Reproductive Potential Impacts Body Maintenance Parameters and Global DNA Methylation in Honeybee Workers (Apis mellifera L.)
Abstract
Simple Summary
Abstract
1. Introduction
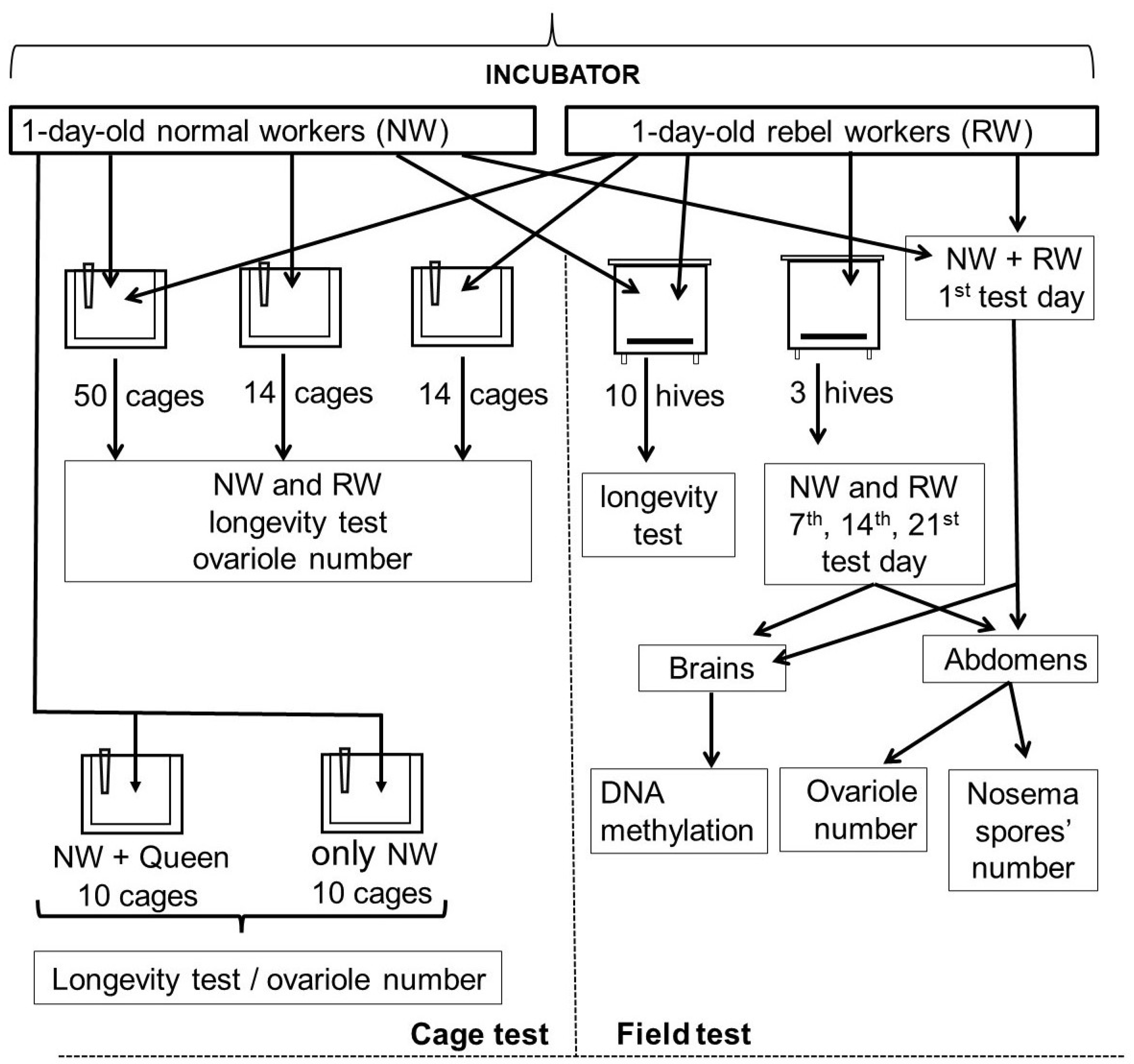
2. Materials and Methods
2.1. Preliminary Procedures and Hive Tests
2.2. Cage Tests
2.3. Laboratory Protocols
2.4. Statistical Analysis
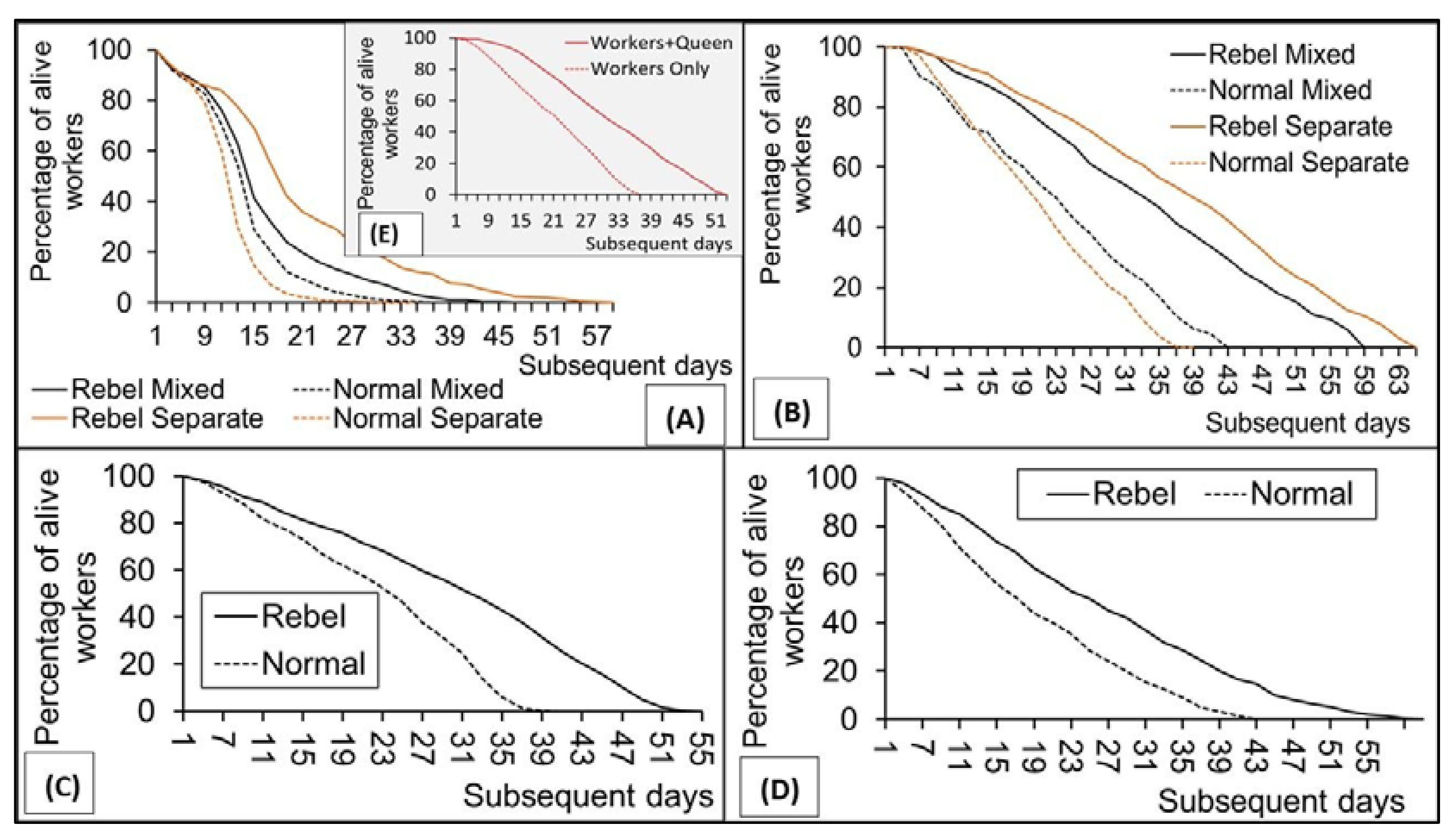
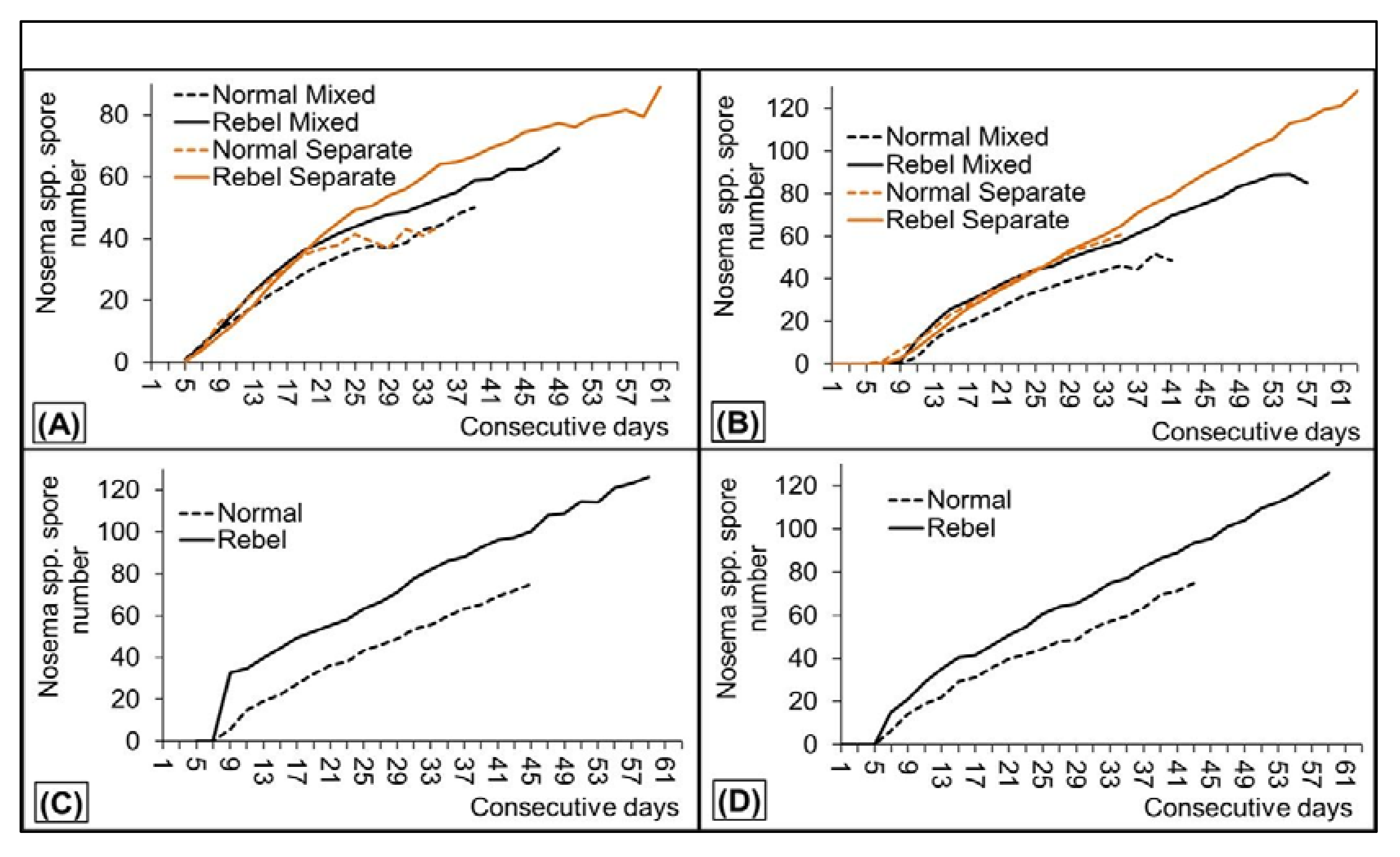
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, G. Pleiotropy, natural selection, and the evolution of senescence. Evolution 1957, 11, 398. [Google Scholar] [CrossRef]
- Fülöp, T.; Dupuis, G.; Witkowski, J.M.; Larbi, A. The Role of Immunosenescence in the Development of Age-Related Diseases. Revista de Investigación Clínica 2016, 68, 84–91. [Google Scholar]
- Lourenço, A.P.; Martins, J.R.; Torres, F.A.S.; Mackert, A.; Aguiar, L.R.; Hartfelder, K.; Bitondi, M.M.G.; Simões, Z.L.P. Immunosenescence in honey bees (Apis mellifera L.) is caused by intrinsic senescence and behavioral physiology. Exp. Gerontol. 2019, 119, 174–183. [Google Scholar] [CrossRef]
- Paleolog, J.; Wilde, J.; Miszczak, A.; Gancarz, M.; Strachecka, A. Antioxidation defenses of Apis mellifera queens and workers respond to imidacloprid in different age-dependent ways: Old queens are resistant, foragers are not. Animals 2021, 11, 1246. [Google Scholar] [CrossRef]
- Kennedy, A.; Herman, J.; Rueppell, O. Reproductive activation in honeybee (Apis mellifera) workers protects against abiotic and biotic stress. Philos. Trans. R. Soc. B 2021, 376, 20190737. [Google Scholar] [CrossRef]
- Elekonich, M.M.; Roberts, S.P. Honey bees as a model for understanding mechanisms of life history transitions. Comp. Biochem. Physiol. Part A 2005, 141, 362–371. [Google Scholar] [CrossRef]
- Münch, D.; Amdam, G.; Wolschin, F. Aging in a eusocial insect: Molecular and physiological characteristics of life span plasticity in the honey bee. Funct. Ecol. 2008, 22, 407–421. [Google Scholar] [CrossRef]
- Lemanski, N.J.; Bansal, S.; Fefferman, N.H. The sensitivity of a honeybee colony to worker mortality depends on season and resource availability. BMC Evol. Biol. 2020, 20, 139. [Google Scholar] [CrossRef]
- Woyciechowski, M.; Kuszewska, K. Swarming generates rebel workers in honeybees. Curr. Biol. 2012, 22, 707–711. [Google Scholar] [CrossRef][Green Version]
- Kuszewska, K.; Miler, K.; Rojek, W.; Woyciechowski, M. Honeybee workers with higher reproductive potential live longer lives. Exp. Gerontol. 2017, 98, 8–12. [Google Scholar] [CrossRef]
- Kuszewska, K.; Wącławska, A.; Woyciechowski, M. Reproduction of rebel workers in honeybee (Apis mellifera) colonies. Apidologie 2018, 49, 162–171. [Google Scholar] [CrossRef]
- Woyciechowski, M.; Kuszewska, K.; Pitorak, J.; Kierat, J. Honeybee worker larvae perceive queen pheromones in their food. Apidologie 2017, 48, 144–149. [Google Scholar] [CrossRef]
- Paris, L.; El Alaoui, H.; Delbac, F.; Diogon, M. Effects of the gut parasite Nosema ceranae on honey bee physiology and behavior. Curr. Opin. Insect Sci. 2018, 26, 149–154. [Google Scholar] [CrossRef]
- Lyko, F.; Foret, S.; Kucharski, R.; Wolf, S.; Falckenhayn, C.; Maleszka, R. Correction: The Honey Bee Epigenomes: Differential Methylation of Brain DNA in Queens and Workers. PLoS Biol. 2011, 9, e1000506. [Google Scholar] [CrossRef]
- Oldroyd, B.P.; Yagound, B. The role of epigenetics, particularly DNA methylation, in the evolution of caste in insect societies. Philos. Trans. R. Soc. B 2021, 376, 20200115. [Google Scholar] [CrossRef]
- Marshall, H.; Lonsdale, Z.N.; Mallon, E.B. Methylation and gene expression differences between reproductive and sterile bumblebee workers. Evol. Lett. 2019, 3, 485–499. [Google Scholar] [CrossRef]
- Lyko, F.; Maleszka, R. Insects as innovative models for functional studies of DNA methylation. Trends Genet. 2011, 27, 127–164. [Google Scholar] [CrossRef]
- Paleolog, J.; Wilde, J.; Siuda, M.; Bąk, B.; Wójcik, Ł.; Strachecka, A. Imidacloprid markedly affects hemolymph proteolysis, biomarkers, DNA global methylation, and the cuticle proteolytic layer in western honeybees. Apidologie 2020, 51, 620–630. [Google Scholar] [CrossRef]
- Strachecka, A.; Krauze, M.; Olszewski, K.; Borsuk, G.; Paleolog, J.; Merska, M.; Chobotow, J.; Bajda, M.; Grzywnowicz, K. Unexpectedly strong effect of caffeine on the vitality of western honeybees (Apis mellifera). Biochem. Mosc. 2014, 79, 1192–1201. [Google Scholar] [CrossRef]
- Strachecka, A.; Olszewski, K.; Paleolog, J. Curcumin stimulates biochemical mechanisms of Apis mellifera resistance and extends the apian life-span. J. Apic. Sci. 2015, 59, 129–141. [Google Scholar]
- Cardoso-Júnior, C.A.M.; Guidugli-Lazzarini, K.R.; Hartfelder, K. DNA methylation affects the lifespan of honey bee (Apis mellifera L.) workers—Evidence for a regulatory module that involves vitellogenin expression but is independent of juvenile hormone function. Insect Biochem. Mol. Biol. 2018, 92, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Sulborska, A.; Horecka, B.; Cebrat, M.; Kowalczyk, M.; Skrzypek, T.H.; Kazimierczak, W.; Trytek, M.; Borsuk, G. Microsporidia Nosema spp.—obligate bee parasites are transmitted by air. Sci. Rep. 2019, 9, 14376. [Google Scholar] [CrossRef] [PubMed]
- Bosua, H.J.; Nicolson, S.W.; Archer, C.R.; Pirk, C. Effects of cage volume and bee density on survival and nutrient intake of honeybees (Apis mellifera L.) under laboratory conditions. Apidologie 2018, 49, 734–746. [Google Scholar] [CrossRef]
- Fries, I.; Chauzat, M.P.; Chen, Y.P.; Doublet, V.; Generesch, E.; Gisder, S.; Higes, M.; McMahon, D.P.; Martin-Hernandez, R.; Natsopoulou, M.; et al. Standard methods for Nosema research. J. Apic. Res. 2013, 51, 1–28. [Google Scholar] [CrossRef]
- Martin-Hernandez, R.; Meana, A.; Prieto, L.; Salvador, A.M.; Garrido-Bailon, E.; Higes, M. Outcome of the colonization of Apis mellifera by Nosema ceranae. App. Environ. Microbiol. 2007, 73, 6331–6338. [Google Scholar] [CrossRef]
- Tyler, P.; Amdam, G.; Rueppell, O. Honeybee workers as models of aging. In Conn’s Handbook of Models for Human Aging, 2nd ed.; Ram, J., Conn, P.M., Eds.; Academic Press: London, UK, 2018; pp. 533–547. [Google Scholar]
- Paleolog, J.; Wilczyńska, R. The influence of queen pheromones, environment factors, genotypes and their interactions on length of life of caged workers in labolatory tests in bees. Pszczelnicze Zeszyty Naukowe 1998, 42, 65–66. [Google Scholar]
- Paleolog, J.; Borsuk, G.; Olszewski, K. Some factor influencing the results of the cage tests of a life span and food intake in Apis mellifera workers. Ann. Univ. Mariae Curie-Skłodowska Lub.-Pol. Sect. EE 2003, 66, 95–104. [Google Scholar]
- Keith, S.; John, R. Effect of queenlessness on worker survival, honey gain and defence behaviour in honeybees. J. Apic. Res. 1987, 26, 37–42. [Google Scholar]
- Majoe, M.; Libbrecht, R.; Foitzik, S.; Nehring, V. Queen loss increases worker survival in leaf-cutting ants under paraquat-induced oxidative stress. Philos. Trans. R. Soc. B 2021, 376, 20190735. [Google Scholar] [CrossRef]
- Kuszewska, K.; Woyciechowski, M. Age at which larvae are orphaned determines their development into typical or rebel workers in the honeybee (Apis mellifera L.). PLoS ONE 2015, 10, e0123404. [Google Scholar]
- Strachecka, A.; Olszewski, K.; Kuszewska, K.; Chobotow, J.; Wójcik, Ł.; Paleolog, J.; Woyciechowski, M. Segmentation of the subcuticular fat body in Apis mellifera females with different reproductive potentials. Sci. Rep. 2021, 11, 13887. [Google Scholar] [CrossRef]
- Woyciechowski, M.; Moroń, D. Life expectancy and onset of foraging in the honeybee (Apis mellifera). Insectes Soc. 2009, 56, 193–201. [Google Scholar] [CrossRef]
- Botías, C.; Martín-Hernández, R.; Barrios, L.; Meana, A.; Higes, M. Nosema spp. infection and its negative effects on honey bees (Apis mellifera iberiensis) at the colony level. Vet. Res. 2013, 44, 25. [Google Scholar] [CrossRef]
- Garrido, P.M.; Porrini, M.P.; Antúnez, K.; Branchiccela, B.; Martínez-Noël, G.M.A.; Zunino, P.; Salerno, G.; Eguaras, M.J.; Ieno, E. Sublethal effects of acaricides and Nosema ceranae infection onimmune related gene expression in honey bees. Vet. Res. 2016, 47, 51. [Google Scholar] [CrossRef] [PubMed]
- Goblirsch, M. Nosema ceranae disease of the honey bee (Apis mellifera). Apidologie 2018, 49, 131–150. [Google Scholar] [CrossRef]
- Higes, M.; García-Palencia, P.; Martín-Hernández, R.; Meana, A. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invert. Pathol. 2007, 94, 211–217. [Google Scholar] [CrossRef]
- Antúnez, K.; Martín-Hernández, R.; Prieto, L.; Meana, A.; Zunino, P.; Higes, M. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ. Microbiol. 2009, 11, 2284–2290. [Google Scholar] [CrossRef]
- Chaimanee, V.; Chantawannakul, P.; Chen, Y.; Evans, J.D.; Pettis, J.S. Effects of host age on susceptibility to infection and immune gene expression in honey bee queens (Apis mellifera) inoculated with Nosema ceranae. Apidologie 2014, 45, 451–463. [Google Scholar] [CrossRef]
- Alaux, C.; Folschweiller, M.; McDonnell, C.; Beslay, D.; Cousin, M.; Dussaubat, C.; Brunet, J.L.; Conte, Y.L. Pathological effects of the microsporidium Nosema ceranae on honey bee queen physiology (Apis mellifera). J. Invert. Pathol. 2011, 106, 380–385. [Google Scholar] [CrossRef]
- Branchiccela, B.; Arredondo, D.; Higes, M.; Invernizzi, C.; Martín-Hernández, R.; Tomasco, I.; Zunino, P.; Antúnez, K. Characterization of Nosema ceranae genetic variants from different geographic origins. Microbial Ecol. 2017, 73, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Juarranz, A.; Dias-Almeida, J.; Lucena, S.; Botias, C.; Meana, A.; García-Palencia, P.; Martín-Hernández, R. Apoptosis in the pathogenesis of Nosema ceranae (Microsporidia: Nosematidae) in honey bees (Apis mellifera). Environ. Microbiol. Rep. 2013, 5, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Kurze, C.; Le Conte, Y.; Dussaubat, C.; Erler, S.; Kryger, P.; Lewkowski, O.; Müller, T.; Widder, M.; Moritz, R.F.A. Nosema Tolerant Honeybees (Apis mellifera) Escape Parasitic Manipulation of Apoptosis. PLoS ONE 2015, 10, e0140174. [Google Scholar] [CrossRef]
- Xing, W.; Zhou, D.; Long, Q.; Sun, M.; Guo, R.; Wang, L. Immune Response of Eastern Honeybee Worker to Nosema ceranae Infection Revealed by Transcriptomic Investigation. Insects 2021, 12, 728. [Google Scholar] [CrossRef]
- Kucharski, R.; Maleszka, J.; Foret, S.; Maleszka, R. Nutritional control of reproductive status in honeybees via DNA methylation. Science 2008, 319, 1827–1830. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, Y.; Zheng, H.Q.; Cao, L.F.; Niu, D.F.; Yu, D.L.; Sun, Y.Q.; Hu, S.N.; Hu, F.L. Transcriptome comparison between honey bee queen- and worker-destined larvae. Insect Biochem. Mol. Biol. 2012, 42, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Y.; Yan, W.Y.; Huang, Z.Y.; Wang, Z.L.; Wu, X.B.; Zeng, Z.J. Genome wide analysis indicates that queen larvae have lower methylation levels in the honey bee (Apis mellifera). Naturwissenschaften 2013, 100, 193–197. [Google Scholar] [CrossRef]
- Amarasinghe, H.E.; Clayton, C.I.; Mallon, E.B. Methylation and worker reproduction in the bumble-bee (Bombus terrestris). Proc. R. Soc. B 2014, 281, 20132502. [Google Scholar]
- Strachecka, A.; Olszewski, K.; Paleolog, J.; Borsuk, G.; Bajda, M.; Krauze, M.; Merska, M.; Chobotow, J. Coenzyme Q10 treatments influence the lifespan and key biochemical resistance systems in the honeybee, Apis mellifera. Archiv. Insect Biochem. Physiol. 2014, 86, 165–179. [Google Scholar] [CrossRef]
- Strachecka, A.; Olszewski, K.; Paleolog, J. Varroa treatment with bromfenvinphos markedly suppresses honeybee biochemical defence levels. Entomol. Exp. Appl. 2016, 160, 57–71. [Google Scholar] [CrossRef]
- Schulz, M.; Łoś, A.; Grzybek, M.; Ścibior, R.; Strachecka, A. Piperine as a new natural supplement with beneficial effects on the life-span and defence system of honeybees. J. Agric. Sci. 2019, 157, 1–10. [Google Scholar] [CrossRef]
- Strachecka, A.; Borsuk, G.; Olszewski, K.; Paleolog, J.; Gagoś, M.; Chobotow, J.; Nawrocka, A.; Gryzińska, M.; Bajda, M. The effect of amphotericin B on the lifespan, body-surface protein concentrations, and DNA methylation levels of the honey bees (Apis mellifera). J. Apic. Sci. 2012, 56, 107–113. [Google Scholar] [CrossRef][Green Version]
- Bebane, P.; Hunt, B.; Pegoraro, M.; Jones, A.; Marshall, H.; Rosato, E.; Mallon, E. The effects of the neonicotinoid imidacloprid on gene expression and DNA methylation in the buff-tailed bumblebee Bombus terrestris. Proc. R. Soc. B 2019, 286, 20190718. [Google Scholar] [CrossRef]
- Olivares-Castro, G.; Cáceres-Jensen, L.; Guerrero-Bosagna, C.; Villagra, C. Insect epigenetic facing antropogenic-derived contamination, an overview. Insects 2021, 12, 780. [Google Scholar] [CrossRef]
- Strachecka, A.; Chobotow, J.; Paleolog, J.; Łoś, A.; Schulz, M.; Teper, D.; Kucharczyk, H.; Grzybek, M. Insights into the biochemical defence and methylation of the solitary bee Osmia rufa L: A foundation for examining eusociality development. PLoS ONE 2017, 12, e0176539. [Google Scholar] [CrossRef]
- Sivalingam, K.; Samikkannu, T. Neuroprotective effect of piracetam against Cocaine-induced neuro epigenetic modification of DNA methylation in astrocytes. Brain Sci. 2020, 10, 611. [Google Scholar] [CrossRef] [PubMed]
- Strachecka, A.; Borsuk, G.; Olszewski, K.; Paleolog, J. A new detection method for a newly revealed mechanism of pyrethroid resistance development in Varroa destructor. Parasitol Res. 2015, 114, 3999–4004. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.; Rayssa, A.; Costa, L.; Barroso, H.; Oliveira, N. Global and gene-specific DNA methylation and hydroxymethylation in human skin exposed and not exposed to sun radiation. Ann. Bras. Dermatol. 2017, 92, 793–800. [Google Scholar]
- Hunt, B.G.; Brisson, J.A.; Yi, S.V.; Goodisman, M.A.D. Functional conservation of DNA methylation in the pea aphid and the honeybee. Genome Biol. Evol. 2010, 2, 719–728. [Google Scholar] [CrossRef]
- Glastad, K.M.; Hunt, B.G.; Goodisman, M.A. Epigenetics in insects: Genome regulation and the generation of phenotypic diversity. Annu. Rev. Entomol. 2019, 64, 185–203. [Google Scholar] [CrossRef] [PubMed]
- Bewick, A.J.; Vogel, K.J.; Moore, A.J.; Schmitz, R.J. Evolution of DNA methylation across insects. Mol. Biol. Evol. 2017, 34, 654–665. [Google Scholar] [CrossRef]
- Li, S.; Tollefsbol, T. DNA methylation methods: Global DNA methylation and methylomic analyses. Methods 2021, 187, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Júnior, C.; Fujimura, P.; Santos-Júnior, C.; Borges, N.; Ueira-Vieira, C.; Hartfelder, K.; Goulart, L.; Bonetti, A. Epigenetic modifications and their relation to caste and sex determination and adult division of labor in the stingless bee Melipona scutellaris. Genet. Mol. Biol. 2017, 40, 61–68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yagound, B.; Remnant, E.; Buchmann, G.; Oldroyd, B. Intergenerational transfer of DNA methylation marks in the honey bee. Proc. Natl. Acad. Sci. USA 2020, 117, 32519–32527. [Google Scholar] [CrossRef]
- Jiang He, X.; Wei, H.; Jiang, W.; Liu, Y.; Wu, X.; Zeng, Z. Honeybee (Apis mellifera) maternal effect causes alternation of DNA methylation regulating queen development. Sociobiology 2021, 68, e5935. [Google Scholar]
- Negroni, M.A.; Feldmeyer, B.; Foitzik, S. Experimental increase in fecundity causes upregulation of fecundity and body maintenance genes in the fat body of ant queens. Biol. Lett. 2021, 17, 20200909. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Bonasio, R.; Simola, D.F.; Liebig, J.; Berger, S.L.; Reinberg, D. DNA Methylation in Social Insects: How Epigenetics Can Control Behavior and Longevity. Ann. Rev. Entomol. 2015, 60, 435–452. [Google Scholar] [CrossRef]
- Kevin, B.; Wolschin, F.; Amdam, G.V. The Role of Methylation of DNA in Environmental Adaptation. Integr. Comp. Biol. 2013, 53, 359–372. [Google Scholar]
- Sieber, K.R.; Dorman, T.; Newell, N.; Yan, H. (Epi)Genetic Mechanisms Underlying the Evolutionary Success of Eusocial Insects. Insects 2021, 12, 498. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paleolog, J.; Kuszewska, K.; Woyciechowski, M.; Strachecka, A. Reproductive Potential Impacts Body Maintenance Parameters and Global DNA Methylation in Honeybee Workers (Apis mellifera L.). Insects 2021, 12, 1021. https://doi.org/10.3390/insects12111021
Paleolog J, Kuszewska K, Woyciechowski M, Strachecka A. Reproductive Potential Impacts Body Maintenance Parameters and Global DNA Methylation in Honeybee Workers (Apis mellifera L.). Insects. 2021; 12(11):1021. https://doi.org/10.3390/insects12111021
Chicago/Turabian StylePaleolog, Jerzy, Karolina Kuszewska, Michał Woyciechowski, and Aneta Strachecka. 2021. "Reproductive Potential Impacts Body Maintenance Parameters and Global DNA Methylation in Honeybee Workers (Apis mellifera L.)" Insects 12, no. 11: 1021. https://doi.org/10.3390/insects12111021
APA StylePaleolog, J., Kuszewska, K., Woyciechowski, M., & Strachecka, A. (2021). Reproductive Potential Impacts Body Maintenance Parameters and Global DNA Methylation in Honeybee Workers (Apis mellifera L.). Insects, 12(11), 1021. https://doi.org/10.3390/insects12111021






