Combined Effect of Different Flower Stem Features on the Visiting Frequency of the Generalist Ant Lasius niger: An Experimental Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Insects
2.2. Microscopy
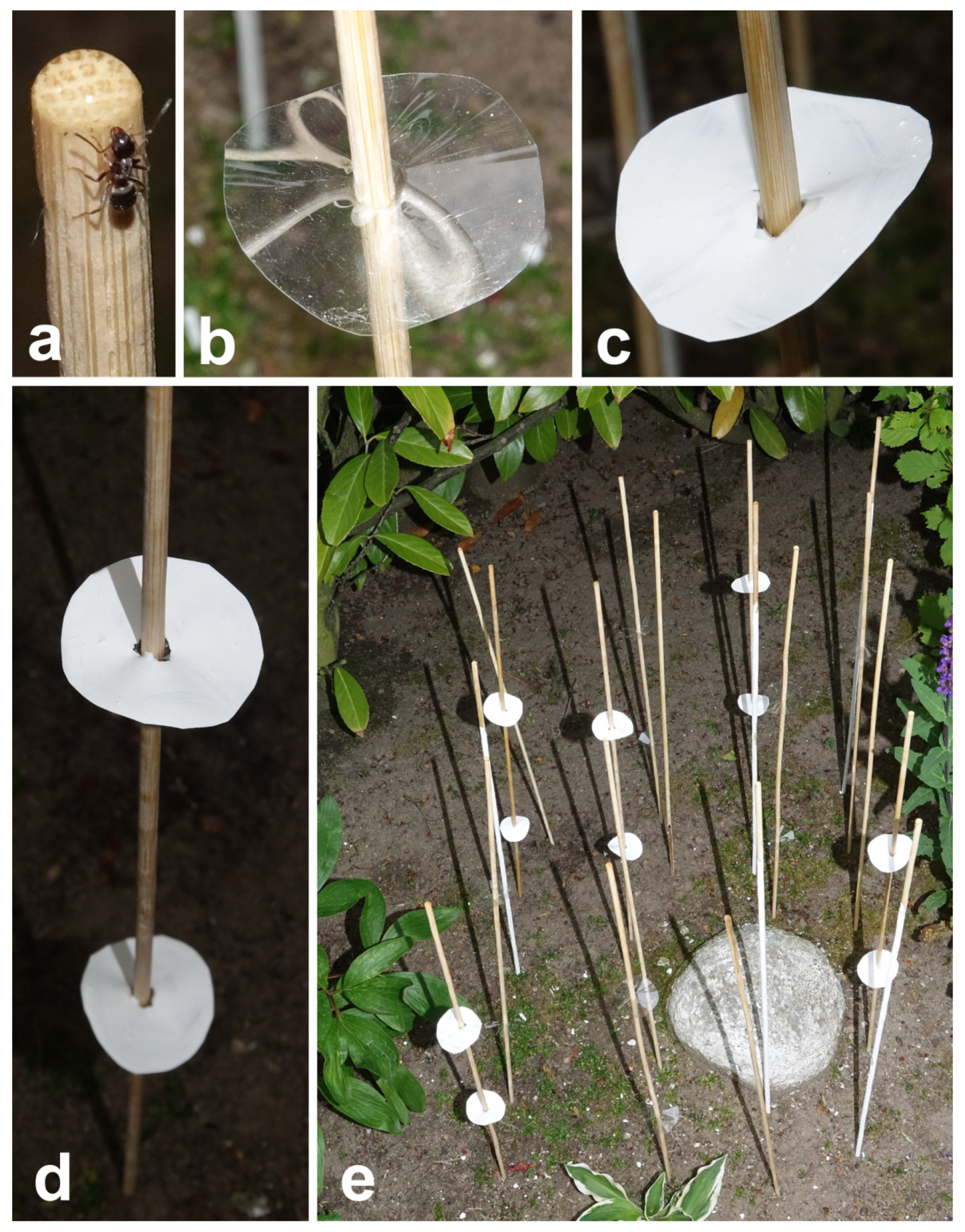
2.3. Experiment
3. Results
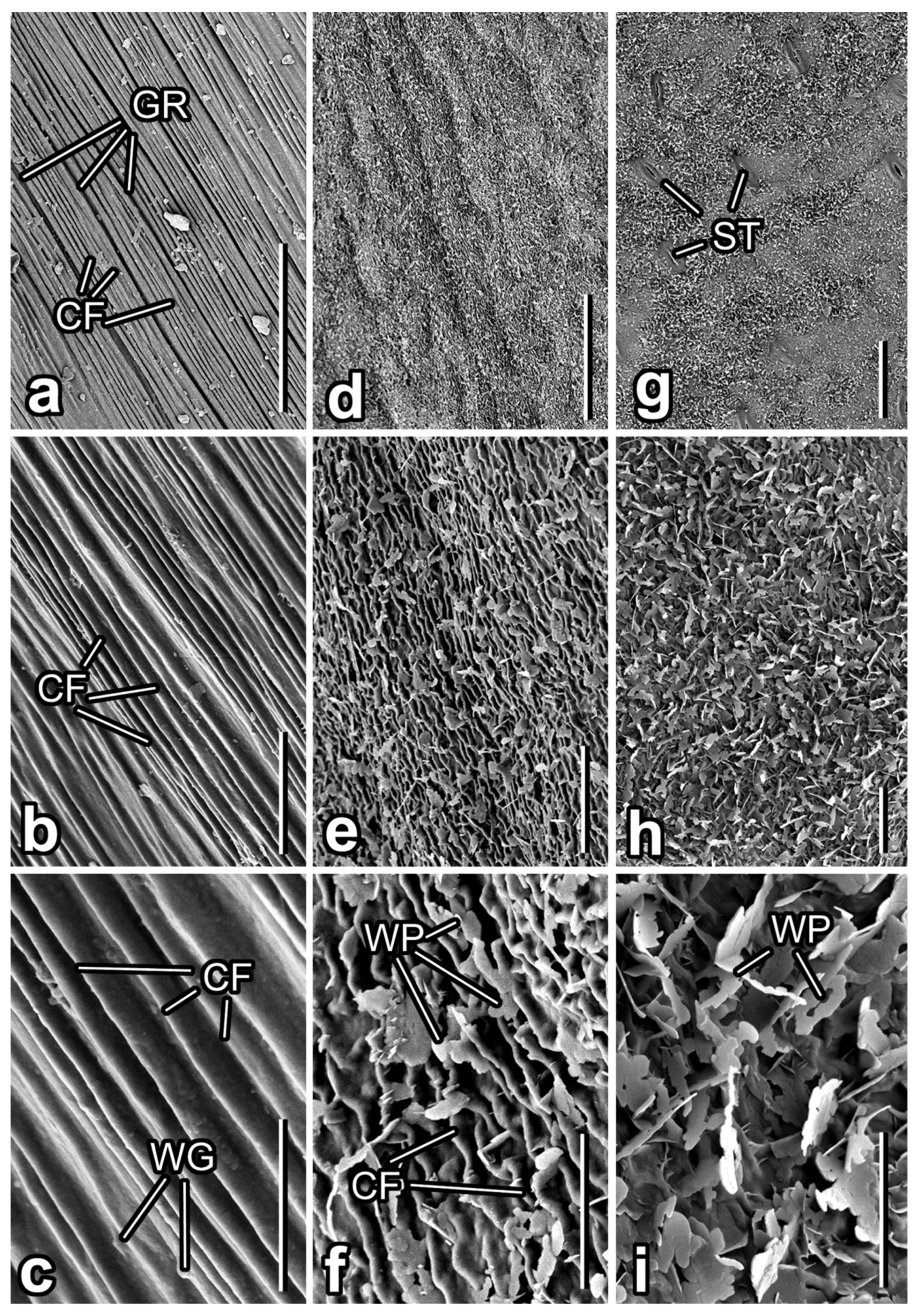
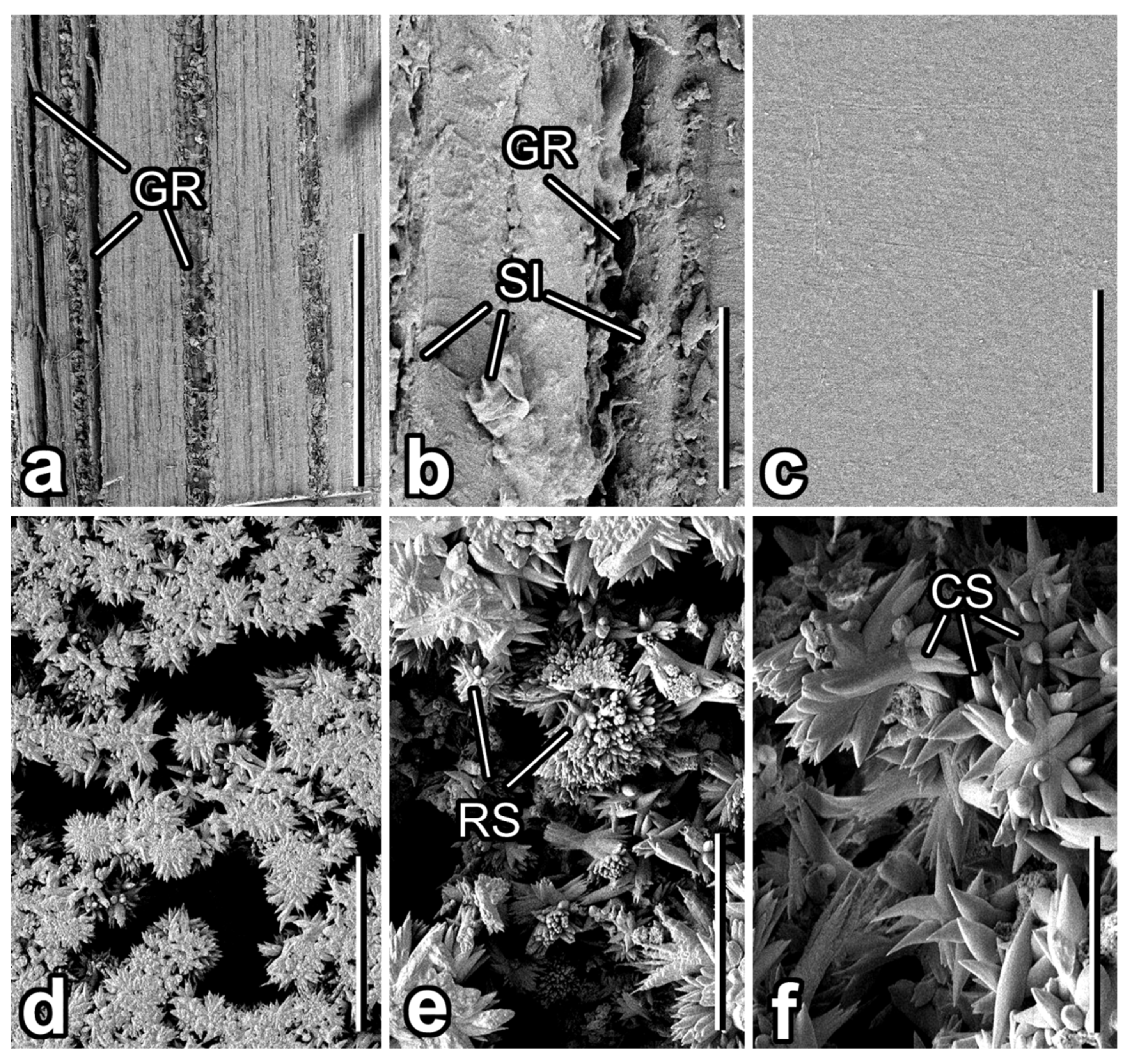
3.1. Micromorphology of Plant Surfaces
3.2. Microtexture of Samples Used in the Experiment
3.3. Ant Visits to Different Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Waser, N.M. Specialization and generalization in plant-pollinator interactions: A historical perspective. In Plant-Pollinator Interactions: From Specialization to Generalization; Waser, N.M., Ollerton, J., Eds.; The University of Chicago Press: Chicago, IL, USA, 2006; pp. 3–17. [Google Scholar]
- Van der Pijl, L. Some remarks on myrmecophytes. Phytomorphology 1955, 5, 190–200. [Google Scholar]
- Janzen, D.H. Why don’t ants visit flowers? Biotropica 1977, 9, 252. [Google Scholar] [CrossRef]
- Guerrant, E.O.; Fiedler, P.L. Flower defenses against nectar-pilferage by ants. Biotropica 1981, 13, 25–33. [Google Scholar] [CrossRef]
- Junker, R.R.; Blüthgen, N. Floral scents repel potentially nectar-thieving ants. Evol. Ecol. Res. 2008, 10, 295–308. [Google Scholar]
- Junker, R.R.; Gershenzon, J.; Unsicker, S.B. Floral odor bouquet loses its ant repellent properties after inhibition of terpene biosynthesis. J. Chem. Ecol. 2011, 37, 1323–1331. [Google Scholar] [CrossRef]
- Galen, C.; Cuba, J. Down the tube: Pollinators, predators, and the evolution of flower shape in the alpine skypilot, Polemonium viscosum. Evolution 2001, 55, 1963–1971. [Google Scholar] [CrossRef]
- Tagawa, K. Repellence of nectar-thieving ants by a physical barrier: Adaptive role of petal hairs on Menyanthes trifoliata (Menyanthaceae). J. Asia Pac. Entomol. 2018, 21, 1211–1214. [Google Scholar] [CrossRef]
- Villamil, N.; Boege, K.; Stone, G.N. Testing the distraction hypothesis: Do extrafloral nectaries reduce ant-pollinator conflict? J. Ecol. 2019, 107, 1377–1391. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Handel, S.N.; Beattie, A. The evidence for, and importance of, ant pollination. In Ant-Plant Interactions; Huxley, C.R., Cutler, D.E., Eds.; Oxford University Press: Oxford, UK, 1991; pp. 421–429. [Google Scholar]
- Willmer, P.G.; Nuttman, C.V.; Raine, N.E.; Stone, G.N.; Pattrick, J.G.; Henson, K.; Stillman, P.; McIlroy, L.; Potts, S.G.; Knudsen, J.T. Floral volatiles controlling ant behaviour. Func. Ecol. 2009, 23, 888–900. [Google Scholar] [CrossRef]
- Tsuji, K.; Hasyim, A.; Harlion; Nakamura, K. Asian weaver ants, Oecophylla smaragdina, and their repelling of pollinators. Ecol. Res. 2004, 19, 669–673. [Google Scholar] [CrossRef]
- Ness, J.H. A mutualism’s indirect costs: The most aggressive plant bodyguards also deter pollinators. Oikos 2006, 113, 506–514. [Google Scholar] [CrossRef]
- Lach, L. Argentine ants displace floral arthropods in a biodiversity hotspot. Divers. Distrib. 2008, 14, 281–290. [Google Scholar] [CrossRef]
- Hansen, D.M.; Müller, C.B. Invasive ants disrupt gecko pollination and seed dispersal of the endangered plant Roussea simplex in Mauritius. Biotropica 2009, 41, 202–208. [Google Scholar] [CrossRef]
- Cembrowski, A.R.; Tan, M.G.; Thomson, J.D.; Frederickson, M.E. Ants and ant scent reduce bumblebee pollination of artificial flowers. Am. Nat. 2014, 183, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Kerner von Marilaun, A. Flowers and their Unbidden Guests; C. Kegan Paul & Co.: London, UK, 1878. [Google Scholar]
- Harley, R.M. Evolution and distribution of Eriope (Labiatae) and its relatives in Brazil. In Proceedings of a Workshop on Neotropical Distributions; Academia Brasileira de Ciencias: Rio de Janeiro, Brazil, 1988; pp. 71–120. [Google Scholar]
- Harley, R. The greasy pole syndrome. In Ant-Plant Interactions; Huxley, C.R., Cutler, D.E., Eds.; Oxford University Press: Oxford, UK, 1991; pp. 430–433. [Google Scholar]
- Juniper, B.E. Waxes on plant surfaces and their interactions with insects. In Waxes: Chemistry, Molecular Biology and Functions; Hamilton, R.J., Ed.; Oily Press West Ferry: Dundee, UK, 1995; pp. 157–174. [Google Scholar]
- Gorb, E.; Gorb, S. How a lack of choice can force ants to climb up waxy plant stems. Arthropod-Plant Interact. 2011, 5, 297–306. [Google Scholar] [CrossRef]
- Gorb, S.N.; Gorb, E.V. Frequency of plant visits by the generalist ant Lasius niger depends on the surface microstructure of plant stems. Arthropod-Plant Interact. 2019, 13, 311–320. [Google Scholar] [CrossRef]
- Strid, A. Mountain Flora of Greece; Cambridge University Press: Cambridge, UK, 1986; 852p. [Google Scholar]
- Fiori, A. Nuova Flora Analitica d’Italia; Tipografia di M. Ricci: Florence, Italy, 1925; p. 95. [Google Scholar]
- Kiehn, M. Pflanzen mit invasivem Potenzial in Botanischen Gärten X: Smyrnium perfoliatum L. (Apiaceae). Carinthia II (Klagenfurt, Austria) 2015, 295/125, 73–82. [Google Scholar]
- Papiomioglou, V. Wild Flowers of Greece; Mediterraneo Editions: Rethymno, Greece, 2006; p. 181. [Google Scholar]
- Unterarten von Smyrnium perfoliatum. Available online: http://www.mittelmeerflora.de/Zweikeim/Apiaceae/smyr_perfoliatum.htm (accessed on 10 March 2020).
- Jäger, E.J.; Reckardt, K. Beiträge zur Wuchsform und Biologie der Gefäßpflanzen des herzynischen Raumes. 2: Smyrnium perfoliatum L. (Apiaceae). Hercynia N. F. 1998, 31, 103–116. [Google Scholar]
- Klotz, J.H.; Hansen, L.; Pospischil, R.; Rust, M. Urban Ants of North America and Europe: Identification, Biology, and Management; Cornell University Press: Ithaca, NY, USA, 2008; 201p. [Google Scholar]
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, MA, USA, 1990; 732p. [Google Scholar]
- Barthlott, W.; Neinhuis, C.; Cutler, D.; Ditsch, F.; Meusel, I.; Theisen, I.; Wilhelmi, H. Classification and terminology of plant epicuticular waxes. Bot. J. Linn. Soc. 1998, 126, 237–260. [Google Scholar] [CrossRef]
- Ritzmann, R.E.; Quinn, R.D.; Fischer, M.S. Convergent evolution and locomotion through complex terrain by insects, vertebrates and robots. Arthropod Struct. Dev. 2004, 33, 361–379. [Google Scholar] [CrossRef]
- Bußhardt, P.; Gorb, S.N.; Wolf, H. Activity of the claw retractor muscle in stick insects in wall and ceiling situations. J. Exp. Biol. 2011, 214, 1676–1684. [Google Scholar] [CrossRef]
- Bohn, H.F.; Thornham, D.G.; Federle, W. Ants swimming in pitcher plants: Kinematics of aquatic and terrestrial locomotion in Camponotus schmitzi. J. Comp. Physiol. 2012, 198, 465–476. [Google Scholar] [CrossRef]
- Stork, N.E. Experimental analysis of adhesion of Chrysolina polita (Chrysomelidae, Coleoptera) on a variety of surfaces. J. Exp. Biol. 1980, 88, 91–107. [Google Scholar] [CrossRef]
- Barthlott, W.; Ehler, N. Raster-Elektronenmikroskopie der Epidermisoberflächen von Spermatophyten. Trop. Subtrop. Pflanzenwelt 1977, 19, 367–467. [Google Scholar]
- Prüm, B.; Seidel, R.; Bohn, H.F.; Speck, T. Plant surfaces with cuticular folds are slippery for beetles. J. R. Soc. Interface 2011, 9, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Prüm, B.; Bohn, H.F.; Seidel, R.; Rubach, S.; Speck, T. Plant surfaces with cuticular folds and their replicas: Influence of microstructuring and surface chemistry on the attachment of a leaf beetle. Acta Biomater. 2013, 9, 6360–6368. [Google Scholar] [CrossRef] [PubMed]
- Voigt, D.; Schweikart, A.; Fery, A.; Gorb, S. Leaf beetle attachment on wrinkled surfaces: Isotropic friction on anisotropic surfaces. J. Exp. Biol. 2012, 215, 1975–1982. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bräuer, P.; Neinhuis, C.; Voigt, D. Attachment of honeybees and greenbottle flies to petal surfaces. Arthropod-Plant Interact. 2017, 11, 171–192. [Google Scholar] [CrossRef]
- Salerno, G.; Rebora, M.; Piersanti, S.; Saitta, V.; Kovalev, A.; Gorb, E.; Gorb, S. Reduction in insect attachment caused by different nanomaterials used as particle films (kaolin, zeolite, calcium carbonate). Sustainability 2021, 13, 8250. [Google Scholar] [CrossRef]
- Glenn, D.M.; Puterka, G.J. Particle films: A new technology for agriculture. Hort. Rev. 2005, 31, 1–44. [Google Scholar]
- Agricultural Lime. Available online: https://en.wikipedia.org/wiki/Agricultural_lime (accessed on 31 July 2020).
- Yee, W.L. Behavioural responses by Rhagoletis indifferens (Dipt., Tephritidae) to sweet cherry treated with kaolin- and limestone-based products. J. Appl. Entomol. 2012, 136, 124–132. [Google Scholar] [CrossRef]
- Barata, J.M.S.; Santos, J.L.F.; Da Rosa, J.A.; Gomes, R.D. Evaluation of triatomine behavior under the effect of contact with calcium hydroxide CaOH2: Mortality rates of Triotoma infestans and Rhodnius neglectus (Hemiptera, Reduviidae). An. Soc. Entomol. Bras. 1992, 21, 169–177. [Google Scholar] [CrossRef]
- Boucher, J.; Adams, R.; Johnson, F.; Pachauskas, R. Eggplant: Hydrated lime as an insect repellent. Insectic. Acaricidae Tests 1993, 18, 131. [Google Scholar] [CrossRef]
- Strack, T.; Cahenzli, F.; Daniel, C. Kaolin, lime and rock dusts to control Drosophila suzukii. In Ökologschen Landbau Weiterdenken: Verantwortung Übernehmen, Vertrauen Stärken; Wolfrum, S., Heuwinkel, H., Wiesinger, K., Reents, H.J., Hülsbergen, K.-J., Eds.; Verlag Dr. Köster: Berlin, Germany, 2017; pp. 262–263. [Google Scholar]
- Gorb, E.; Hosoda, N.; Miksch, C.; Gorb, S. Slippery pores: Anti-adhesive effect of nanoporous substrates on the beetle attachment system. J. R. Soc. Interface 2010, 7, 1571–1579. [Google Scholar] [CrossRef]
- Gorb, E.V.; Lemke, W.; Gorb, S.N. Porous substrate affects a subsequent attachment ability of the beetle Harmonia axyridis (Coleoptera, Coccinellidae). J. R. Soc. Interface 2019, 16, 20180696. [Google Scholar] [CrossRef]
- Gorb, E.V.; Gorb, S.N. Anti-adhesive effects of plant wax coverage on insect attachment. J. Exp. Bot. 2017, 68, 5323–5337. [Google Scholar] [CrossRef] [PubMed]
- Gorb, E.V.; Gorb, S.N. Attachment ability of the beetle Chrysolina fastuosa on various plant surfaces. Entomol. Exp. Appl. 2002, 105, 13–28. [Google Scholar] [CrossRef]
- Gorb, E.V.; Purtov, J.; Gorb, S.N. Adhesion force measurements on the two wax layers of the waxy zone in Nepenthes alata pitchers. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorb, E.V.; Gorb, S.N. Combined Effect of Different Flower Stem Features on the Visiting Frequency of the Generalist Ant Lasius niger: An Experimental Study. Insects 2021, 12, 1026. https://doi.org/10.3390/insects12111026
Gorb EV, Gorb SN. Combined Effect of Different Flower Stem Features on the Visiting Frequency of the Generalist Ant Lasius niger: An Experimental Study. Insects. 2021; 12(11):1026. https://doi.org/10.3390/insects12111026
Chicago/Turabian StyleGorb, Elena V., and Stanislav N. Gorb. 2021. "Combined Effect of Different Flower Stem Features on the Visiting Frequency of the Generalist Ant Lasius niger: An Experimental Study" Insects 12, no. 11: 1026. https://doi.org/10.3390/insects12111026
APA StyleGorb, E. V., & Gorb, S. N. (2021). Combined Effect of Different Flower Stem Features on the Visiting Frequency of the Generalist Ant Lasius niger: An Experimental Study. Insects, 12(11), 1026. https://doi.org/10.3390/insects12111026






