The Fitness of Mass Rearing Food on the Establishment of Chrysopa pallens in a Banker Plant System under Fluctuating Temperature Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Evaluation of Population Colonization of Chrysopa pallens Using Life Tables and Predation Rates
2.3. Life Table Analysis
2.4. Predation Rate Analysis
2.5. Statistical Analysis
3. Results
3.1. Age-Stage, Two-Sex Life Table of Chrysopa pallens
3.2. Population Parameters of Chrysopa pallens under Fluctuating Temperature Conditions in a Greenhouse
3.3. Predation Rate of Chrysopa pallens under Fluctuating Temperature Conditions in a Greenhouse
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. Biocontrol 2012, 57, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.W.; Zang, L.S.; Wang, S.; Guo, J.Y.; Xu, H.X.; Zhang, F.; Wan, F.H. Biological pest management by predators and parasitoids in the greenhouse vegetables in China. Biol. Control 2014, 68, 92–102. [Google Scholar] [CrossRef]
- Huang, N.X.; Jaworski, C.C.; Desneux, N.; Zhang, F.; Yang, P.Y.; Wang, S. Long-term and large-scale releases of Trichogramma promote pesticide decrease in maize in northeastern China. Entomol. Gen. 2020, 40, 331–335. [Google Scholar] [CrossRef]
- Zang, L.S.; Wang, S.; Zhang, F.; Desneux, N. Biological control with Trichogramma in China: History, present status and perspectives. Annu. Rev. Entomol. 2021, 66, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Symondson, W.O.C.; Sunderland, K.D.; Greenstone, M.H. Can generalist predators be effective biocontrol agents? Annu. Rev. Entomol. 2002, 47, 561–594. [Google Scholar] [CrossRef] [Green Version]
- Desneux, N.; O’Neil, R.J.; Yoo, H.J.S. Suppression of population growth of the soybean aphid, Aphis glycines Matsumura, by predators: The identification of a key predator, and the effects of prey dispersion, predator density and temperature. Environ. Entomol. 2006, 35, 1342–1349. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.H.; Wu, K.M.; Jiang, Y.Y.; Guo, Y.Y.; Desneux, N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 2012, 487, 362–365. [Google Scholar] [CrossRef]
- Yang, X.K. Discussion on the scientific name of Chrysopa pallens (Rambur) and related questions. Acta Entomol. Sin. 1998, 41, 106–107. (In Chinese) [Google Scholar]
- Sterling, W.L.; El-Zik, K.M.; Wilson, L.T. Biological control of pest populations. In Integrated Pest Management Systems and Cotton Production; Frisbie, R.E., El-Zik, K.M., Wilson, L.T., Eds.; John Wiley, Son: New York, NJ, USA, 1989; pp. 155–189. [Google Scholar]
- Wang, Y.; Zhang, R.; Wang, M.; Zhang, L.; Shi, C.-M.; Li, J.; Fan, F.; Geng, S.; Liu, X.; Yang, D. The first chromosome-level genome assembly of a green lacewing Chrysopa pallens and its implication for biological control. Mol. Ecol. Resour. 2021. [Google Scholar] [CrossRef]
- Tsukaguchi, S. Chrysopidae of Japan (Insecta, Neuroptera); Yutaka Publish: Hyogo, Japan, 1995. [Google Scholar]
- Boo, K.S.; Chung, I.B.; Han, K.S.; Pickett, J.A.; Wadhams, L.J. Response of the lacewing Chrysopa cognata, to pheromones of its aphid prey. J. Chem. Ecol. 1998, 24, 631–643. [Google Scholar] [CrossRef]
- Canard, M.; Volkovich, T.A. Outlines of lacewing development. In Lacewings in the Crop Environment; McEwen, P.K., New, R., Whittington, A.E., Eds.; Cambridge University Press: Cambridge, MA, USA, 2001; pp. 130–153. [Google Scholar]
- Nakahira, K.; Arakawa, R. Effect of photoperiod on the development and diapause of the green lacewing Chrysopa pallens (Neuroptera: Chrysopidae). Entomol. Sci. 2005, 8, 133–135. [Google Scholar] [CrossRef]
- Sarkar, S.C.; Wang, E.; Zhang, Z.; Wu, S.; Lei, Z. Laboratory and glasshouse evaluation of the green lacewing, Chrysopa pallens (Neuroptera: Chrysopidae) against the western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae). Appl. Entomol. Zool. 2019, 54, 115–121. [Google Scholar] [CrossRef]
- Choi, M.Y.; Lee, G.H.; Paik, C.H.; Lee, J.J. Development of artificial diets for green lacewing, Chrysopa pallens (Rambur), by addition of natural products. Korean J. Appl. Entomol. 2000, 39, 99–103. [Google Scholar]
- Lee, K.S.; Lee, J.H. Rearing of Chrysopa pallens (Rambur) (Neuroptera: Chrysopidae) on artificial diet. Entomol. Res. 2005, 35, 183–188. [Google Scholar] [CrossRef]
- Liu, F.; Liu, C.; Zeng, F. Effects of an artificial diet on development, reproduction and digestive physiology of Chrysopa septempunctata. Biocontrol 2013, 58, 789–795. [Google Scholar] [CrossRef]
- Shi, A.J.; Xu, H.F.; Liu, Z.D.; Zhao, J.; Zhang, F.; Xu, Y.Y. Effect of photoperiod on induction of prepupal diapause and larval development in Chrysopa pallens (Rambur) (Neuroptera: Chrysopidae). Acta Ecol. Sin. 2008, 28, 3854–3859. (In Chinese) [Google Scholar]
- Ali, I.; Zhang, S.; Iqbal, M.; Ejaz, S.; Cui, J.J. Trypsinized Cry1Fa and Vip3Aa have no detrimental effects on the adult green lacewing Chrysopa pallens (Neuroptera: Chrysopidae). Appl. Entomol. Zool. 2017, 52, 321–327. [Google Scholar] [CrossRef]
- Ali, I.; Zhang, S.; Sajjad, A.; Rehman, H.M.U.; Cui, J.J. Artificial diet based investigation on the impact of purified Cry1Ac, Cry1Fa and Cry2Ab on the survival and reproductive performance of adult green lacewing, Chrysopa pallens (Rambur) (Neuroptera: Chrysopidae). Phytoparasitica 2018, 46, 127–135. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef] [Green Version]
- Parolin, P.; Bresch, C.; Poncet, C.; Desneux, N. Functional characteristics of secondary plants for increased pest management. Int. J. Pest Manag. 2012, 58, 369–377. [Google Scholar] [CrossRef]
- Frank, S.D. Biological control of arthropod pests using banker plant systems: Past progress and future directions. Biol. Control 2010, 52, 8–16. [Google Scholar] [CrossRef]
- Huang, N.X.; Enkegaard, A.; Osborne, L.S.; Ramakers, P.M.J.; Messelink, G.J.; Pijnakker, J.; Murphy, G. The banker plant method in biological control. Crit. Rev. Plant Sci. 2011, 30, 259–278. [Google Scholar] [CrossRef]
- Zheng, X.S.; Lu, Y.H.; Zhu, P.Y.; Zhang, F.; Tian, J.C.; Xu, H.; Chen, G.; Nansen, C.; Lv, Z.X. Use of banker plant system for sustainable management of the most important insect pest in rice field in China. Sci. Rep. 2017, 7, 45581. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jaworski, C.C.; Dai, H.; Liang, Y.Y.; Guo, X.J.; Wang, S.; Zang, L.S.; Desneux, N. Combining banker plants to achieve long-term pest control in multi-pest and multi-natural enemy cropping systems. J. Pest Sci. 2021. [Google Scholar] [CrossRef]
- Sanchez, J.A.; López-Gallego, E.; Pérez-Marcos, M.; Perera-Fernández, L. The effect of banker plants and pre-plant release on the establishment and pest control of Macrolophus pygmaeus in tomato greenhouses. J. Pest Sci. 2021, 94, 297–307. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zhang, G.H.; Tian, C.B.; Liu, M.X.; Liu, Y.Q.; Liu, H.; Wang, J.J. Does long–term feeding on alternative prey affect the biological performance of Neoseiulus barkeri (Acari: Phytoseiidae) on the target spider mites? J. Econ. Entomol. 2017, 110, 915–923. [Google Scholar] [CrossRef]
- Yu, L.Y.; Chen, Z.Z.; Zheng, F.Q.; Shi, A.J.; Guo, T.T.; Yeh, B.H.; Chi, H.; Xu, Y.Y. Demographic analysis, a comparison of the jackknife and bootstrap methods, and predation projection: A case study of Chrysopa pallens (Rambur) (Neuroptera: Chrysopidae). J. Econ. Entomol. 2013, 106, 1–9. [Google Scholar] [CrossRef]
- Canale, A.; Benelli, G. Impact of mass–rearing on the host seeking behaviour and parasitism by the fruit fly parasitoid Psyttalia concolor (Szépligeti) (Hymenoptera: Braconidae). J. Pest Sci. 2012, 85, 65–74. [Google Scholar] [CrossRef]
- Cocuzza, G.E.; Clercq, P.; Veire, M.; Cock, A.; Degheele, D.; Vacante, V. Reproduction of Orius laevigatus and Orius albidipennis on pollen and Ephestia kuehniella eggs. Entomol. Exp. Appl. 1997, 82, 101–104. [Google Scholar] [CrossRef]
- Pitcher, S.A.; Hoffmann, M.P.; Gardner, J.; Wright, M.G.; Kuhar, T.P. Cold storage of Trichogramma ostriniae reared on Sitotroga cerealella eggs. Biocontrol 2002, 47, 525–535. [Google Scholar] [CrossRef]
- Zanuncio, J.C.; Molina–Rugama, A.J.; Serrao, J.; Pratissoli, D. Nymphal development and reproduction of Podisus nigrispinus (Heteroptera: Pentatomidae) fed with combinations of Tenebrio molitor (Coleoptera: Tenebrionidae) pupae and Musca domestica (Diptera: Muscidae) larvae. Biocontrol Sci. Technol. 2001, 11, 331–337. [Google Scholar] [CrossRef]
- Khanzada, M.S.; Wang, S.; Huang, N.X.; Pang, H.; Tan, X.L.; Khanzada, S.R. Optimization of microencapsulated artificial diets for mass rearing of the predacious big eyed bug, Geocoris pallidipennis. Entomol. Gen. 2019, 39, 353–363. [Google Scholar] [CrossRef]
- Tan, X.L.; Wang, S.; Zhang, F. Optimization an optimal artificial diet for the predatory bug Orius sauteri (Hemiptera: Anthocoridae). PLoS ONE 2013, 8, e61129. [Google Scholar]
- Yang, L.W.; Zhang, F.; Zhao, J.; Li, S.; Wang, S. The effects of short–time food acclimation on the functional response of Orius sauteri reared to Corcyra cephalonica eggs. Acta Phytophylacica Sin. 2014, 41, 705–710. [Google Scholar]
- Liang, W.X.; Wang, Z.H.; Huang, J. Effect of alternative feeding with artificial diets and natural prey on oviposition of Delphastus catalinae (Horn). Chin. J. Biol. Control 2014, 30, 600–605. [Google Scholar]
- Alasady, M.A.A.; Omar, D.B.; Ibrahim, Y.B.; Ibrahim, R.B. Life table of the green lacewing Apertochrysa sp. (Neuroptera: Chrysopidae) reared on rice moth Corcyra cephalonica (Lepidoptera: Pyralidae). Int. J. Agric. Biol. 2010, 12, 266–270. [Google Scholar]
- Khuhro, N.H.; Chen, H.Y.; Zhang, Y.; Zhang, L.S.; Wang, M.Q. Effect of different prey species on the life history parameters of Chrysoperla sinica (Neuroptera: Chrysopidae). Eur. J. Entomol. 2012, 109, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Y.; Lei, Q.; Hua, H.Q.; Song, H.F.; Wang, S.; Ramirez-Romero, R.; Dai, H.J.; Li, J.T.; Li, Y.X. Impact of host suitability on oviposition preference toward fertilized and unfertilized host eggs in two Trichogramma parasitoid species. Entomol. Gen. 2019, 39, 313–323. [Google Scholar] [CrossRef]
- Lü, X.; Han, S.C.; Li, Z.; Li, L.Y. Biological characters of Trichogramma dendrolimi (Hymenoptera: Trichogrammatidae) reared in vitro versus in vivo for thirty generations. Sci. Rep. 2017, 7, 17928. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zou, Z.P.; Hou, Y.Y.; Yang, X.B.; Wang, S.; Dai, H.J.; Xu, Y.Y.; Zang, L.S. Manually–extracted unfertilized eggs of Chinese oak silkworm, Antheraea pernyi, enhance mass production of Trichogramma parasitoids. Entomol. Gen. 2020, 40, 397–406. [Google Scholar] [CrossRef]
- Yu, J.Z.; Chi, H.; Chen, B.H. Comparison of the life tables and predation rates of Harmonia dimidiate (F.) (Coleoptera: Coccinellidae) fed on Aphis gossypii Glover (Hemiptera: Aphididae) at different temperatures. Biol. Control 2013, 64, 1–9. [Google Scholar] [CrossRef]
- Chi, H.; You, M.S.; Atlihan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.J.; Fu, J.W.; Xu, Y.Y.; et al. Age–stage, two–sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Wang, S.X.; Di, N.; Chen, X.; Zhang, F.; Biondi, A.; Desneux, N.; Wang, S. Life history and functional response to prey density of the flower bug Orius sauteri attacking the fungivorous sciarid fly Lycoriella pleuroti. J. Pest Sci. 2018, 92, 715–722. [Google Scholar] [CrossRef]
- Xiao, D.; Zhao, J.; Guo, X.J.; Chen, H.Y.; Qu, M.M.; Zhai, W.G.; Desneux, N.; Biondi, A.; Zhang, F.; Wang, S. Sublethal effects of imidacloprid on the predatory seven–spot ladybird beetle Coccinella septempunctata. Ecotoxicology 2015, 25, 1782–1793. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Atlihan, R.; Chi, H.; Chu, D. Demographic analysis of progeny fitness and timing of resurgence of Laodelphax striatellus after insecticides exposure. Entomol. Gen. 2019, 39, 221–230. [Google Scholar] [CrossRef]
- Ding, H.Y.; Lin, Y.Y.; Tuan, S.J.; Tang, L.C.; Chi, H.; Atlıhan, R.; Özgökçe, M.S.; Güncan, A. Integrating demography, predation rate, and computer simulation for evaluation of Orius strigicollis as biological control agent against Frankliniella intonsa. Entomol. Gen. 2021, 42, 179–196. [Google Scholar] [CrossRef]
- Tuan, S.J.; Yeh, C.C.; Atlihan, R.; Chi, H. Linking life table and predation rate for biological control: A comparative study of Eocanthecona furcellata fed on Spodoptera litura and Plutella xylostella. J. Econ. Entomol. 2016, 109, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Li, S.; Gao, X.W.; Zhang, F.; Wang, S. Comparison of life tables of Cheilomenes sexmaculata (Coleoptera: Coccinellidae) under laboratory and greenhouse conditions. J. Econ. Entomol. 2015, 108, 1700–1707. [Google Scholar] [CrossRef]
- Milosavljevi´c, I.; McCalla, K.A.; Ratkowsky, D.A.; Hoddle, M.S. Effects of constant and fluctuating temperatures on development rates and longevity of Diaphorencyrtus aligarhensis (Hymenoptera: Encyrtidae). J. Econ. Entomol. 2019, 112, 1062–1072. [Google Scholar] [CrossRef]
- Chen, L.; Enkegaard, A.; Sørensen, J.G. Temperature affects biological control efficacy: A microcosm study of Trichogramma achaeae. Insects 2021, 12, 95. [Google Scholar] [CrossRef]
- Cheng, L.Y.; Liao, X.J.; Xu, L.X.; Sun, L.; Chen, Z.Z.; Xu, Y.Y. Two–sex life table and predation of Chrysopa pallens (Rambur) feeding on Megoura japonica (Matsumura). Acta Phytophylacica Sin. 2014, 41, 680–686. [Google Scholar]
- Zhang, F.; Wang, S.; Wang, B.; Guo, X.J.; Zhang, J.M.; Xiao, D. Breeding the Larvae Chrysopa Pallens with Rice Moth Eggs. Application No. CN105746435A, 13 July 2016. [Google Scholar]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. 1985, 24, 225–240. [Google Scholar]
- Chi, H.; Yang, T.C. Two-sex life table and predation rate of Propylaea japonica Thunberg (Coleoptera: Coccinellidae) fed on Myzus persicae (Sulzer) (Homoptera: Aphididae). Environ. Entomol. 2003, 32, 327–333. [Google Scholar] [CrossRef] [Green Version]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis; National Chung Hsing University: Taichung, Taiwan. Available online: http://140.120.197.173/Ecology/prod02.htm (accessed on 25 December 2019).
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Chi, H. CONSUME-MSChart: A Computer Program for Consumption Rate Analysis Based on the Age Stage, Two-Sex Life Table; National Chung Hsing University: Taichung, Taiwan. Available online: http://140.120.197.173/Ecology/prod02.htm (accessed on 26 July 2019).
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman & Hall Inc.: New York, NY, USA, 1993. [Google Scholar]
- Zhang, R.; Ji, D.; Zhang, Q.; Jin, L. Evaluation of eleven plant species as potential banker plants to support predatory Orius sauteri in tea plant systems. Insects 2021, 12, 162. [Google Scholar] [CrossRef]
- Wang, S.; Chi, H.; Liu, T. Demography and parasitic effectiveness of Aphelinus asychis reared from Sitobion avenae as a biological control agent of Myzus persicae reared on chili pepper and cabbage. Biol. Control 2016, 92, 111–119. [Google Scholar] [CrossRef]
- Wang, Y.S.; Yao, F.L.; Soares, M.A.; Basiri, S.E.; Amiens-Desneux, E.; Campos, M.R.; Lavoir, A.V.; Desneux, N. Effects of four non-crop plants on life history traits of the lady beetle Harmonia axyridis. Entomol. Gen. 2020, 40, 243–252. [Google Scholar] [CrossRef]
- Nagasaka, K.; Sagisaka, A.; Moriya, S.; Mitsunaga, T. Host-range study about four aphid parasitoid species among 16 aphid species for constructing bankercplant systems. Appl. Entomol. Zool. 2020, 55, 249–257. [Google Scholar] [CrossRef]
- Yu, J.Z.; Chen, B.H.; Güncan, A.; Atlıhan, R.; Gökçe, A.; Smith, C.L.; Gümüs, E.; Chi, H. Demography and mass–rearing Harmonia dimidiata (Coleoptera: Coccinellidae) using Aphis gossypii (Hemiptera: Aphididae) and eggs of Bactrocera dorsalis (Diptera: Tephritidae). J. Econ. Entomol. 2018, 111, 595–602. [Google Scholar] [CrossRef]
- McCalla, K.A.; Keçeci, M.; Milosavljević, I.; Ratkowsky, D.A.; Hoddle, M.S. The Influence of Temperature Variation on Life History Parameters and Thermal Performance Curves of Tamarixia radiata (Hymenoptera: Eulophidae), a Parasitoid of the Asian Citrus Psyllid (Hemiptera: Liviidae). J. Econ. Entomol. 2019, 112, 1560–1574. [Google Scholar] [CrossRef] [PubMed]
- Pizzol, J.; Pintureau, B.; Khoualdia, O.; Desneux, N. Temperature-dependent differences in biological traits between two strains of Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). J. Pest Sci. 2010, 83, 447–452. [Google Scholar] [CrossRef]
- Tuan, S.J.; Lee, C.C.; Chi, H. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age–stage, two–sex life table. Pest Manag. Sci. 2014, 70, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xiao, D.; Du, X.Y.; Zhang, F.; Zang, L.; Wang, S. Impact of polymorphism and abiotic conditions on prey consumption by Harmonia axyridis. Entomol. Gen. 2019, 39, 251–258. [Google Scholar] [CrossRef]
- Wang, S.S.; Chen, X.; Li, Y.; Pan, B.Y.; Wang, S.G.; Dai, H.J.; Wang, S.; Tang, B. Effects of changing temperature on the physiological and biochemical properties of Harmonia axyridis larvae. Entomol. Gen. 2020, 40, 229–241. [Google Scholar] [CrossRef]
- Yao, F.L.; Ding, X.L.; Mei, W.J.; Zheng, Y.; Desneux, N.; He, Y.X.; Weng, Q.Y. Impact of heat stress on the development of egg and adult coccinellid Serangium japonicum: Evidence for cross-stage and cross-generation effects. Entomol. Gen. 2020, 40, 365–376. [Google Scholar] [CrossRef]
- Mu, J.Y.; Wang, N.C.; Fan, Y.G. Studies on the life histories and bionomics of four species of green lacewings. Acta Phytophylacica Sin. 1980, 15, 123–127. [Google Scholar]
- Zhao, J.Z. Studies on the bionomics of Chrysopa septempunctata Wesmoer. Acta Phytophylacica Sin. 1988, 15, 123–127. [Google Scholar]
- Milosavljević, I.; McCalla, K.A.; Morgan, D.J.W.; Hoddle, M.S. The effects of constant and fluctuating temperatures on development of Diaphorina citri (Hemiptera: Liviidae), the Asian citrus psyllid. J. Econ. Entomol. 2020, 113, 633–645. [Google Scholar] [CrossRef]
- Castellanos, N.L.; Bueno, A.F.; Haddi, K.; Silveira, E.C.; Rodrigues, H.S.; Hirose, E.; Smagghe, G.; Oliveira, E.E. The fitness and economic benefits of rearing the parasitoid Telenomus podisi under fluctuating temperature regime. Neotrop. Entomol. 2019, 48, 934–948. [Google Scholar] [CrossRef]
- Braz, É.C.; Bueno, A.D.; Colombo, F.C.; de Queiroz, A.P. Temperature impact on Telenomus podisi emergence in field releases of unprotected and encapsulated parasitoid pupae. Neotrop. Entomol. 2021, 50, 462–469. [Google Scholar] [CrossRef] [PubMed]
- El-Serafi, H.A.K.; Abdel-Salam, A.H.; Abdel-Baky, N.F. Effect of four aphid species on certain biological characteristics and life table parameters of Chrysoperla carnea Stephen and Chrysopa septempunctata Wesmael (Neuroptera: Chrysopidae) under laboratory conditions. Pak. J. Biol. Sci. 2000, 3, 239–245. [Google Scholar]
- Khanamani, M.; Fathipour, Y.; Talebi, A.A.; Mehrabadi, M. Quantitative analysis of long–term mass rearing of Neoseiulus californicus (Acari: Phytoseiidae) on almond pollen. J. Econ. Entomol. 2017, 110, 1442–1450. [Google Scholar] [CrossRef]
- Henry, L.M.; May, N.; Acheampong, S.; Gillespie, D.R.; Roitberg, B.D. Host–adapted parasitoids in biological control: Does source matter? Ecol. Appl. 2010, 20, 242–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julian, R.G.; Jian, J.D.; Kaitlin, R.; Judith, H.G.; Ellen, A.A. Laboratory adaptation of a native North American parasitoid to an exotic wood-boring beetle: Implications for biological control of invasive pests. J. Pest Sci. 2019, 92, 1179–1186. [Google Scholar]
- Pan, M.Z.; Liu, T.X.; Nansen, C. Avoidance of parasitized host by female wasps of Aphidius gifuensis (Hymenoptera: Braconidae). The role of natal rearing effects and host availability? Insect Sci. 2018, 25, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Ghaemmaghami, E.; Fathipour, Y.; Bagheri, A.; Talebi, A.A.; Reddy, G.V.P. Quality control of the parasitoid wasp Trichogramma brassicae (Hymenoptera: Trichogrammatidae) over 45 generations of rearing on Sitotroga Cerealella. Insect Sci. 2021, 28, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.S.; Dixon, A.F.G.; Jarosik, V. Costs and benefits of prey specialization in a generalist insect predator. J. Anim. Ecol. 2002, 71, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhao, C.L.; Yang, X.; Chi, H.; Dai, P.; Desneux, N.; Benelli, G.; Zang, L.S. Yacon as an alternative host plant for Encarsia formosa mass-rearing: Validating a multinomial theorem for bootstrap technique in life table research. Pest Manag. Sci. 2021, 77, 2324–2336. [Google Scholar] [CrossRef]
- Lumbierres, B.; Madeira, F.; Roca, M.; Pons, X. Effects of temperature and diet on the development and reproduction of the ladybird Oenopia Conglobate. Entomol. Gen. 2021, 41, 197–208. [Google Scholar] [CrossRef]
- Ghaderi, S.; Minaei, K.; Kavousi, A.; Akrami, M.A.; Aleosfoor, M.; Ghadamyari, M. Demographic analysis of the effect of Fenpyroximate on Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae). Entomol. Gen. 2013, 34, 225–233. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sub-lethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
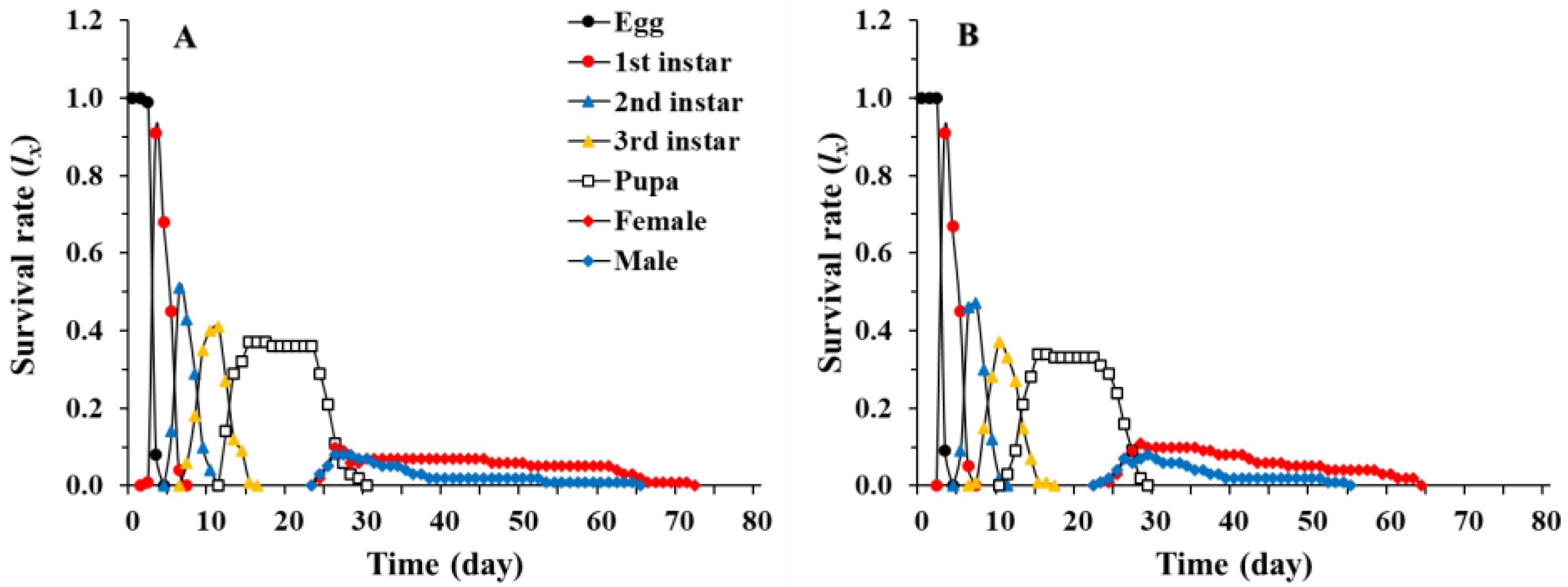
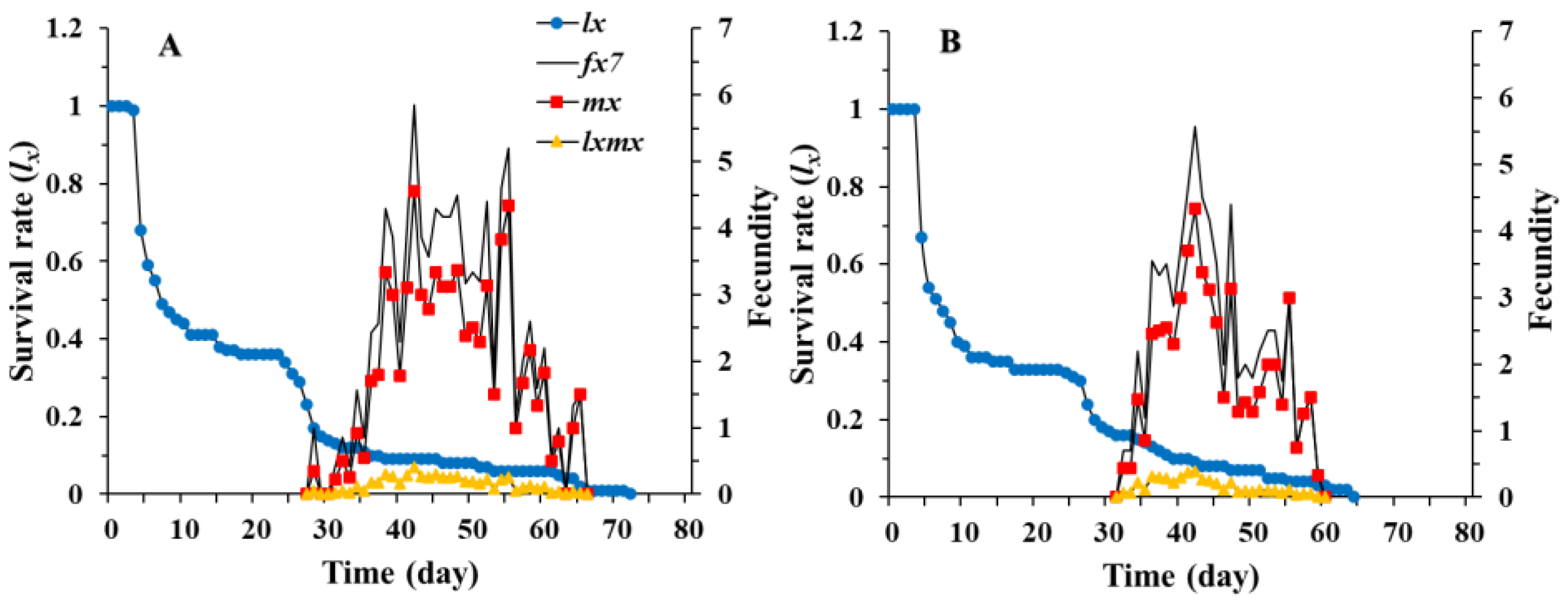
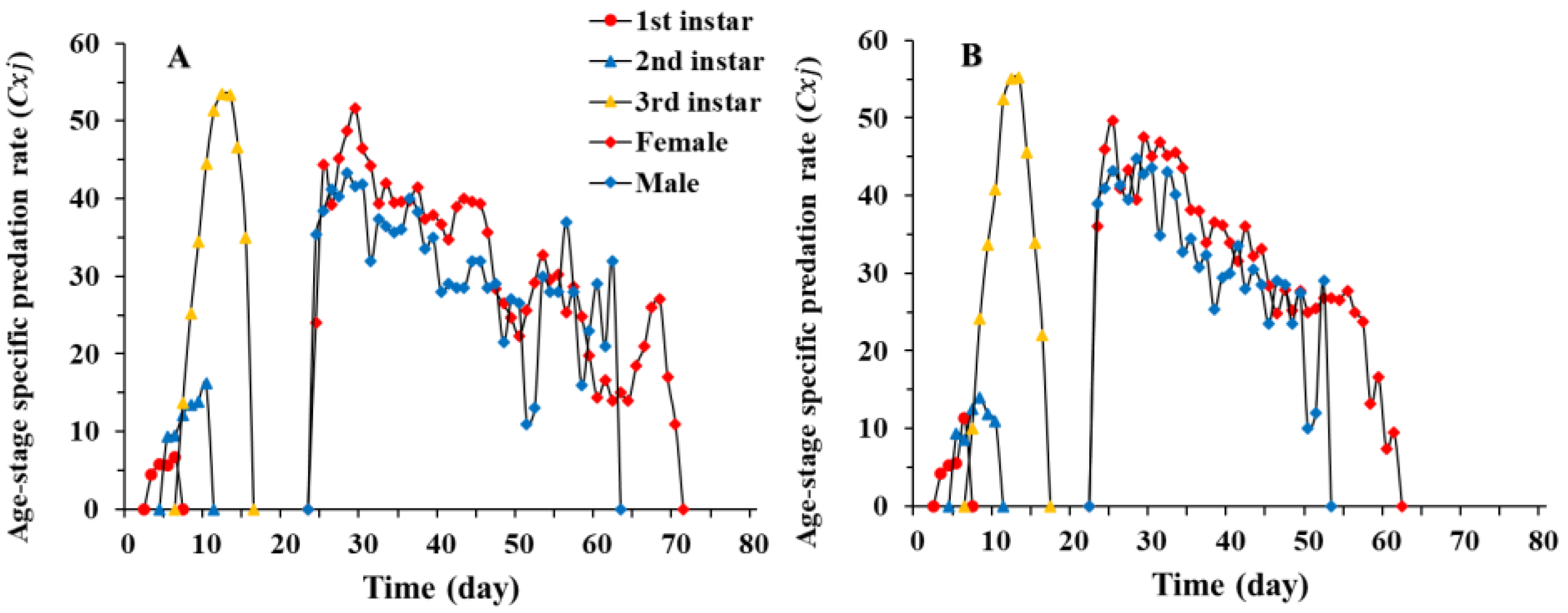
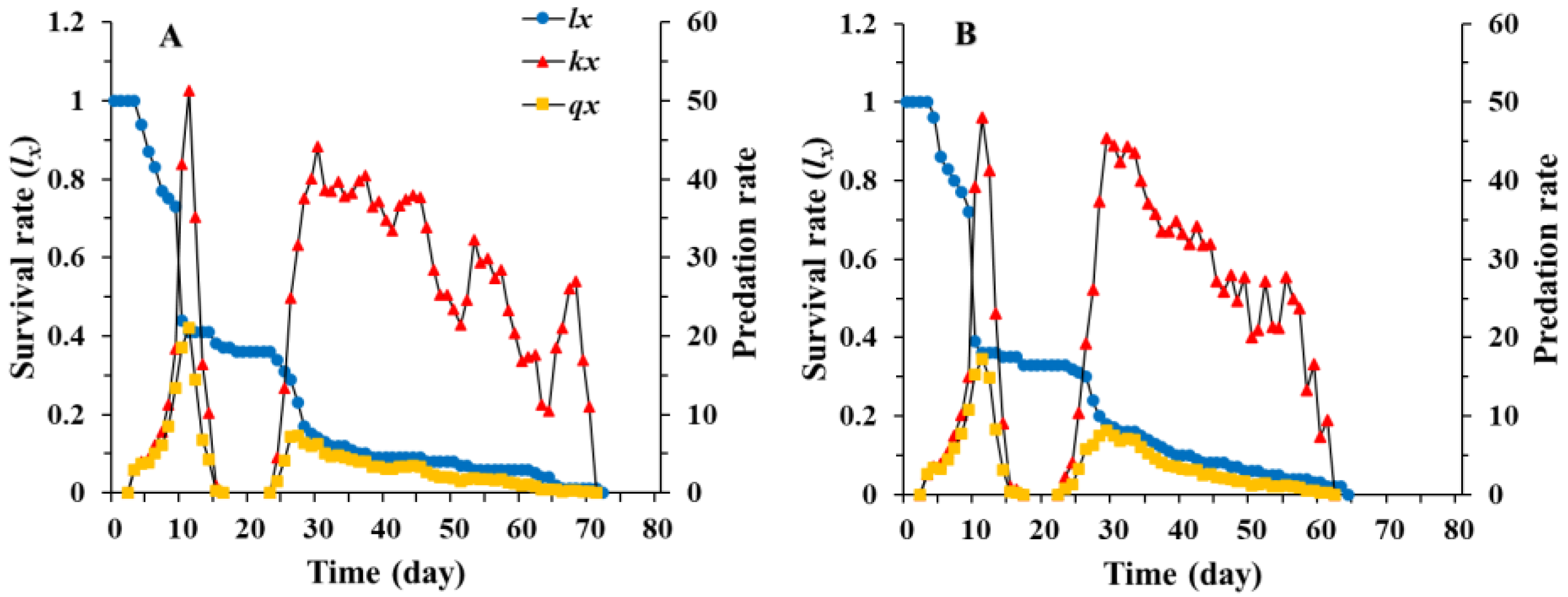
| Parameters | Stage | M. japonica | C. cephalonica Eggs | P | ||
|---|---|---|---|---|---|---|
| n | Mean ± SE | n | Mean ± SE | |||
| Developmental time (days) | Egg | 100 | 3.1 ± 0.1 a | 100 | 3.1 ± 0.1 a | 0.623 |
| 1st instar | 54 | 2.7 ± 0.1 a | 51 | 2.8 ± 0.1 a | 0.080 | |
| 2nd instar | 45 | 3.0 ± 0.2 a | 42 | 3.1 ± 0.1 a | 0.644 | |
| 3rd instar | 38 | 4.3 ± 0.2 a | 34 | 4.2 ± 0.1 a | 0.511 | |
| Pupa | 30 | 13.0 ± 0.2 a | 28 | 13.1 ± 0.2 a | 0.620 | |
| Adult longevity (days) | Female | 16 | 15.8 ± 4.4 a | 15 | 17.7 ± 3.7 a | 0.739 |
| Male | 14 | 8.5 ± 2.7 a | 14 | 7.7 ± 2.3 a | 0.820 | |
| APOP of female (days) | Female | 7 | 6.6 ± 0.9 a | 10 | 7.3 ± 0.3 a | 0.433 |
| TPOP of female (days) | Female | 7 | 33.3 ± 1.0 a | 10 | 33.7 ± 0.4 a | 0.710 |
| Fecundity (eggs/female) | Female | 16 | 35.5 ± 14.8 a | 14 | 33.6 ± 11.7 a | 0.910 |
| Population Parameter | M. japonica | C. cephalonica Eggs | P |
|---|---|---|---|
| Intrinsic rate of increase (r) (day–1) | 0.0379 ± 0.0119 a | 0.0359 ± 0.0109 a | 0.916 |
| Finite rate of increase (λ) (day–1) | 1.0386 ± 0.0122 a | 1.0366 ± 0.0112 a | 0.916 |
| Net reproduction rate (R0) (offspring individual–1) | 5.68 ± 2.64 a | 4.67 ± 1.96 a | 0.753 |
| Mean generation time (T) (day) | 45.83 ± 1.72 a | 42.90 ± 1.14 a | 0.071 |
| Gross reproduction rate (GRR) (offspring) | 72.58 ± 24.29 a | 55.61 ± 16.32 a | 0.560 |
| Parameter | Stage | M. japonica | C. cephalonica Eggs | P |
|---|---|---|---|---|
| Mean ± SE | Mean ± SE | |||
| Predation rate(aphids/predator) | 1st instar | 14.9 ± 0.9 a | 15.1 ± 0.7 a | 0.837 |
| 2nd instar | 35.5 ± 2.4 a | 35.5 ± 2.2 a | 0.987 | |
| 3rd instar | 191.1 ± 5.7 a | 190.8 ± 6.3 a | 0.954 | |
| Female | 539.0 ± 150.0 a | 609.0 ± 120.0 a | 0.715 | |
| Male | 291.1 ± 79.8 a | 270.2 ± 68.1 a | 0.836 | |
| 236.00 ± 39.66 a | 220.97 ± 36.33 a | 0.776 | ||
| Transformation rate | 54.22 ± 43.93 a | 55.97 ± 29.41 a | 0.945 | |
| Stable predation rate ψ | 9.19 ± 0.73 a | 8.92 ± 0.78 a | 0.799 | |
| Finite predation rate ω | 9.53 ± 0.80 a | 9.23 ± 0.87 a | 0.802 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, S.; Yang, J.; Guo, M.; Dai, H.; Ramirez-Romero, R.; Jin, Z.; Wang, S. The Fitness of Mass Rearing Food on the Establishment of Chrysopa pallens in a Banker Plant System under Fluctuating Temperature Conditions. Insects 2021, 12, 1014. https://doi.org/10.3390/insects12111014
Wang J, Li S, Yang J, Guo M, Dai H, Ramirez-Romero R, Jin Z, Wang S. The Fitness of Mass Rearing Food on the Establishment of Chrysopa pallens in a Banker Plant System under Fluctuating Temperature Conditions. Insects. 2021; 12(11):1014. https://doi.org/10.3390/insects12111014
Chicago/Turabian StyleWang, Jie, Shu Li, Jun Yang, Mingcheng Guo, Huijie Dai, Ricardo Ramirez-Romero, Zhenyu Jin, and Su Wang. 2021. "The Fitness of Mass Rearing Food on the Establishment of Chrysopa pallens in a Banker Plant System under Fluctuating Temperature Conditions" Insects 12, no. 11: 1014. https://doi.org/10.3390/insects12111014
APA StyleWang, J., Li, S., Yang, J., Guo, M., Dai, H., Ramirez-Romero, R., Jin, Z., & Wang, S. (2021). The Fitness of Mass Rearing Food on the Establishment of Chrysopa pallens in a Banker Plant System under Fluctuating Temperature Conditions. Insects, 12(11), 1014. https://doi.org/10.3390/insects12111014








