Integrated Pest Management for Stored Grain: Potential Natural Biological Control by a Parasitoid Wasp Community
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Survey
2.3. Data Analysis
3. Results
3.1. Parasitoid Distribution by Family
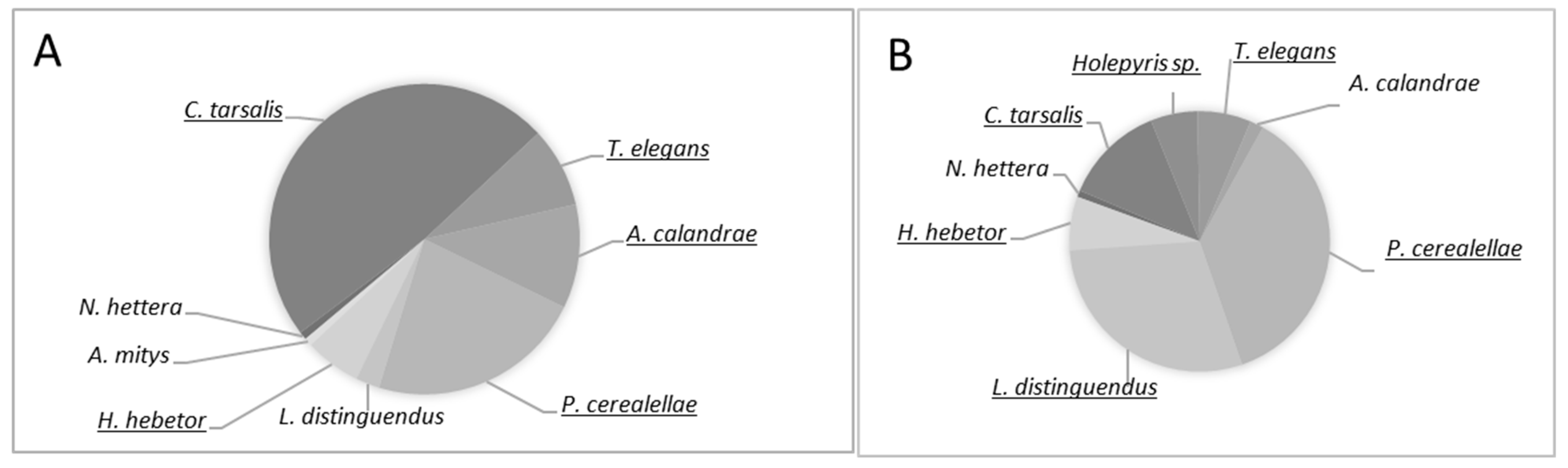
3.2. Parasitoid Dominancy and Abundance
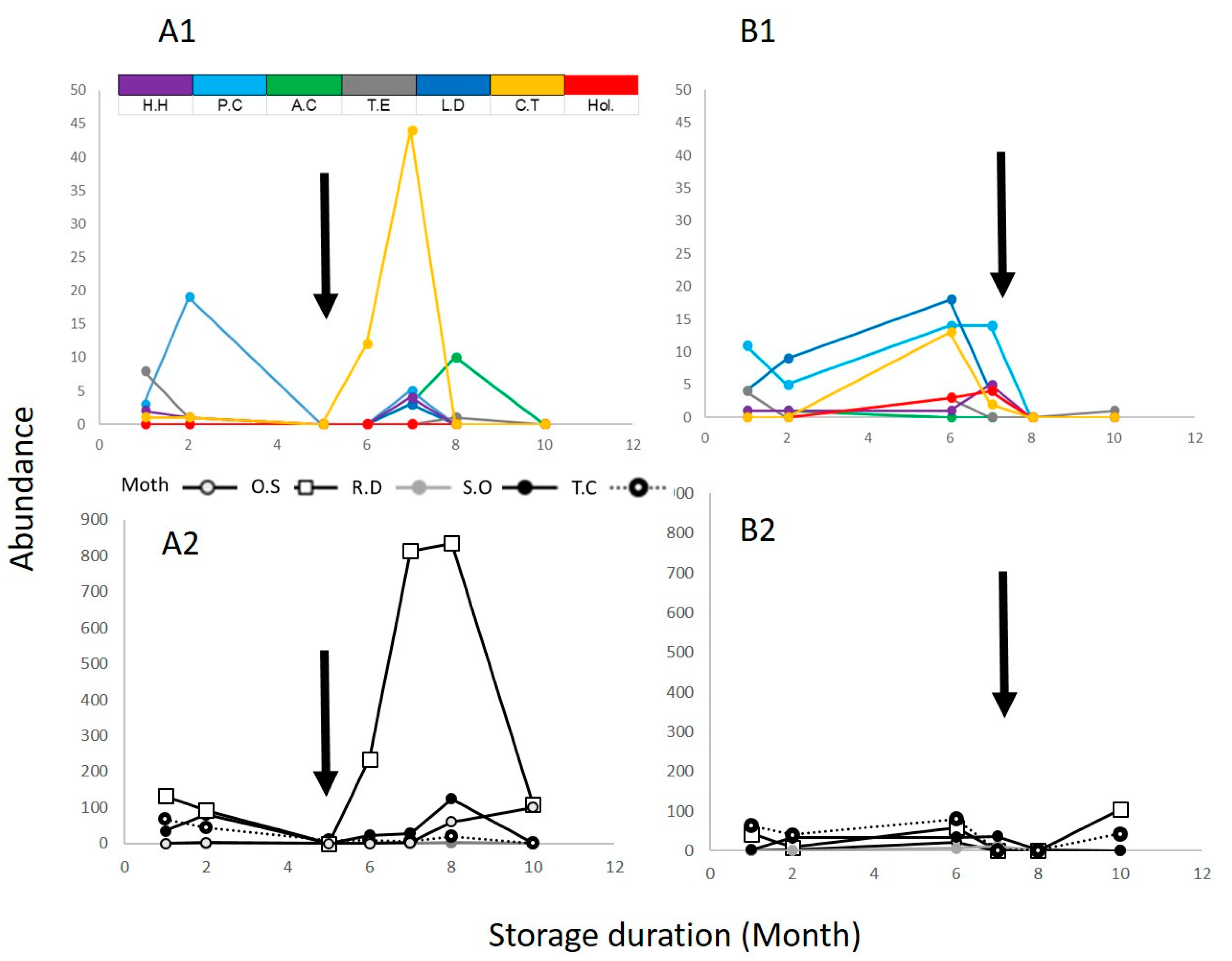
3.3. Parasitoids Affect the Abundance of Pest Species
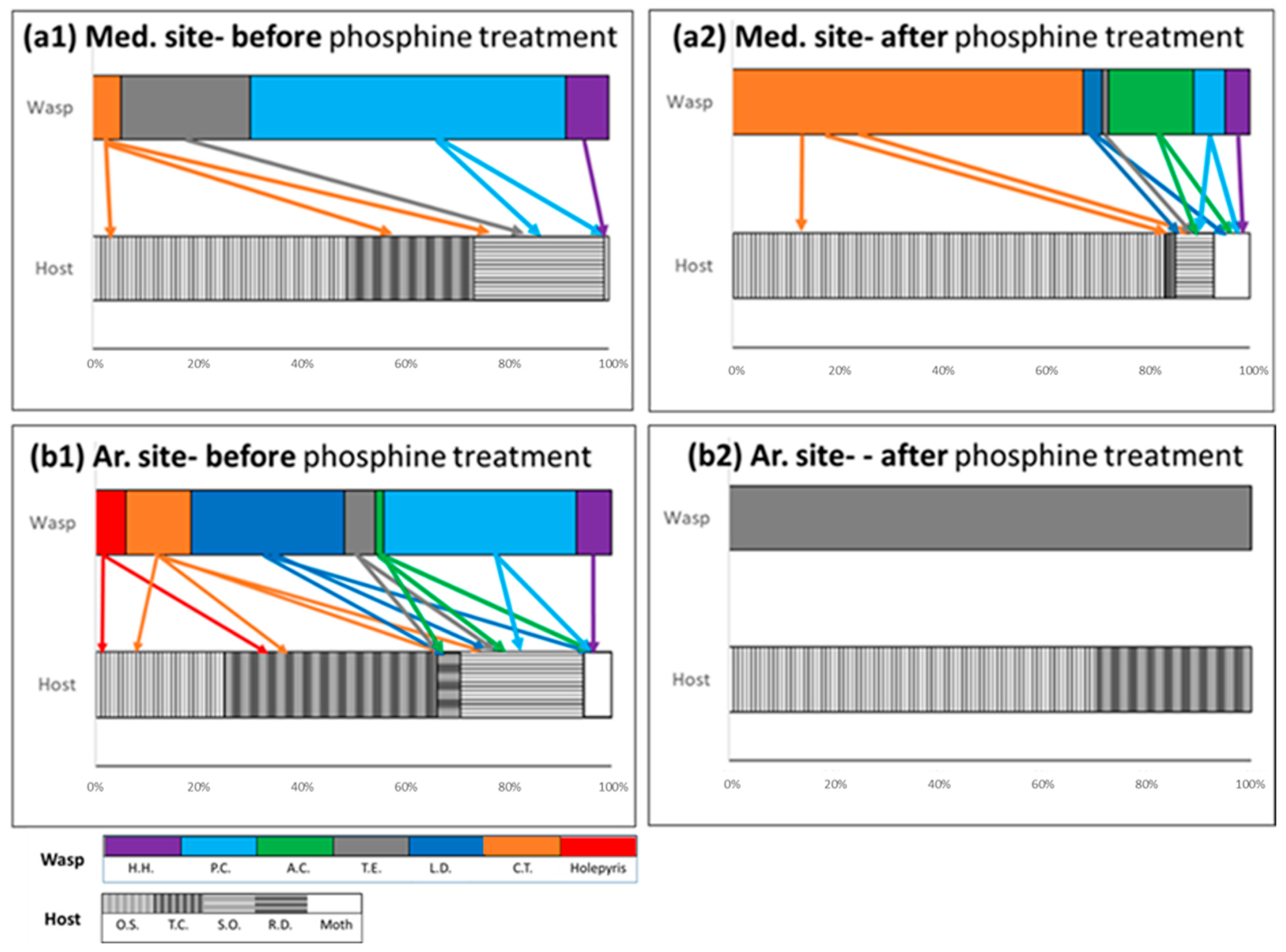
3.4. Effect of Phosphine Treatment on the Composition of the Parasitoid and Pest Community
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anukiruthika, T.; Jian, F.; Jayas, D.S. Movement and behavioral response of stored product insects under stored grain environments-A review. J. Stored Prod. Res. 2021, 90, 101752. [Google Scholar] [CrossRef]
- Nayak, M.K.; Holloway, J.C.; Emery, R.N.; Pavic, H.; Bartlet, J.; Collins, P.J. Strong resistance to phosphine in the rusty grain beetle, Cryptolestes ferrugineus (Stephens) (Coleoptera: Laemophloeidae): Its characterization, a rapid assay for diagnosis and its distribution in Australia. Pest Manag. Sci. 2012, 69, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Suma, P.; Amante, M.; Bella, S.; La Pergola, A.; Russo, A. Stored-product insect natural enemies in wheat industry in Sicily. IOBC-WPRS Bull. 2014, 98, 227–233. [Google Scholar]
- Schöller, M.; Prozell, S.; Suma, P.; Russo, A. Biological control of stored-product insects. In Recent Advances in Stored Product Protection; Springer: Berlin/Heidelberg, Germany, 2018; pp. 183–209. [Google Scholar]
- Savidan-Neiderer, A. Tritrophic Interactions in Maize Storage Systems. Ph.D. Thesis, Université de Neuchâtel, Neuchâtel, Switzerland, 2002. [Google Scholar]
- Schöller, M. Integration of biological and non-biological methods for controlling arthropods infesting stored products. Postharvest News Inf. 1998, 9, 15–20. [Google Scholar]
- Schöller, M. Prospects for biological control of stored-product pests. Julius-Kühn-Archive 2010, 429, 25. [Google Scholar]
- Eliopoulos, P.A.; Athanaaiou, C.G.; Buchelos, C.H. Occurrence of hymenopterous parasitoids of stored product pests in Greece. IOBC-WPRS Bull. 2002, 25, 3–5. [Google Scholar]
- Schöller, M.; Flinn, P.W. Parasitoids and predators. In Alternatives to Pesticides in Stored-Product IPM; Springer: Boston, MA, USA, 2000; pp. 229–271. [Google Scholar]
- Khan, B.A.; Anwarullah, M. Predators and parasites associated with stored grain pests in Karachi, West Pakistan. Sci. Ind. 1970, 7, 45–49. [Google Scholar]
- Abdel-Rahman, H.A.; Shaumar, N.F.; Soliman, Z.A.; El-Agoze, M.M. Survey and taxonomy of parasites and predators of stored grain and grain products insects. Bull. Soc. Entomol. D’egypte 1977, 61, 53–74. [Google Scholar]
- Sedlacek, J.D.; Price, B.D.; Sharkey, M.J.; Hill, S.J.; Weston, P.A. Parasitoids found in on-farm shelled corn in Kentucky. J. Agr. Entomol. 1998, 15, 223–230. [Google Scholar]
- Rosa, P.; Lotfalizadeh, H.; Pourrafei, L. First checklist of the chrysidid wasps (Hymenoptera: Chrysididae) of Iran. Zootaxa 2013, 3700, 1–47. [Google Scholar] [CrossRef][Green Version]
- Agrafioti, P.; Kaloudis, E.; Bantas, S.; Sotiroudas, V.; Athanassiou, C.G. Phosphine distribution and insect mortality in commercial metal shipping containers using wireless sensors and CFD modeling. Comput. Electron. Agric. 2021, 184, 106087. [Google Scholar] [CrossRef]
- Vidan, E.; Quinn, E.; Trostanetsky, A.; Rapaport, A.; Doron, J.; Harush, A.; Kostyukovsky, M.; Gottlieb, D. How do similar community dynamics yield different population dynamics and spatial distributions of species? J. Stored Prod. Res. 2020, 87, 101621. [Google Scholar] [CrossRef]
- Adarkwah, C.; Anankware, J.P.; Obeng-Ofori, D.; Ulrichs, C.; Schöller, M. Insect infestation and quality loss of major stored products in Ghana. In Proceedings of the 12th International Working Conference on Stored Product Protection (IWCSPP), Berlin, Germany, 7–11 October 2018. [Google Scholar]
- Steidle, J.L.M.; Schöller, M. Fecundity and ability of the parasitoid Lariophagus distinguendus (Hymenoptera: Pteromalidae) to find larvae of the granary weevil Sitophilus granaries (Coleoptera: Curculionidae) in bulk grain. J. Stored Prod. Res. 2002, 38, 43–53. [Google Scholar] [CrossRef]
- Bouček, Z.; Rasplus, J.Y. Illustrated key to West-Palearctic Genera of Pteromalidae (Hymenoptera: Chalcidoidea); INRA: Paris, France, 1991. [Google Scholar]
- Perkins, J.F. Handbooks for the Identification of British Insects. Hymenoptera Bethyloidea (Excluding Chrysididae); Royal Entomological Society: London, UK, 1976; Volume 6, p. 38. [Google Scholar]
- Medvedev, G.S. Keys to the Insects of the European Part of the USSR, Volume III, Hymenoptera, Part II; Translation of Publication in Russian by Nauka Publishers: Leningrad, Russia, 1978; Oxonian Press: New Delhi, India, 1987. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2014, 67, 1–48. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Barton, K. Mu-MIn: Multi-Model Inference. R Package Version 1.15. 6. 2016. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 1 August 2021).
- Grömping, U. Relative importance for linear regression in R: The package relaimpo. J. Stat. Softw. 2006, 17, 1–27. [Google Scholar] [CrossRef]
- Hallett, L.M.; Jones, S.K.; MacDonald, A.A.; Jones, M.B.; Flynn, D.F.; Ripplinger, J.; Slaughter, P.; Gries, C.; Collins, S.L. codyn: An r package of community dynamics metrics. Methods Ecol. Evol. 2016, 7, 1146–1151. [Google Scholar] [CrossRef]
- Eliopoulos, P.A.; Harvey, J.A.; Athanassiou, C.G.; Stathas, G.J. Effect of biotic and abiotic factors on reproductive parameters of synovigenic endoparasitoid Venturia canescens. Physiol. Entomol. 2003, 28, 268–275. [Google Scholar] [CrossRef]
- Eliopoulos, P.A. Life table parameters of the parasitoid Cephalonomia tarsalis (Hymenoptera: Bethylidae) and its host the saw-toothed grain beetle Oryzaephilus surinamensis (Coleoptera: Silvanidae). J. Plant Prot. Res. 2019, 59, 544–551. [Google Scholar]
- Moravvej, S.A.; Shishehbor, P.; Lotfalizadeh, H. A checklist of Chalcidoidea (Insecta: Hymenoptera) of Khuzestan in southwestern Iran. J. Insect Biodivers. Syst. 2016, 2, 121–142. [Google Scholar]
- Mbata, G.N.; Warsi, S. Habrobracon hebetor and Pteromalus cerealellae as tools in post-harvest integrated pest management. Insects 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.I.; Pikart, T.G.; Ramalho, F.S.; Manickavasagam, S.; Serrão, J.E.; Zanuncio, J.C. Antrocephalus mitys (Hymenoptera: Chalcididae) in laboratory cultures of Tenebrio molitor (Coleoptera: Tenebrionidae), and possible role in biological control of Ephestia cautella (Lepidoptera: Pyralidae). Fla. Entomol. 2013, 96, 634–637. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Arthur, F.H. Economic Theory Versus Reality in Stored Grain IPM: Theory and Practice in Stored Product Management. In Recent Advances in Stored Product Protection; Springer: Berlin, Germany, 2018; p. 273. [Google Scholar]
- Xu, H.Y.; Yang, N.W.; Wan, F.H. Competitive interactions between parasitoids provide new insight into host suppression. PLoS ONE 2013, 8, e82003. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, M.A. Fumigation of Farm Stored Grain and Structures; Technical Report; Department of Plant, Soil and Insect Sciences, The University of Wyoming: Laramie, WY, USA, 1996. [Google Scholar]
- Lorenz, S.; Adler, C.; Reichmuth, C. Penetration ability of Holepyris sylvanidis into the feeding substrate of its host Tribolium confusum. Julius-Kühn-Archiv 2010, 425, 721. [Google Scholar]
| Species in the Grain Storehouses | Curculionidae | Bostrichidae | Tenebrionidae | Silvanidae | Moth Larvae | |
|---|---|---|---|---|---|---|
| Sitophilus oryzae (Linnaeus) | Rhyzopertha dominica (Fabricius) | Tribolium sp. (Macleay) | Oryzophilus surinamensis (Linnaeus) | |||
| Bethylidae | Cephalonomia tarsalis (Ashmead) | [26] | [26] | [26] | [26,27] | |
| Holepyris sp. | [26] | [26] | ||||
| Braconidae | Habrobracon hebetor (Say) | [26] | ||||
| Chalcididae | Antrocephalus mitys (Walker) | [28] | ||||
| Neohybothorax hettera (Walker) | Unknown [29] | Unknown [29] | Unknown [29] | Unknown [29] | Unknown [29] | |
| Pteromalidae | Lariophagus distinguendus (Förster) | [26,30] | [30] | [26] | ||
| Pteromalus cerealellae (Ashmead) | [31] | [26] | ||||
| Theocolax elegans (Westwood) | [26,30] | [26,30] | [26] | |||
| Anisopteromalus calandrae (Howard) | [26,30] | [26,30] | [26] | |||
| Pest (Host) | Abiotic Conditions | Parasitoid Wasps | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Location in Storehouse | Temperature | Grain Moisture | Site | Holepyris sp. | C. tarsalis | L. distinguendus | T. elegans | A. calandrae | P. cerealellae | H. hebetor | |
| O. surinamensis | <0.0001 | <0.0001 | <0.0001 | <0.0001 | NS | <0.0001 | |||||
| T. castaneum | <0.0001 | <0.0001 | <0.0001 | NS | NS | <0.05 * | |||||
| R. dominica | NS | <0.05 | <0.001 | <0.01 | NS | NS | NS | <0.01 | |||
| S. oryzae | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.05 | <0.01 | <0.0001 | <0.01 | ||
| Moth | <0.0001 | <0.0001 | <0.0001 | <0.0001 | NS | NS | NS | NS | NS | <0.0001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harush, A.; Quinn, E.; Trostanetsky, A.; Rapaport, A.; Kostyukovsky, M.; Gottlieb, D. Integrated Pest Management for Stored Grain: Potential Natural Biological Control by a Parasitoid Wasp Community. Insects 2021, 12, 1038. https://doi.org/10.3390/insects12111038
Harush A, Quinn E, Trostanetsky A, Rapaport A, Kostyukovsky M, Gottlieb D. Integrated Pest Management for Stored Grain: Potential Natural Biological Control by a Parasitoid Wasp Community. Insects. 2021; 12(11):1038. https://doi.org/10.3390/insects12111038
Chicago/Turabian StyleHarush, Avichai, Elazar Quinn, Anatoly Trostanetsky, Aviv Rapaport, Moshe Kostyukovsky, and Daphna Gottlieb. 2021. "Integrated Pest Management for Stored Grain: Potential Natural Biological Control by a Parasitoid Wasp Community" Insects 12, no. 11: 1038. https://doi.org/10.3390/insects12111038
APA StyleHarush, A., Quinn, E., Trostanetsky, A., Rapaport, A., Kostyukovsky, M., & Gottlieb, D. (2021). Integrated Pest Management for Stored Grain: Potential Natural Biological Control by a Parasitoid Wasp Community. Insects, 12(11), 1038. https://doi.org/10.3390/insects12111038





