The Filippi’s Glands of Giant Silk Moths: To Be or Not to Be?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Light Microscopy
2.3. Masson Trichrome Staining
3. Results
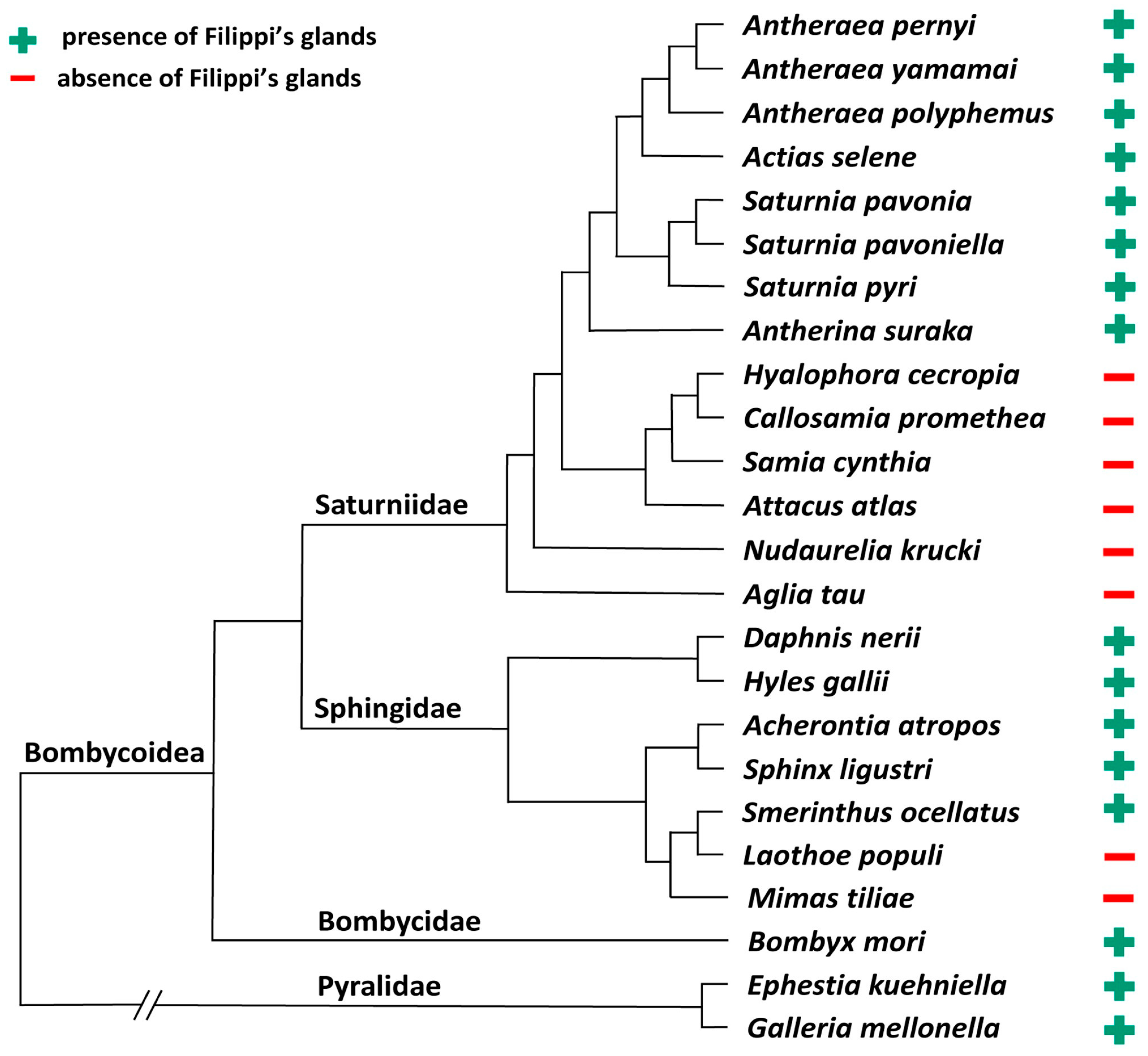
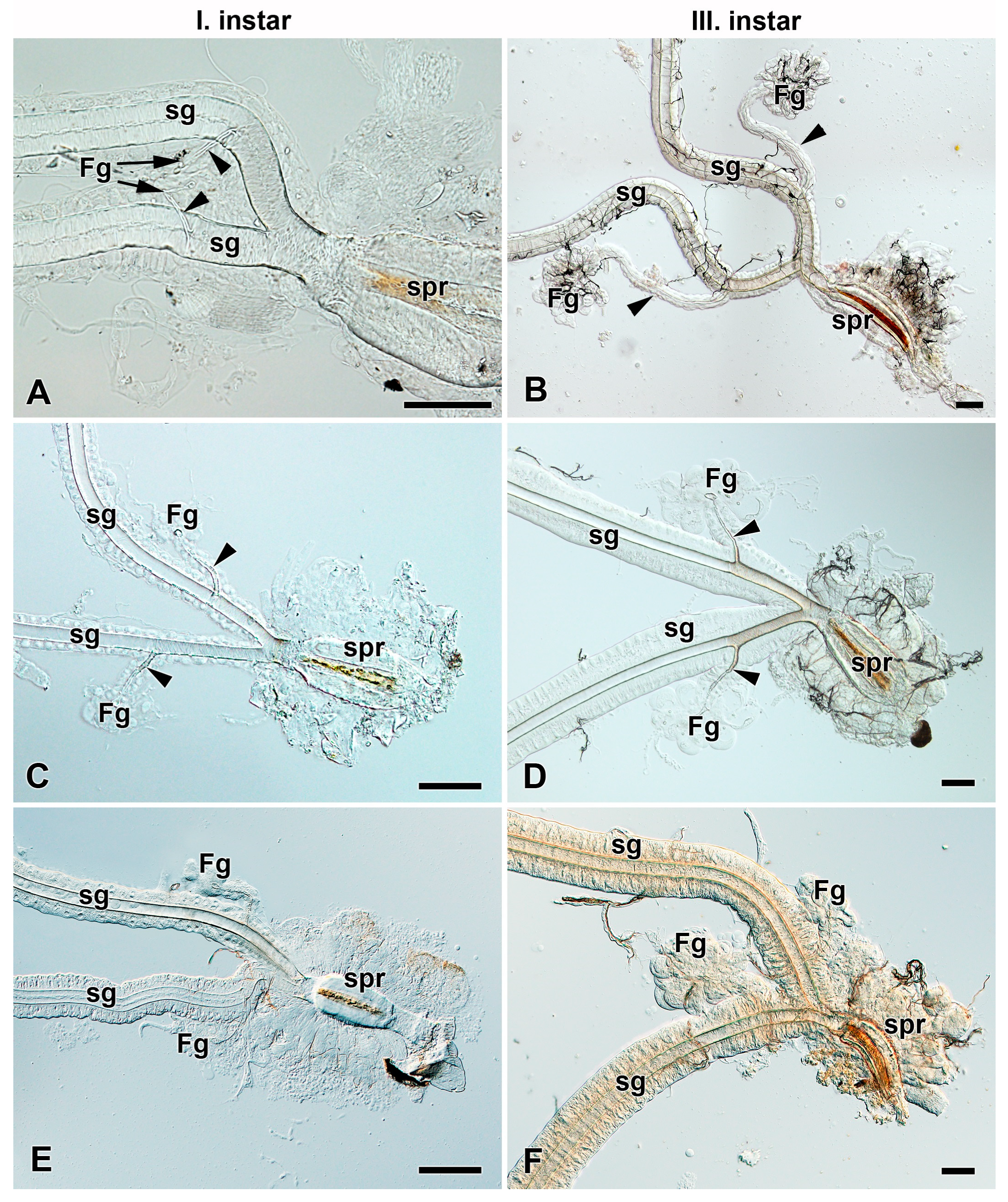
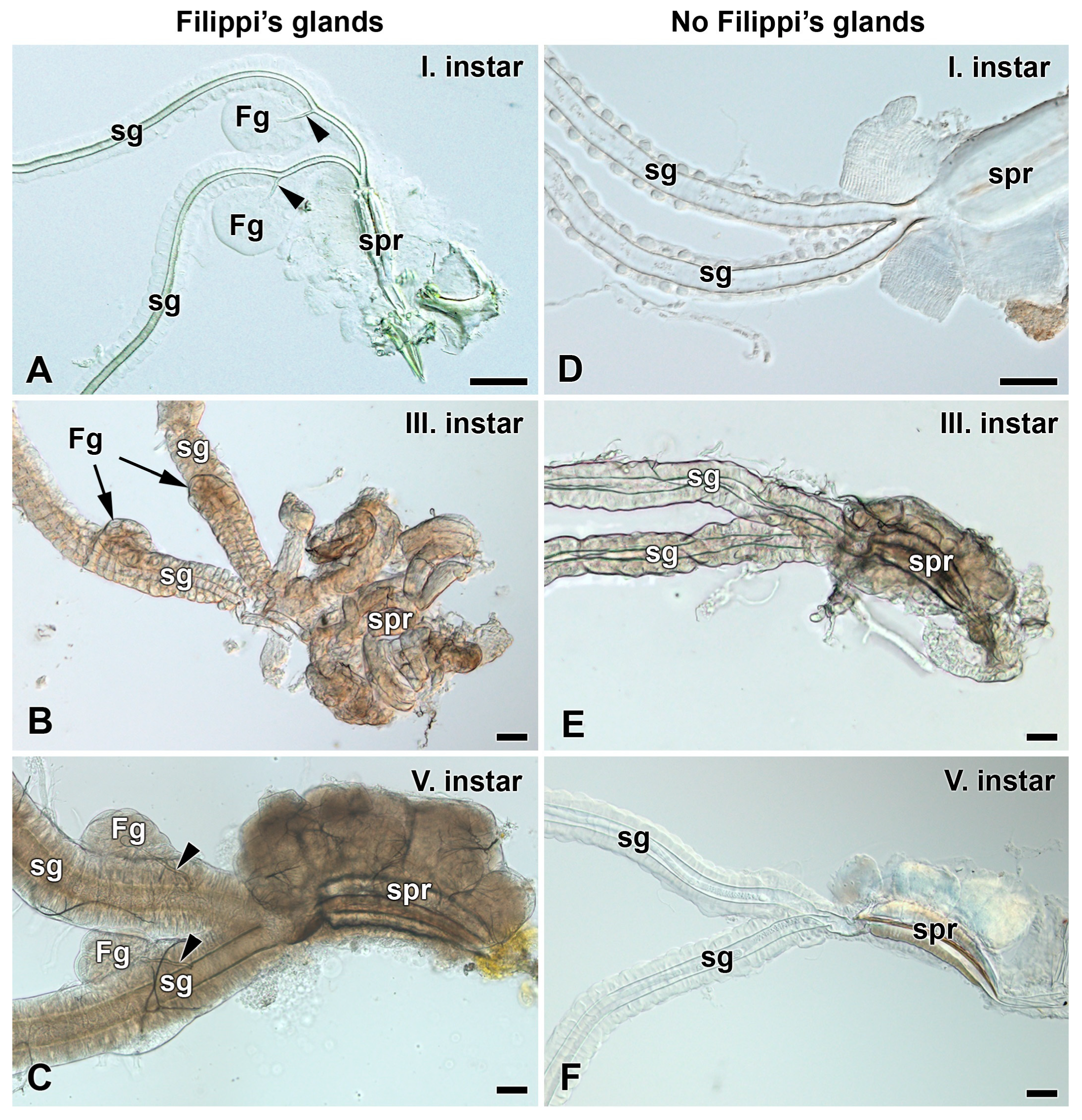
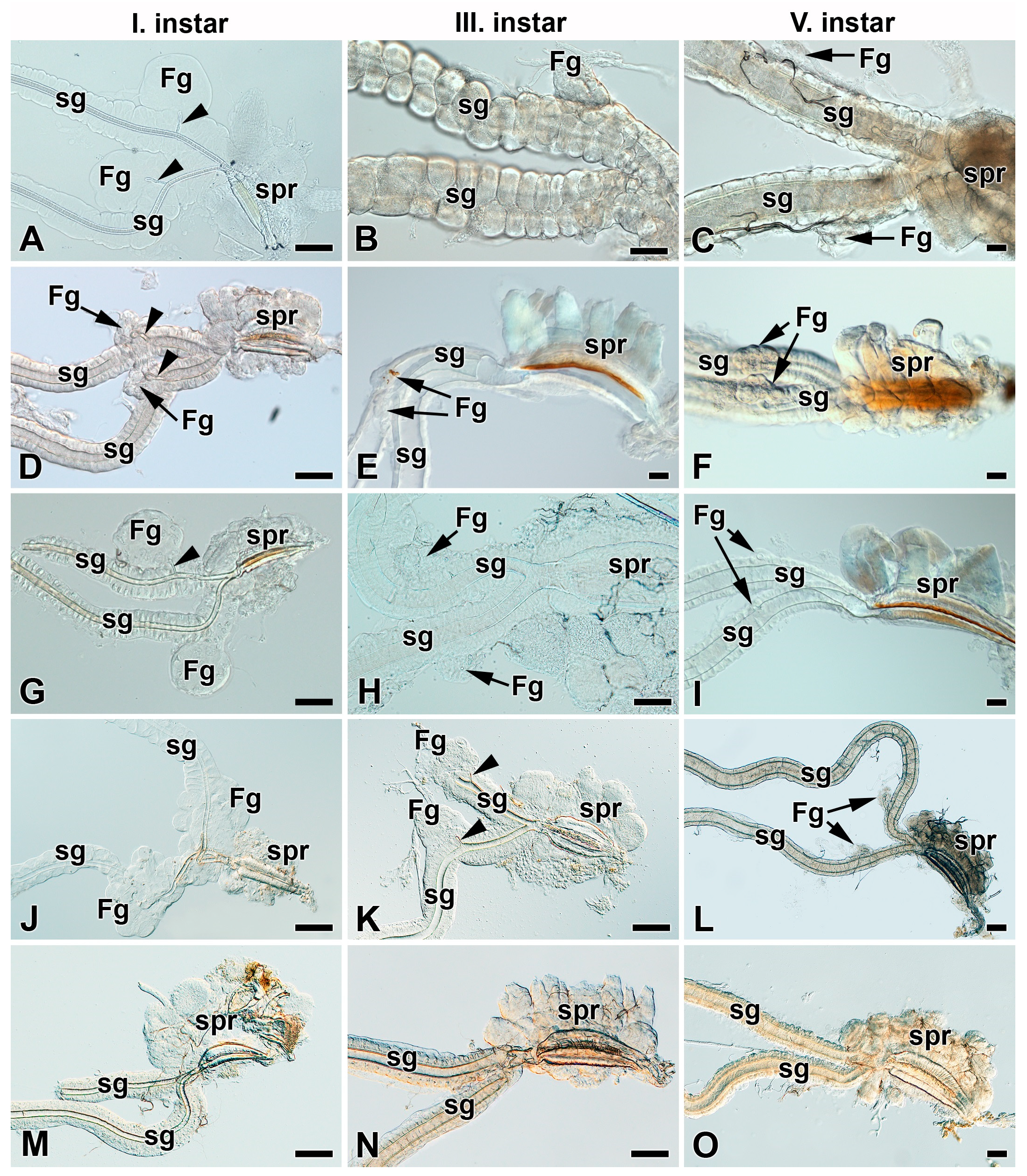
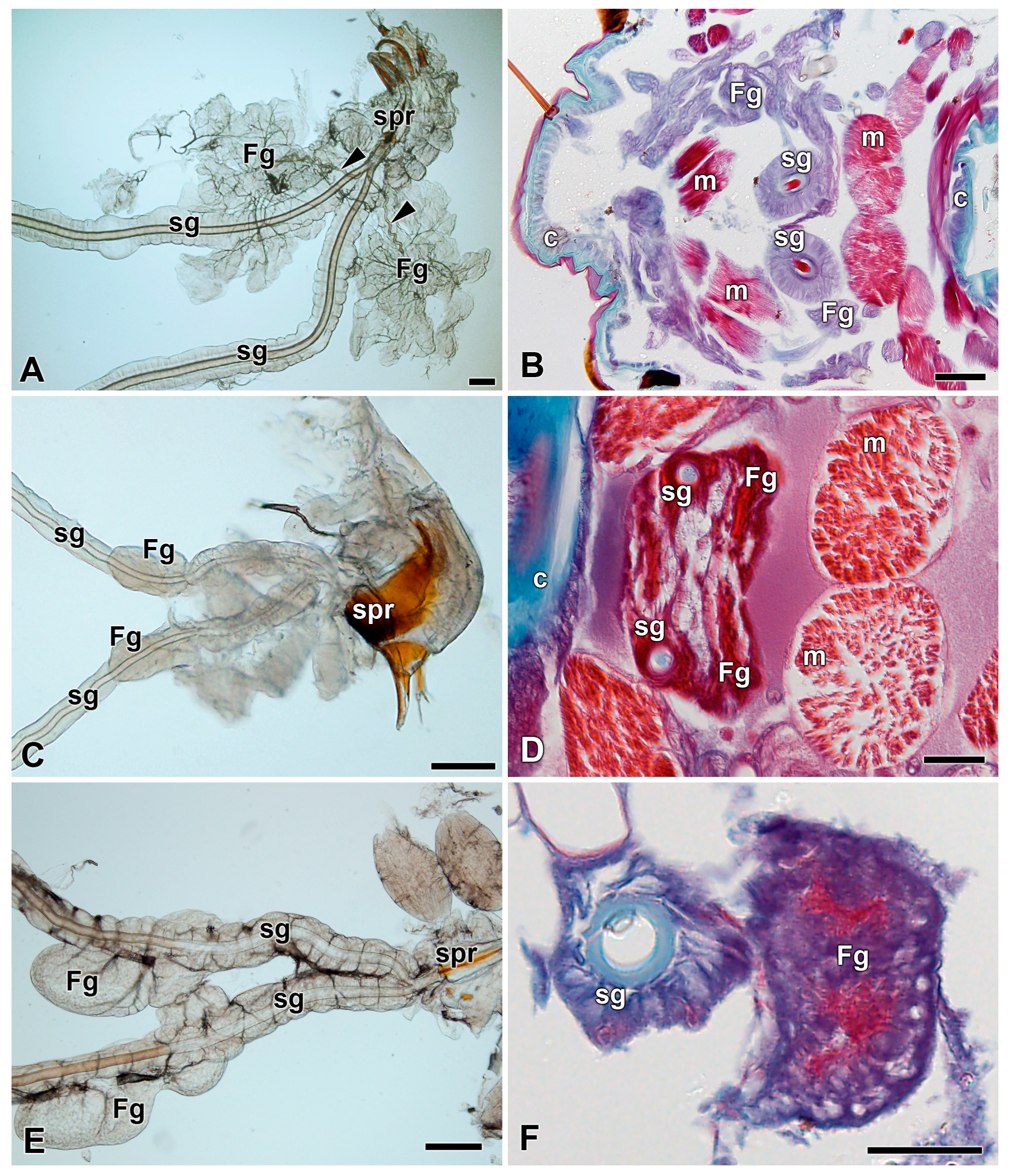
3.1. Absence of Filippi’s Glands in Some Giant Silk Moth Species (Saturniidae)
3.2. Development of Filippi’s Glands in Saturniidae
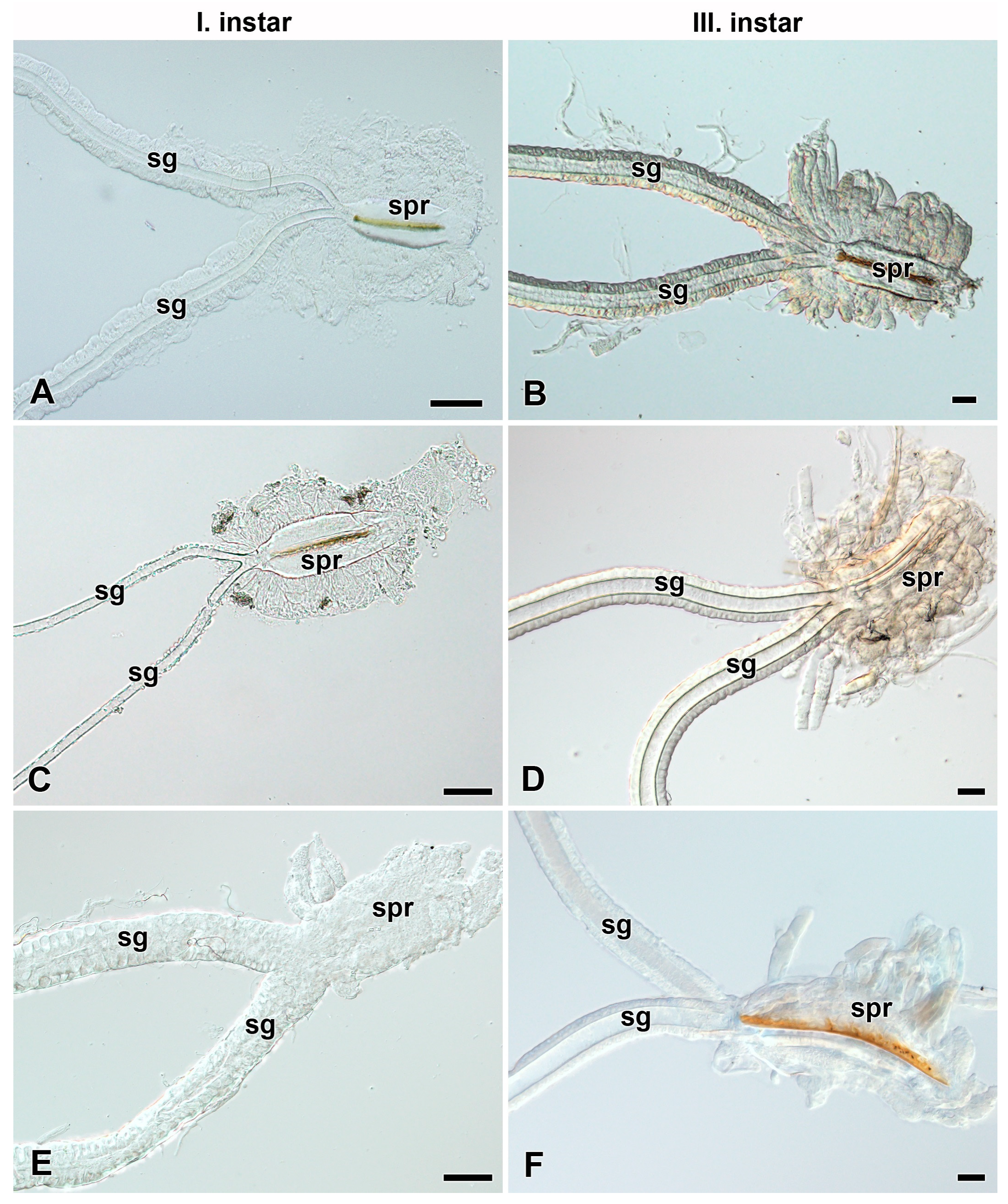
3.3. Heterogeneity of Filippi’s Glands Occurrence in Hawk Moths (Sphingidae)
3.4. Filippi’s Glands in Other Bombycoidea Species
3.5. Filippi’s Glands in Some Ancestral Lepidopteran Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Helm, E. Über die Spinndrüsen der Lepidopteren; Wilhelm Engelmann: Leipzig, Germany, 1876. [Google Scholar]
- Day, M.F.; Waterhouse, D.F. Chapter 12 in Insect Physiology; Roeder, K., Ed.; John Wiley and Sons, Inc.: New York, NY, USA, 1953. [Google Scholar]
- Wigglesworth, V.B. The Principles of Insect Physiology; Chapman and Hall: London, UK, 1972. [Google Scholar]
- Wang, X.; Li, Y.; Peng, L.; Chen, H.; Xia, Q.; Zhao, P. Comparative transcriptome analysis of Bombyx mori spinnerets and Filippi’s glands suggests their role in silk fiber formation. Insect Biochem. Mol. Biol. 2016, 68, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Liu, Q.; Tan, X.; Xie, X.; Xia, Q.; Zhao, P. GC/MS-based metabolomics analysis reveals active fatty acids biosynthesis in the Filippi’s gland of the silkworm, Bombyx mori, during silk spinning. Insect Biochem. Mol. Biol. 2019, 105, 1–9. [Google Scholar] [CrossRef]
- Patra, S.; Singh, R.N.; Raziuddin, M. Morphology and histology of Lyonet’s gland of the tropical tasar silkworm, Antheraea mylitta. J. Insect Sci. 2012, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Machida, Y. Studies on the silk glands of the silkworm, Bombyx mori L. I. Morphological and functional studies of Filippi’s glands in the silkworm. Sci. Bull. Fac. Agric. Kyushu Univ. 1965, 22, 95–108. [Google Scholar]
- Sehnal, F.; Akai, H. Insect Silk Glands—Their Types, Development and Function, and Effects of Environmental-Factors and Morphogenetic Hormones on Them. Int. J. Insect Morphol. 1990, 19, 79–132. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Hamano, K.; Mukaiyama, F. Morphological studies of the Filippi’s glands in several lepidopterous insects. J. Sericic. Sci. Jpn. 1987, 56, 449–456. [Google Scholar]
- Waku, Y.; Sumimoto, K.I. Ultrastructure of Lyonet’s gland in the silkworm (Bombyx mori L.). J. Morphol. 1974, 142, 165–185. [Google Scholar] [CrossRef]
- Paudel, S.; Mikó, I.; Deans, A.; Rajotte, E.; Felton, G. Lyonet’s gland of the tomato fruitworm, Helicoverpa zea (Lepidoptera: Noctuidae). Res. Ideas Outcomes 2018, 5, e35906. [Google Scholar] [CrossRef]
- Vegliante, F. Larval head anatomy of Heterogynis penella (Zygaenoidea, Heterogynidae), and a general discussion of caterpillar head structure (Insecta, Lepidoptera). Acta Zool. 2005, 86, 167–194. [Google Scholar] [CrossRef]
- Sorour, J.; Larink, A.; Ramadan, A.; Shalaby, A.; Osman, S. Anatomical, histological and electron microscopical studies on the salivary glands of larvae of Spodoptera littoralis Boids (Lepidoptera: Noctuidae). I. The anterior and posterior secretory parts. Zool. Jahrbücher Anat. Ontog. Tiere 1990, 120, 251–257. [Google Scholar]
- Chi, C.; Drew, W.A.; Young, J.H.; Curd, M.R. Comparative morphology and histology of the larval digestive system of two genera of Noctuidae (Lepidoptera): Heliothis and Spodoptera. Ann. Entomol. Soc. Am. 1975, 68, 371–380. [Google Scholar] [CrossRef]
- Victoriano, E.; Gregorio, E.A. Ultrastructure of the Lyonet’s glands in larvae of Diatraea saccharalis Fabricius (Lepidoptera: Pyralidae). Biocell 2004, 28, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Drecktrah, H.G.; Knight, K.L.; Brindley, T.A. Morphological investigations of the european corn borer. Iowa State J. Sci. 1966, 40, 257–286. [Google Scholar]
- Miko, I.; Rahman, S.R.; Jones, A.; Townley, M.A.; Gominho, B.; Paudel, S.; Stupski, S.D.; Hines, H.M.; Schilder, R.J. From Spinning Silk to Spreading Saliva: Mouthpart Remodeling in Manduca sexta (Lepidoptera: Sphingidae). Insect Syst. Diver. 2019, 3, 2. [Google Scholar] [CrossRef]
- Diss, A.L.; Kunkel, J.G.; Montgomery, M.E.; Leonard, D.E. Effects of maternal nutrition and egg provisioning on parameters of larval hatch, survival and dispersal in the gypsy moth, Lymantria dispar L. Oecologia 1996, 106, 470–477. [Google Scholar] [CrossRef]
- Perovic, D.J.; Johnson, M.L.; Scholz, B.; Zalucki, M.P. The mortality of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) neonate larvae in relation to drop-off and soil surface temperature: The dangers of bungy jumping. Aust. J. Entomol. 2008, 47, 289–296. [Google Scholar] [CrossRef]
- Reinecke, J.P.; Buckner, J.S.; Grugel, S.R. Life-Cycle of Laboratory-Reared Tobacco Hornworms, Manduca-Sexta—Study of Development and Behavior, Using Time-Lapse Cinematography. Biol. Bull. 1980, 158, 129–140. [Google Scholar] [CrossRef]
- Goodman, W.G.; Carlson, R.O.; Nelson, K.L. Analysis of Larval and Pupal Development in the Tobacco Hornworm (Lepidoptera, Sphingidae), Manduca-Sexta. Ann. Entomol. Soc. Am. 1985, 78, 70–80. [Google Scholar] [CrossRef]
- Goodman, W.G.; Jeanne, R.; Sutherland, P. Teaching about behavior with the tobacco hornworm. Am. Biol. Teach. 2001, 63, 258–261. [Google Scholar] [CrossRef]
- Sehadova, H.; Guerra, P.A.; Sauman, I.; Reppert, S.M. A re-evaluation of silk measurement by the cecropia caterpillar (Hyalophora cecropia) during cocoon construction reveals use of a silk odometer that is temporally regulated. PLoS ONE 2020, 15, e0228453. [Google Scholar]
- Hamilton, C.A.; St Laurent, R.A.; Dexter, K.; Kitching, I.J.; Breinholt, J.W.; Zwick, A.; Timmermans, M.J.T.N.; Barber, J.R.; Kawahara, A.Y. Phylogenomics resolves major relationships and reveals significant diversification rate shifts in the evolution of silk moths and relatives. BMC Evol. Biol. 2019, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sehnal, F. Kritisches Studium der Bionomie und Biometrik der in verschiedenen Lebensbedingungen gezüchteten Wachsmotte, Galleria mellonella L. (Lepidoptera). Z. Wiss. Zool. 1966, 174, 53–82. [Google Scholar]
- Marec, F. Genetic control of pest Lepidoptera: Induction of sex-linked recessive lethal mutations in Ephestia-kuehniella (Pyralidae). Acta Entomol. Bohemos. 1990, 87, 445–458. [Google Scholar]
- Kamioka, S.; Mukaiyama, F.; Takei, T.; Ito, T. Digestion and utilization of artificial diet by the silkworm, Bombyx mori, with special references to the efficiency of the diet at varying levels of dietary soybean meal. J. Sericic. Sci. Jpn. 1971, 40, 473–483. [Google Scholar]
- Meister, F. A Guide to the Breeding of Tropical Silk Moths; Verlag Dr. Friedrich Pheil: Munich, Germany, 2011. [Google Scholar]
- Gardiner, B.O.C. A Silkmoth Rearer’s Handbook; The Amateur Entomologists’ Society: Hanworth, UK, 1982. [Google Scholar]
- Levine, J.D.; Sauman, I.; Imbalzano, M.; Reppert, S.M.; Jackson, F.R. Period Protein from the Giant Silkmoth Antheraea-Pernyi Functions as a Circadian Clock Element in Drosophila-Melanogaster. Neuron 1995, 15, 147–157. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.-C.; Hao, D.; Liu, Z.; Meng, P.; Xu, F. The research on morphology of SG and FG of 18 species in lepidoptera. J. Shenyang Agric. Univ. 1997, 28, 104–108. [Google Scholar]
- Tuskes, P.M.; Tuttle, J.P.; Collins, M.M. The Wild Silk Moths of North America: A Natural History of the Saturniidae of the United States and Canada; Cornell University Press: Ithaca, NY, USA, 1996. [Google Scholar]
- Lampe, R.E.J. Saturniidae of the World: Their Life Stages from the Eggs to the Adults; Verlag Dr. Friedrich Pfeil: Munich, Germany, 2010. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sehadova, H.; Zavodska, R.; Zurovec, M.; Sauman, I. The Filippi’s Glands of Giant Silk Moths: To Be or Not to Be? Insects 2021, 12, 1040. https://doi.org/10.3390/insects12111040
Sehadova H, Zavodska R, Zurovec M, Sauman I. The Filippi’s Glands of Giant Silk Moths: To Be or Not to Be? Insects. 2021; 12(11):1040. https://doi.org/10.3390/insects12111040
Chicago/Turabian StyleSehadova, Hana, Radka Zavodska, Michal Zurovec, and Ivo Sauman. 2021. "The Filippi’s Glands of Giant Silk Moths: To Be or Not to Be?" Insects 12, no. 11: 1040. https://doi.org/10.3390/insects12111040
APA StyleSehadova, H., Zavodska, R., Zurovec, M., & Sauman, I. (2021). The Filippi’s Glands of Giant Silk Moths: To Be or Not to Be? Insects, 12(11), 1040. https://doi.org/10.3390/insects12111040






