: Evidence indicating anti-desiccant and cryoprotectant functions of this polyol and a role for the brain in coordinating the response. J. Insect Physiol. 2006, 52, 202–214. [Google Scholar] [CrossRef]
- Guo, H.; Wang, H.; Keyhani, N.O.; Xia, Y.; Peng, G. Disruption of an adenylate-forming reductase required for conidiation, increases virulence of the insect pathogenic fungus Metarhizium acridum by enhancing cuticle invasion. Pest Manag. Sci. 2020, 76, 758–768. [Google Scholar] [CrossRef]
- Gao, Q.; Shang, Y.; Huang, W.; Wang, C. Glycerol-3-phosphate acyltransferase contributes to triacylglycerol biosynthesis, lipid droplet formation, and host invasion in Metarhizium robertsii. Appl. Environ. Microbiol. 2013, 79, 7646–7653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gołębiowski, M.; Cerkowniak, M.; Ostachowska, A.; Naczk, A.M.; Boguś, M.I.; Stepnowski, P. Effect of Conidiobolus coronatus on the cuticular and internal lipid composition of Tettigonia viridissima males. Chem. Biodivers. 2016, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Grebenok, R.J.; Behmer, S.T. Diet micronutrient balance matters: How the ratio of dietary sterols/steroids affects development, growth and reproduction in two lepidopteran insects. J. Insect Physiol. 2014, 67, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Bouvaine, S.; Faure, M.L.; Grebenok, R.J.; Behmer, S.T.; Douglas, A.E. A dietary test of putative deleterious sterols for the aphid Myzus persicae. PLoS ONE 2014, 9, e86256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behmer, S.T.; David Nes, W. Insect sterol nutrition and nhysiology: A global overview. Adv. Insect Phys. 2003, 31, 1–72. [Google Scholar] [CrossRef]
- Jing, X.; Grebenok, R.J.; Behmer, S.T. Sterol/steroid metabolism and absorption in a generalist and specialist caterpillar: Effects of dietary sterol/steroid structure, mixture and ratio. Insect Biochem. Mol. Biol. 2013, 43, 580–587. [Google Scholar] [CrossRef]
- Michaud, M.R.; Denlinger, D.L. Oleic acid is elevated in cell membranes during rapid cold-hardening and pupal diapause in the flesh fly, Sarcophaga crassipalpis. J. Insect Physiol. 2006, 52, 1073–1082. [Google Scholar] [CrossRef] [Green Version]
- Hadley, N.F. Cuticle: Ecological Significance. In Biology of the Integument: Invertebrates; Bereiter-Hahn, J., Matoltsy, A.G., Richards, K.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 685–693. ISBN 978-3-642-51593-4. [Google Scholar]
- Hamamura, Y.; Hayashiya, K.; Naito, K.I. Food selection by silkworm larvæ, Bombyx mori: β-sitosterol as one of the biting factors. Nature 1961, 190, 880–881. [Google Scholar] [CrossRef]
- Nagata, S.; Omori, Y.; Nagasawa, H. Dietary sterol preference in the silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 2006, 70, 3094–3098. [Google Scholar] [CrossRef]
- Nagata, S.; Nagasawa, H. Sterol composition in larvae of the silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 2011, 75, 1003–1005. [Google Scholar] [CrossRef]
- Kiprono, P.C.; Kaberia, F.; Keriko, J.M.; Karanja, J.N. The in vitro anti-fungal and anti-bacterial activities of β-sitosterol from Senecio lyratus (Asteraceae). Zeitschrift für Naturforschung C 2000, 55, 485–488. [Google Scholar] [CrossRef]
- Aderiye, B.I.; Ogundana, S.K.; Adesanya, S.A.; Roberts, M.F. The effect of β-sitosterol on spore germination and germ-tube elongation of Aspergillus niger and Botryodiplodia theobromae. Int. J. Food Microbiol. 1989, 8, 73–78. [Google Scholar] [CrossRef]
- Mizuba, S.; Lee, K.; Jiu, J. Three antimicrobial metabolites from Aspergillus caespitosus. Can. J. Microbiol. 1975, 21, 1781–1787. [Google Scholar] [CrossRef]
- Parish, E.J.; Nes, W.D. Biochemistry nd Function of Sterols; CRC Press: Boca Raton, FL, USA, 2020; ISBN 9780849376740. [Google Scholar]
- Wojciechowska, M.; Stepnowski, P.; Gołębiowski, M. Cyfluthrin and deltamethrin induce changes in the fat body composition of Tenebrio molitor larvae, males and females. Chem. Biodivers. 2019, 16, e1800515. [Google Scholar] [CrossRef]
- Iijima, R.; Kurata, S.; Natori, S. Purification, characterization, and cDNA cloning of an antifungal protein from the hemolymph of Sarcophaga peregrina (flesh fly) larvae. J. Biol. Chem. 1993, 268, 12055–12061. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Ali, M.M.; Dorrah, M.A.; Bassal, T.T.M. Mediation of inducible nitric oxide and immune-reactive lysozymes biosynthesis by eicosanoid and biogenic amines in flesh flies. Int. J. Trop. Insect Sci. 2018, 38, 93–104. [Google Scholar] [CrossRef]
- Stanley, D.; Kim, Y. Eicosanoid signaling in Insects: From discovery to plant protection. Crit. Rev. Plant Sci. 2014, 33, 20–63. [Google Scholar] [CrossRef]
- Shrestha, S.; Kim, Y. Various eicosanoids modulate the cellular and humoral immune responses of the beet armyworm, Spodoptera exigua. Biosci. Biotechnol. Biochem. 2009, 73, 2077–2084. [Google Scholar] [CrossRef] [Green Version]
- Yajima, M.; Takada, M.; Takahashi, N.; Kikuchi, H.; Natori, S.; Oshima, Y.; Kurata, S. A newly established in vitro culture using transgenic Drosophila reveals functional coupling between the phospholipase A2-generated fatty acid cascade and lipopolysaccharide-dependent activation of the immune deficiency (IMD) pathway in insect immunity. Biochem. J. 2003, 371, 205–210. [Google Scholar] [CrossRef]
- Clements, J.; Olson, J.M.; Sanchez-Sedillo, B.; Bradford, B.; Groves, R.L. Changes in emergence phenology, fatty acid composition, and xenobiotic-metabolizing enzyme expression is associated with increased insecticide resistance in the Colorado potato beetle. Arch. Insect Biochem. Physiol. 2020, 103, e21630. [Google Scholar] [CrossRef]
- Stanley, D.; Kim, Y. Why most insects have very low proportions of C20 polyunsaturated fatty acids: The oxidative stress hypothesis. Arch. Insect Biochem. Physiol. 2020, 103, e21622. [Google Scholar] [CrossRef]

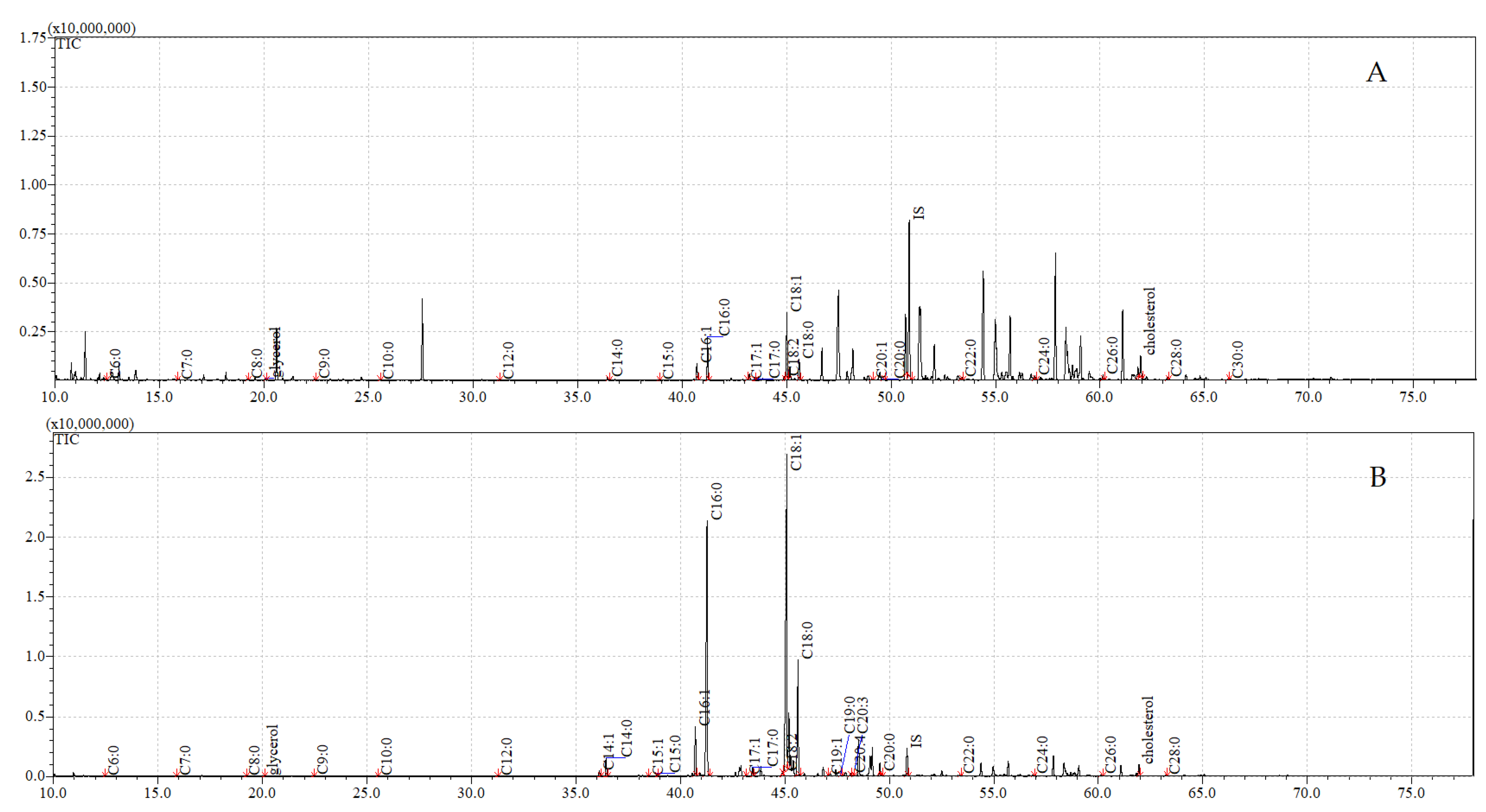
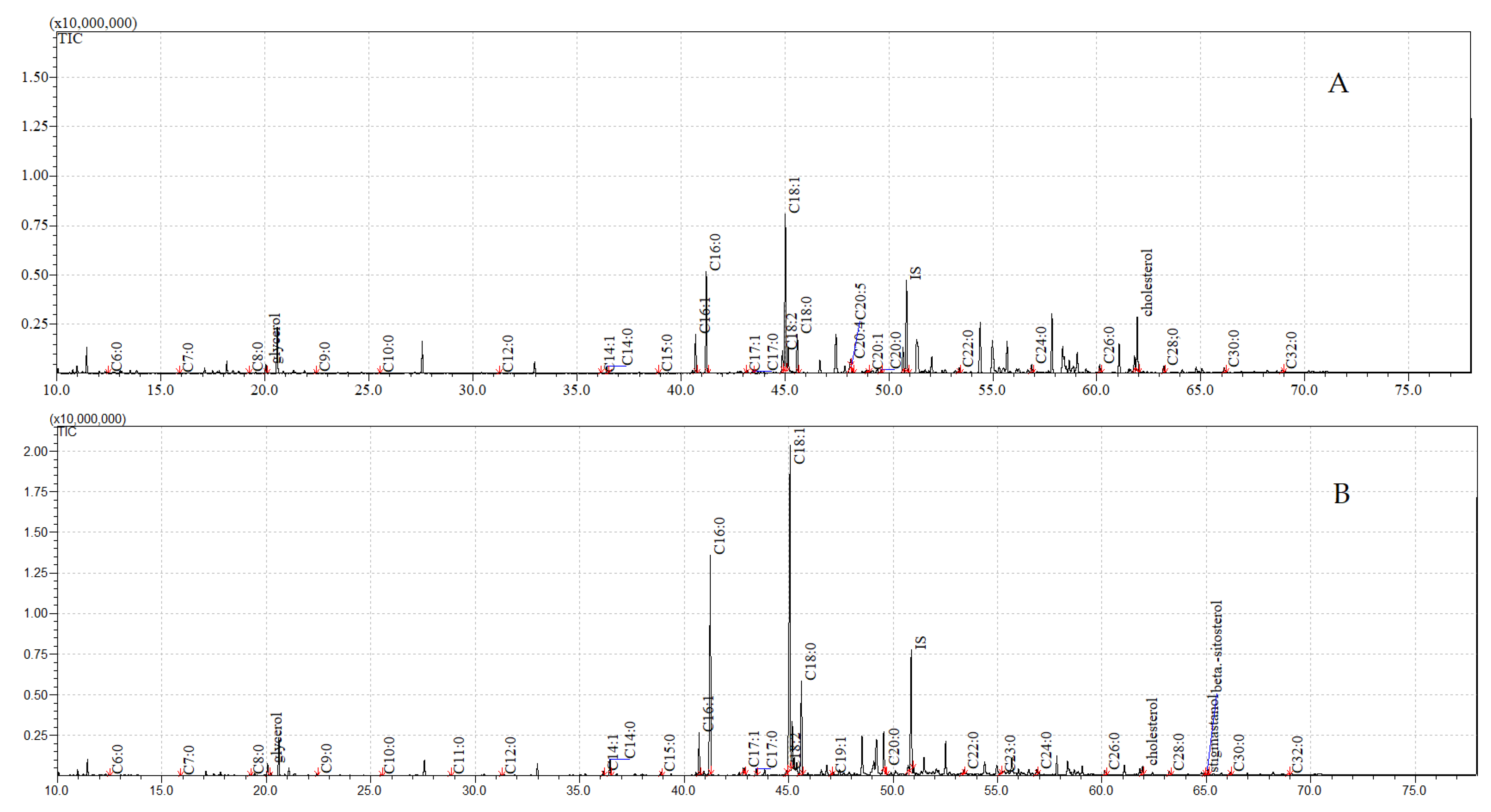

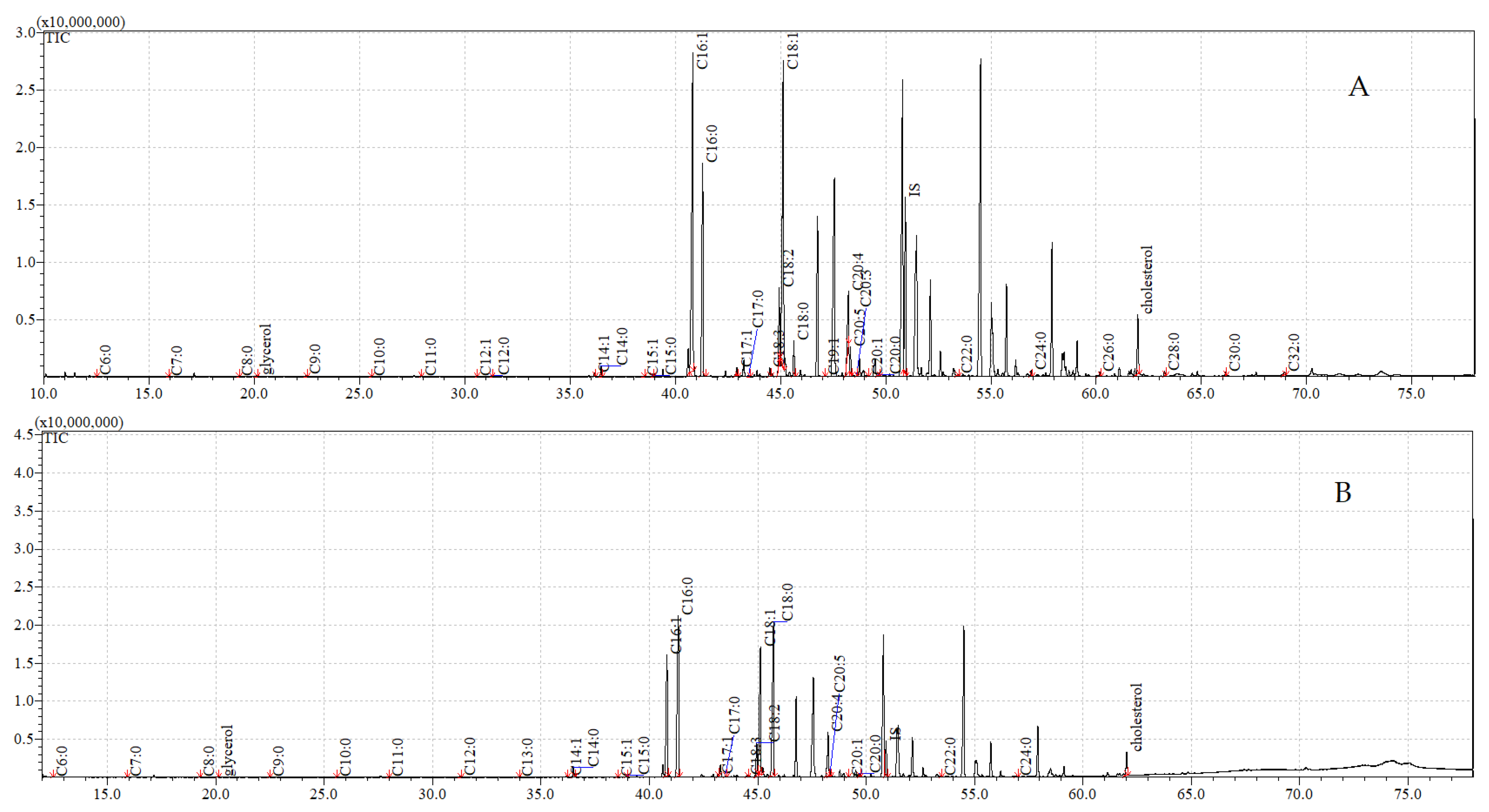
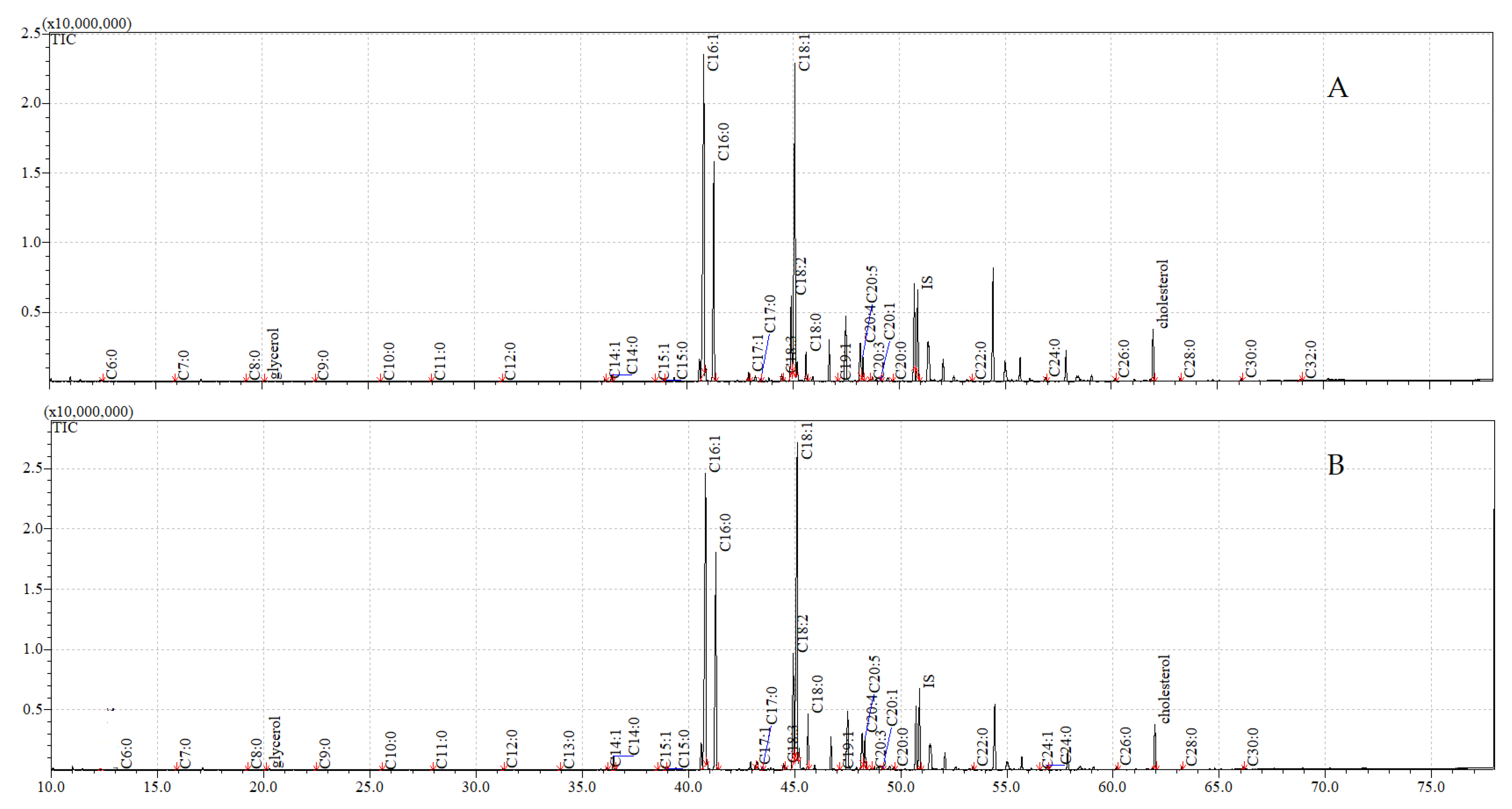
| Treatments: | N | Insects Mass [g] | Extract Mass | ||||||
|---|---|---|---|---|---|---|---|---|---|
| mg | mg/Insect | ||||||||
| I | II | III | I | II | III | ||||
| pupae | control | 40 | 0.83 | 4.53 | 1.12 | 17.37 | 0.113 | 0.028 | 0.434 |
| exposure to C. coronatus | 18 | 0.24 | 2.08 | 0.88 | 0.47 | 0.116 | 0.049 | 0.027 | |
| adults | control | 57 | 5.71 | 5.94 | 8.36 | 25.17 | 0.104 | 0.147 | 0.442 |
| exposure to C. coronatus | 47 | 4.78 | 13.97 | 7.29 | 5.25 | 0.297 | 0.156 | 0.112 | |
| Insect Treatment | N | Percent of Flies Hatched from Pupae [%±SD] | Mortality of Imagines [%±SD] | |
|---|---|---|---|---|
| pupae | control | 30 | 80.00 ± 8.00 | 7.41 ± 12.83 # |
| exposure to C. coronatus | 30 | 80 ± 0.00 | 8.33 ± 7.22 # | |
| adults | control | 30 | 3.33 ± 5.77 * | |
| exposure to C. coronatus | 30 | 60.00 ± 10.00 * | ||
| FFA | Cuticular | Internal | ||
|---|---|---|---|---|
| Control | Exposure to C. coronatus | Control | Exposure to C. coronatus | |
| Hexanoic acid C6:0 | 0.17 ± 0.01 A | 0.37 ± 0.02 A | 0.71 ± 0.06 A | 0.03 ± 0.00 A |
| Heptanoic acid C7:0 | 0.02 ± 0.01 A | 0.06 ± 0.02 B | 0.14 ± 0.02 A,B | 0.01 ± 0.00 B |
| Octanoic acid C8:0 | 0.26 ± 0.06 A | 0.31 ± 0.06 B | 0.33 ± 0.01 C | 0.02 ± 0.00 A,B,C |
| Nonanoic acid C9:0 | 0.28 ± 0.00 A | 1.16 ± 0.08 A | 0.65 ± 0.04 A | 0.04 ± 0.00 A |
| Decanoic acid C10:0 | 0.06 ± 0.00 | 0.19 ± 0.07 A,B | 0.06 ± 0.02 A | 0.01 ± 0.00 B |
| Undecanoic acid C11:0 | ND A | 0.04 ± 0.01 A,B,C | ND B | ND C |
| Dodecanoic acid C12:0 | 0.10 ± 0.01 A | 0.38 ± 0.05 A,B | 0.07 ± 0.02 B | 0.01 ± 0.00 A |
| Tetradecenoic acid C14:1 | 0.01 ± 0.01 A | 6.43 ± 0.41 A,B,C | ND B | 0.03 ± 0.00 C |
| Tetradecanoic acid C14:0 | 0.85 ± 0.02 A | 22.46 ± 1.00 A,B,C | 0.23 ± 0.03 B | 0.13 ± 0.00 C |
| Pentadecenoic acid C15:1 | ND A | 0.10 ± 0.05 A,B,C | ND B | ND C |
| Pentadecanoic acid C15:0 | 0.15 ± 0.01 A | 4.04 ± 0.21 A,B,C | 0.07 ± 0.02 B | 0.03 ± 0.00 C |
| Hexadecenoic acid C16:1 | 4.29 ± 0.12 A | 59.94 ± 3.02 A,B,C | 0.76 ± 0.05 B | 0.36 ± 0.00 C |
| Hexadecanoic acid C16:0 | 11.42 ± 0.12 A | 386.27 ± 12.76 A,B,C | 5.60 ± 0.18 B | 2.41 ± 0.3 C |
| Heptadecenoic acid C17:1 | 0.10 ± 0.08 A | 2.63 ± 0.22 A,B,C | ND B | 0.06 ± 0.00 C |
| Heptadecanoic acid C17:0 | 0.21 ± 0.00 A | 10.85 ± 0.67 A,B,C | ND B | 0.05 ± 0.00 C |
| Octadecadienoic acid C18:2 | 1.50 ± 0.07 A | 2.73 ± 0.71 A,B | 0.39 ± 0.03 B | 0.06 ± 0.00 A |
| Octadecenoic acid C18:1 | 17.73 ± 0.15 A | 517.97 ± 25.16 A,B,C | 4.91 ± 0.23 B | 3.85 ± 0.06 C |
| Octadecanoic acid C18:0 | 5.15 ± 0.11 A | 151.16 ± 7.66 A,B,C | 3.14 ± 0.09 B | 0.93 ± 0.02 C |
| Nonadecenoic acid C19:1 | ND A | 1.05 ± 0.27 A,B,C | ND B | ND C |
| Nonadecanoic acid C19:0 | 0.01 ± 0.00 A | 0.80 ± 0.13 A,B,C | ND B | ND C |
| Eicosapentaenoic acid C20:5 | 0.09 ± 0.00 A,B,C | ND A | ND B | ND C |
| Eicosatetraenoic acid C20:4 | ND A | 3.04 ± 0.30 A,B,C | ND B | ND C |
| Eicosatrienoic acid C20:3 | ND A | 1.03 ± 0.10 A,B,C | ND B | ND C |
| Eicosenoic acid C20:1 | 0.16 ± 0.02 A,B,C | ND A | ND B | ND C |
| Eicosanoic acid C20:0 | 0.29 ± 0.01 A,B | 0.40 ± 0.13 C,D | ND A,C | 0.05 ± 0.00 B,D |
| Docosanoic acid C22:0 | 0.63 ± 0.04 A,B | 1.99 ± 0.24 A,B | ND A | 0.11 ± 0.00 B |
| Tricosanoic acid C23:0 | NDA | 0.10 ± 0.01 A,B | ND B | 0.03 ± 0.00 A,B |
| Tetracosanoic acid C24:0 | 0.97 ± 0.03 A,B | 2.76 ± 0.23 A,B | ND A | 0.13 ± 0.00 B |
| Hexacosanoic acid C26:0 | 1.20 ± 0.04 A,B | 2.57 ± 0.22 A,B | ND A | 0.11 ± 0.00 B |
| Octacosanoic acid C28:0 | 0.84 ± 0.01 A,B | 1.31 ± 0.12 A,B | ND A | 0.05 ± 0.00 B |
| Triacontanoic acid C30:0 | 0.50 ± 0.05 | 0.74 ± 0.05 A,B | ND A | 0.03 ± 0.00 B |
| Dotriacontanoic acid C32:0 | 0.28 ± 0.04 A,B | 0.14 ± 0.03 A,B | ND A | ND B |
| Sum of FFA | 47.27 ± 0.26 A | 1189.69 ± 57.97 A,B,C | 15.87 ± 0.45 B | 8.53 ± 0.07 C |
| Cuticular | Internal | |||
|---|---|---|---|---|
| Control | Exposure to C. coronatus | Control | Exposure to C. coronatus | |
| Glycerol | 0.67 ± 0.03 A | 1.82 ± 0.08 A | 0.92 ± 0.02 A | 0.09 ± 0.00 A |
| Cholesterol | 6.51 ± 0.25 A,B | 14.48 ± 0.70 A,B | 1.26 ± 0.07 A | 0.09 ± 0.01 B |
| β-Sitosterol | ND A | 0.24±0.01 A,B, | ND B | 0.10 ± 0.01 A,B |
| Stigmastanol | ND A | 0.53±0.11 A,B | ND B | 0.32 ± 0.02 A,B |
| FFA | Cuticular | Internal | ||
|---|---|---|---|---|
| Control | Exposure to C. coronatus | Control | Exposure to C. coronatus | |
| Hexanoic acid C6:0 | 0.06 ± 0.00 A | 0.09 ± 0.01 A | 0.04 ± 0.00 A | 0.02 ± 0.00 A |
| Heptanoic acid C7:0 | 0.02 ± 0.00 B | 0.04 ± 0.01 A,B | 0.02 ± 0.00 A | 0.01 ± 0.00 B |
| Octanoic acid C8:0 | 0.04 ± 0.00 B | 0.08 ± 0.01 A,B | 0.03 ± 0.00 A | 0.02 ± 0.00 A,B |
| Nonanoic acid C9:0 | 0.09 ± 0.01 B | 0.16 ± 0.01 A,B | 0.08 ± 0.01 A | 0.04 ± 0.00 A,B |
| Decanoic acid C10:0 | 0.02 ± 0.00 B | 0.03 ± 0.00 A,B | 0.02 ± 0.00 A | trace amount A,B |
| Undecanoic acid C11:0 | 0.05 ± 0.01 A | 0.16 ± 0.01 A,B | ND A,B | 0.05 ± 0.00 B |
| Dodecenoic acid C12:1 | 0.01 ± 0.00 A,B | ND B | ND A | 0.01 ± 0.00 A,B |
| Dodecanoic acid C12:0 | 0.09 ± 0.00 A | 0.34 ± 0.01 A | 0.04 ± 0.01 A | 0.07 ± 0.00 A |
| Tridecanoic acid C13:0 | ND A | 0.18 ± 0.02 A,B | ND B | 0.03 ± 0.00 A,B |
| Tetradecenoic acid C14:1 | 0.25 ± 0.01 A | 0.42 ± 0.01 A | 0.07 ± 0.01 A | 0.10 ± 0.01 A |
| Tetradecanoic acid C14:0 | 0.82 ± 0.00 A | 2.78 ± 0.14 A,B | 0.33 ± 0.01 A,B | 0.97 ± 0.00 B |
| Pentadecenoic acid C15:1 | 0.02 ± 0.00 A,B | 0.06 ± 0.01 A,B | ND A | trace amount B |
| Pentadecanoic acid C15:0 | 0.10 ± 0.00 A | 0.39 ± 0.07 A,B,C | 0.05 ± 0.01 B | 0.08 ± 0.00 C |
| Hexadecenoic acid C16:1 | 46.61 ± 0.46 A,B | 58.90 ± 1.13 A,B | 17.53 ± 0.19 | 17.24 ± 0.19 |
| Hexadecanoic acid C16:0 | 25.60 ± 0.36 A,B | 52.12 ± 8.16 A,B | 10.91 ± 0.14 A | 12.11 ± 0.12 B |
| Heptadecenoic acid C17:1 | 0.80 ± 0.20 A,C | 0.80 ± 0.12 B,D | 0.21 ± 0.01 A,B | 0.18 ± 0.03 C,D |
| Heptadecanoic acid C17:0 | 0.13 ± 0.01 A | 0.34 ± 0.08 A,B,C | 0.05 ± 0.01 B | 0.07 ± 0.00 C |
| Octadecatrienoic acid C18:3 | 0.76 ± 0.01 A,C | 0.79 ± 0.02 B,D | 0.23 ± 0.01 A,B | 0.28 ± 0.00 C,D |
| Octadecadienoic acid C18:2 | 8.93 ± 0.18 A | 16.74 ± 0.33 A | 3.47 ± 0.06 A | 5.75 ± 0.12 A |
| Octadecenoic acid C18:1 | 45.30 ± 0.51 A,B | 66.19 ± 2.54 A,B | 17.58 ± 0.10 A | 19.70 ± 0.10 B |
| Octadecanoic acid C18:0 | 3.39 ± 0.11 A | 26.47 ± 12.14 A,B,C | 1.55 ± 0.02 B | 2.20 ± 0.08 C |
| Nonadecenoic acid C19:1 | 0.03 ± 0.01 A | 0.04 ± 0.00 B | NDA,B,C | 0.02 ± 0.00 C |
| Nonadecanoic acid C19:0 | ND | ND | ND | 0.16 ± 0.21 |
| Eicosapentaenoic acid C20:5 | 1.81 ± 0.27 A | 2.84 ± 0.06 A | 0.64 ± 0.06 A | 1.29 ± 0.12 A |
| Eicosatetraenoic acid C20:4 | 5.28 ± 0.06 A,B | 12.56 ± 0.33 A,B | 1.41 ± 0.15 A | 1.65 ± 0.07 B |
| Eicosatrienoic acid C20:3 | 0.08 ± 0.00 A | 0.05 ± 0.00 A | ND A | 0.04 ± 0.00 A |
| Eicosenoic acid C20:1 | 0.06 ± 0.01 A,C | 0.07 ± 0.01 B,D | ND A,B | 0.02 ± 0.00 C,D |
| Eicosanoic acid C20:0 | 0.13 ± 0.00 A | 0.61 ± 0.09 A,B,C | 0.06 ± 0.01 B | 0.14 ± 0.00 C |
| Docosanoic acid C22:0 | 0.17 ± 0.01 A | 0.42 ± 0.01 A,B | 0.07 ± 0.00 A,B | 0.15 ± 0.00 B |
| Tetracosenoic acid C24:1 | ND A | 0.05 ± 0.01 A,B | ND B | 0.03 ± 0.00 A,B |
| Tetracosanoic acid C24:0 | 0.53 ± 0.02 A | 0.63 ± 0.05 A | 0.18 ± 0.00 A | 0.27 ± 0.00 A |
| Hexacosanoic acid C26:0 | 0.38 ± 0.02 A | 0.28 ± 0.03 A | 0.10 ± 0.01 A | 0.19 ± 0.00 A |
| Octacosanoic acid C28:0 | 0.35 ± 0.02 A | 0.22 ± 0.02 A | 0.08 ± 0.00 A | 0.15 ± 0.01 A |
| Triacontanoic acid C30:0 | 0.29 ± 0.01 A | 0.16 ± 0.02 A | 0.05 ± 0.00 A | 0.10 ± 0.00 A |
| Dotriacontanoic acid C32:0 | 0.23 ± 0.02 A,B | ND A | 0.02 ± 0.01 B | 0.07 ± 0.00 A,B |
| Sum of FFA | 142.42 ± 1.71 A,B | 244.96 ± 17.08 A,B | 54.81 ± 0.47 A | 63.19 ± 0.76 B |
| Cuticular | Internal | |||
|---|---|---|---|---|
| Control | Exposure to C. coronatus | Control | Exposure to C. coronatus | |
| Glycerol | 0.10 ± 0.00 A | 0.19 ± 0.01 A,B | 0.08 ± 0.01 B | 0.07 ± 0.00 A |
| Cholesterol | 6.47 ± 0.08 A | 8.50 ± 0.11 A | 3.12 ± 0.07 A | 3.52 ± 0.03 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarek, A.; Boguś, M.I. The Impact of the Entomopathogenic Fungus Conidiobolus coronatus on the Free Fatty Acid Profile of the Flesh Fly Sarcophaga argyrostoma. Insects 2021, 12, 970. https://doi.org/10.3390/insects12110970
Kaczmarek A, Boguś MI. The Impact of the Entomopathogenic Fungus Conidiobolus coronatus on the Free Fatty Acid Profile of the Flesh Fly Sarcophaga argyrostoma. Insects. 2021; 12(11):970. https://doi.org/10.3390/insects12110970
Chicago/Turabian StyleKaczmarek, Agata, and Mieczysława Irena Boguś. 2021. "The Impact of the Entomopathogenic Fungus Conidiobolus coronatus on the Free Fatty Acid Profile of the Flesh Fly Sarcophaga argyrostoma" Insects 12, no. 11: 970. https://doi.org/10.3390/insects12110970
APA StyleKaczmarek, A., & Boguś, M. I. (2021). The Impact of the Entomopathogenic Fungus Conidiobolus coronatus on the Free Fatty Acid Profile of the Flesh Fly Sarcophaga argyrostoma. Insects, 12(11), 970. https://doi.org/10.3390/insects12110970







