Parasite Prevalence May Drive the Biotic Impoverishment of New England (USA) Bumble Bee Communities
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
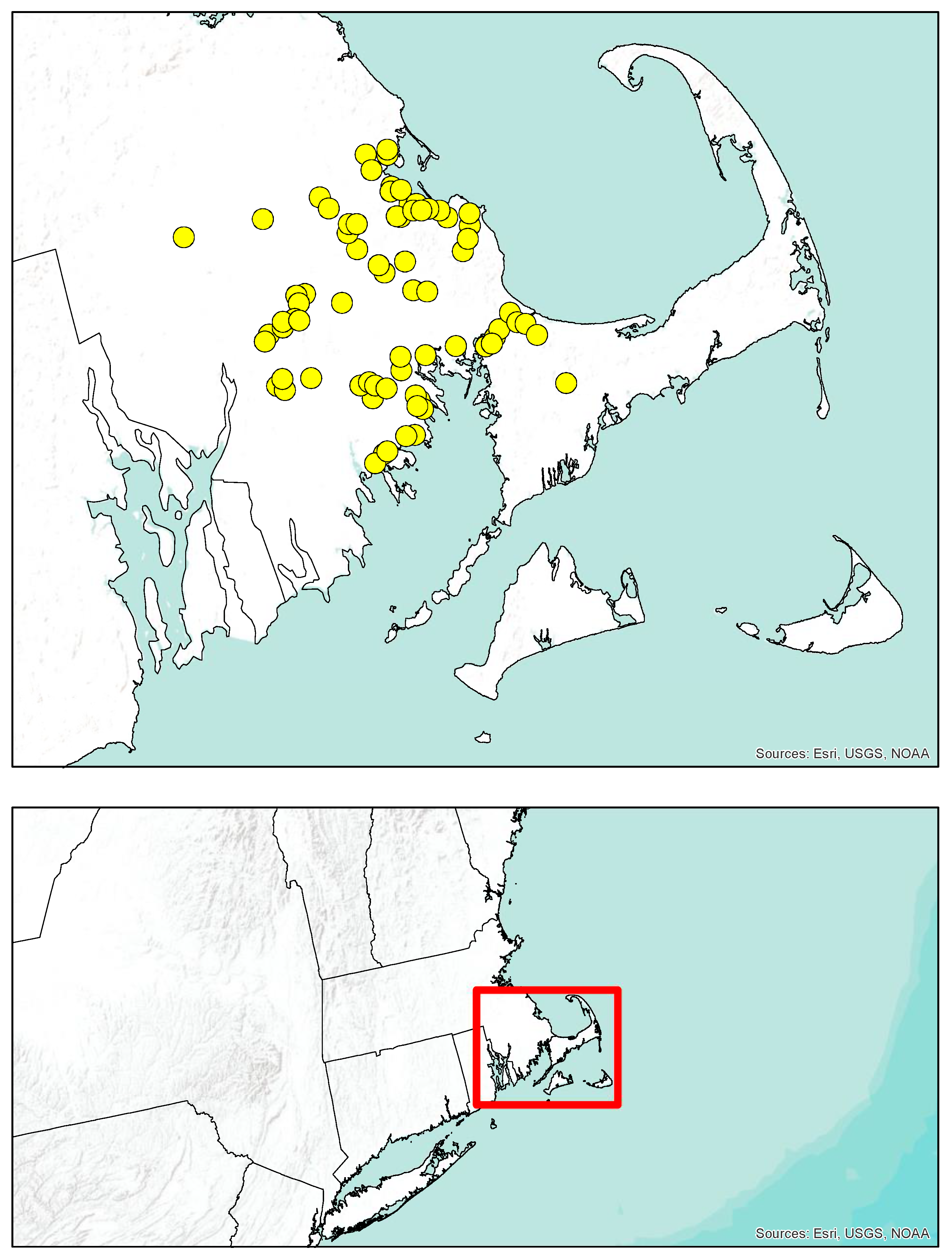
2.1. Study Area
2.2. Collections
2.3. Cryptic Bombus Identifications
2.4. Parasites and Their Assessment
3. Results
3.1. Collections
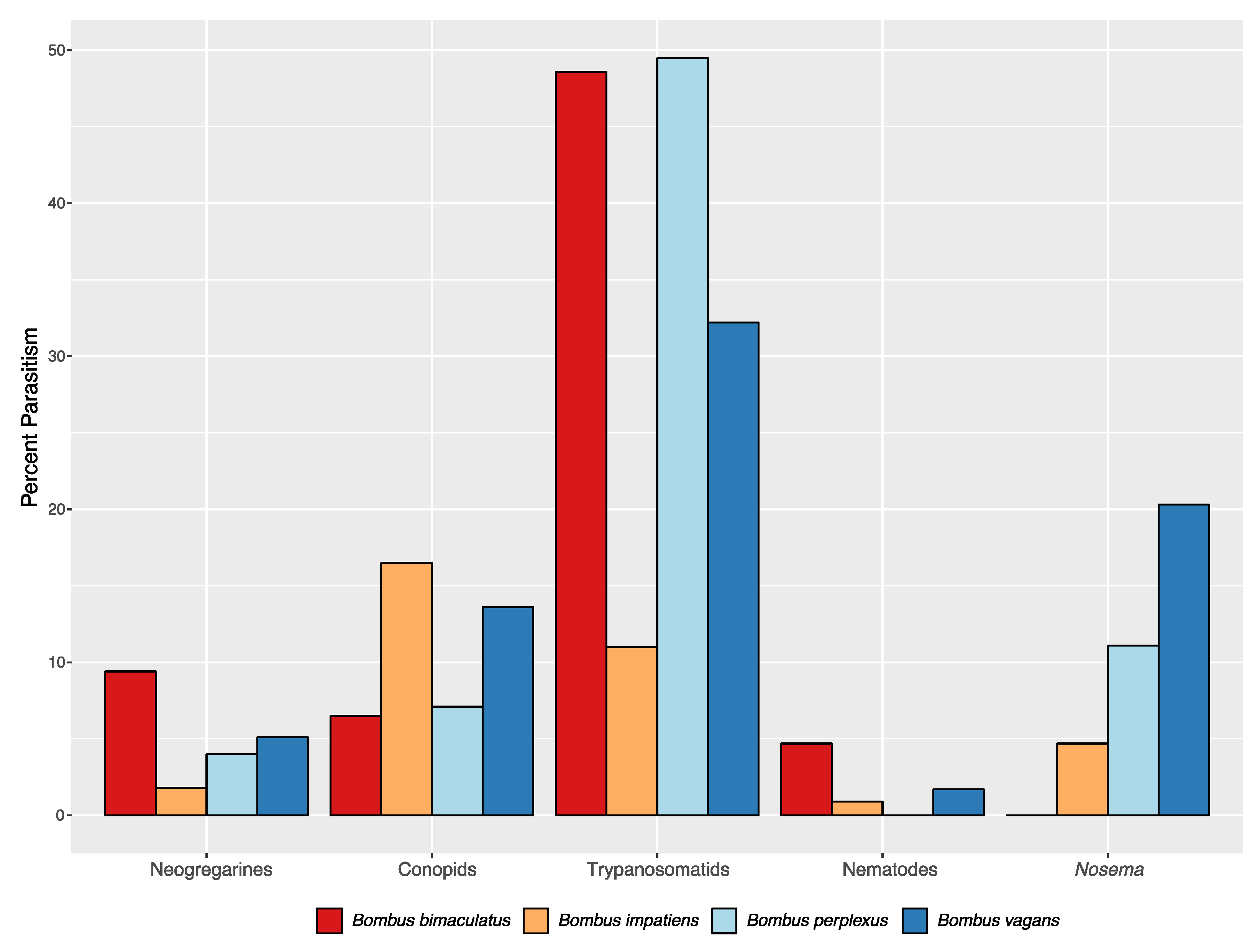
3.2. Parasites and Their Assessments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goulson, D. Bumblebees Behaviour, Ecology, and Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Bartomeus, I.; Gibbs, J.S.; Danforth, B.N.; Wagner, D.L.; Hedtke, S.M.; Winfree, R. Historical changes in northeastern United States bee pollinators related to shared ecological traits. Proc. Natl. Acad. Sci. USA 2013, 110, 4656–4660. [Google Scholar] [CrossRef]
- Dupont, V.L.; Damgarrd, C.; Simonsen, V. Quantitative historical change in bumblebee (Bombus spp.) assemblages of red clover fields. PLoS ONE 2011, 6, e25172. [Google Scholar] [CrossRef]
- Bommarco, R.; Lundin, O.; Smith, H.G.; Rundlof, M. Drastic historic shifts in bumble-bee community composition in Sweden. Proc. R. Soc. Lond. B 2012, 279, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.M.; Tucker, E.M.; Mathiason, M.E.; Rehan, S.M. Decline of bumble bees in northeastern North America, with special focus on Bombus terricola. Biol. Conserv. 2018, 217, 437–445. [Google Scholar] [CrossRef]
- Cane, J.H.; Schiffhauer, D. Dose-response relationships between pollination and fruiting refine pollinator comparisons for cranberry (Vaccinium macrocarpon [Ericaceae]). Am. J. Bot. 2003, 90, 1425–1432. [Google Scholar] [CrossRef]
- MacKenzie, K. The foraging behavior of honey bees (Apis mellifera L.) and bumble bees (Bombus spp.) on cranberry (Vaccinium macrocarpon Ait). Apidologie 1994, 25, 375–383. [Google Scholar] [CrossRef][Green Version]
- Averill, A.L.; Sylvia, M.M.; Hahn, N.; Couto, A.V. Bees (Hymenoptera: Apoidea) foraging on American Cranberry in Massachusetts. Northeast. Nat. 2018, 25, 501–512. [Google Scholar] [CrossRef]
- Franklin, H.J. The Bombidae of the New World. Trans. Am. Entomol. Soc. 1913, 38, 177–486. [Google Scholar]
- Franklin, H.J. Cranberry insects in Massachusetts: Part V. Insects and other animals beneficial in cranberry growing. Mass. Agric. Exp. Sta. Bull. 1950, 445, 55–76. [Google Scholar]
- MacKenzie, K.E.; Averill, A.L. Bee (Hymenoptera: Apoidea) diversity and abundance on cranberry in southeastern Massachusetts. Ann. Entomol. Soc. Amer. 1995, 88, 334–341. [Google Scholar] [CrossRef]
- Brown, E.R. Catastrophic Loss of Bumble Bee (Bombus spp.) Diversity Revealed in 27-Year Survey of Massachusetts Cranberry Pollinators. Bachelor’s Thesis, Commonwealth Honors College, University of Massachusetts Amherst, Amherst, MA, USA, 2018. [Google Scholar]
- Colla, S.R.; Gadallah, F.; Richardson, L.; Wagner, D.; Gall, L. Assessing decline of North American bumble bees (Bombus spp.) using museum specimens. Biodivers. Conserv. 2012, 21, 3585–3595. [Google Scholar] [CrossRef]
- Tucker, E.M.; Rehan, S.M. Wild bee pollination networks in northern New England. J. Insect Conserv. 2016, 20, 325–337. [Google Scholar] [CrossRef]
- Suni, S.S.; Scott, Z.; Averill, A.L.; Whitely, A. Population genetics of wild and managed pollinators: Implications for crop pollination and the genetic integrity of wild bees. Conserv. Genet. 2017, 18, 667–677. [Google Scholar] [CrossRef]
- Looney, C.; Strange, J.P.; Freeman, M.M.; Jennnings, D. Expansion of Bombus impatiens Cresson in the Pacific Northwest and its establishment in Washington State. Biol. Invasions 2019, 21, 1879–1885. [Google Scholar] [CrossRef]
- Inoue, M.N.; Yokoyama, J. Competition for flower resources between Bombus terrestris (L.) and Japanese native bumblebees. Appl. Entomol. Zool. 2010, 45, 29–35. [Google Scholar] [CrossRef][Green Version]
- Morales, C.L.; Arbetman, M.P.; Cameron, S.A.; Aizen, M.A. Rapid ecological replacement of a native bumble bee by invasive species. Front. Ecol. Environ. 2013, 11, 529–534. [Google Scholar] [CrossRef]
- Williams, P.H.; Thorpe, R.W.; Richardson, L.L.; Colla, S.R. Bumble Bees of North America: An Identification Guide; Princeton University Press: Princeton, NJ, USA, 2014. [Google Scholar]
- Soroye, P.; Newbold, T.; Kerr, J. Climate change contributes to widespread declines among bumble bee species across continents. Science 2020, 367, 685–688. [Google Scholar] [CrossRef]
- Novotny, J.L.; Reeher, P.; Varvaro, M.; Lybbert, A.; Smith, J.; Mitchell, R.J.; Goodell, K. Bumble bee species distributions and habitat associations in the Midwestern USA, a region of declining diversity. Biodivers. Conserv. 2021, 30, 865–887. [Google Scholar] [CrossRef]
- Schmid-Hempel, P. Parasites in Social Insects; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Malfi, R.L.; Roulston, T.H. Patterns of parasite infection in bumble bees (Bombus spp.) of Northern Virginia. Ecol. Entomol. 2014, 39, 17–29. [Google Scholar] [CrossRef]
- Brown, M.J.F. The trouble with bumblebees. Nature 2011, 469, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D.; Whitehorn, P.; Fowley, M. Influence of urbanization on the prevalence of protozoan parasites of bumblebees. Ecol. Entomol. 2012, 37, 83–89. [Google Scholar] [CrossRef]
- Cameron, S.A.; Lim, H.C.; Lozier, J.D.; Duennes, M.A.; Thorp, R. Test of the invasive pathogen hypothesis of bumble bee decline in North America. Proc. Natl. Acad. Sci. USA 2016, 113, 4386–4391. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, C.N.; Cameron, S.A.; Thorp, R.W.; White, B.; Solter, L.F. Survey of bumble bee (Bombus) pathogens and parasites in Illinois and selected areas of northern California and southern Oregon. J. Invertebr. Pathol. 2011, 107, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Cordes, N.; Huang, W.-F.; Strange, J.P.; Cameron, S.A.; Griswold, T.L.; Lozier, J.D.; Solter, L.F. Interspecific geographic distribution and variation of the pathogens Nosema bombi and Crithidia species in United States bumble bee populations. J. Invertebr. Pathol. 2012, 109, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, S. Factors affecting parasite prevalence among wild bumblebees. Ecol. Entomol. 2010, 35, 737–747. [Google Scholar] [CrossRef]
- Hall, B.; Motzkin, G.; Foster, D.R.; Syfert, M.; Burk, J. Three hundred years of forest and land-use change in Massachusetts, USA. J. Biogeogr. 2002, 29, 1319–1335. [Google Scholar] [CrossRef]
- Milam, J.; Johnson, D.E.; Andersen, J.C.; Fassler, A.B.; Narango, D.L.; Elkinton, J.S. Validating morphometrics with DNA barcoding to reliably separate three cryptic species of Bombus Cresson (Hymenoptera: Apidae). Insects 2020, 11, 669. [Google Scholar] [CrossRef]
- Otti, O.; Schmid-Hempel, P. A pollinator parasite with detrimental fitness effects. J. Invertebr. Pathol. 2007, 96, 118–124. [Google Scholar] [CrossRef]
- Rutrecht, S.T.; Brown, M.J.F. Differential virulence in a multiple-host parasite of bumble bees: Resolving the paradox of parasite survival? Oikos 2009, 118, 941–949. [Google Scholar] [CrossRef]
- Graystock, P.; Meeus, I.; Smagghe, G.; Goulson, D.; Hughes, W.O.H. The effects of single and mixed infections of Apicystis bombi and deformed wing virus in Bombus terrestris. Parasitology 2016, 143, 358–365. [Google Scholar] [CrossRef]
- Brown, M.J.F.; Schmid-Hempel, R.; Schmid-Hempel, P. Strong context-dependent virulence in a host–parasite system: Reconciling genetic evidence with theory. J. Anim. Ecol. 2003, 72, 994–1002. [Google Scholar] [CrossRef]
- Shykoff, J.A.; Schmid-Hempel, P. Incidence and effects of four parasites in natural populations of bumble bees in Switzerland. Apidologie 1991, 22, 117–125. [Google Scholar] [CrossRef]
- Brown, M.J.F.; Loosli, R.; Schmid-Hempel, P. Condition-dependent expression of virulence in a trypanosome infecting bumblebees. Oikos 2000, 91, 421–427. [Google Scholar] [CrossRef]
- Gegear, R.J.; Otterstatter, M.C.; Thomson, J.D. Does parasitic infection impair the ability of bumblebees to learn flower-handling techniques? Anim. Behav. 2005, 70, 209–215. [Google Scholar] [CrossRef]
- Gegear, R.J.; Otterstatter, M.C.; Thomson, J.D. Bumble-bee foragers infected by a gut parasite have an impaired ability to utilize floral information. Proc. R. Soc. B 2006, 273, 1073–1078. [Google Scholar] [CrossRef]
- Otterstatter, M.C.; Gegear, R.J.; Colla, S.; Thomson, J.D. Effects of parasitic mites and protozoa on the flower constancy and foraging rate of bumble bees. Behav. Ecol. Sociobiol. 2005, 58, 383–389. [Google Scholar] [CrossRef]
- Goulson, D.; O’Connor, S.; Park, K.J. The impacts of predators and parasites on wild bumblebee colonies. Ecol. Entomol. 2018, 43, 163–181. [Google Scholar] [CrossRef]
- Alford, D.V. Bumblebees; Davis–Poynter: London, UK, 1975. [Google Scholar]
- Schmid-Hempel, P.; Schmid-Hempel, R. Transmission of a pathogen in Bombus terrestris, with a note on division of labor in social insects. Behav. Ecol. Sociobiol. 1993, 33, 319–327. [Google Scholar] [CrossRef]
- Durrer, S.; Schmid-Hempel, P. Shared use of flowers leads to horizontal pathogen transmission. Proc. R. Soc. Lond. B 1994, 258, 299–302. [Google Scholar]
- Smith, K.G.; Peterson, B.V. Conopidae. In Manual of Nearctic Diptera; McAlpine, J.F., Peterson, B.V., Shewall, G.E., Teskey, H.H., Vockeroth, J.R., Wood, D.M., Eds.; Research Branch Agriculture Canada: Ottawa, ON, Canada, 1987; Volume 2, pp. 749–756. [Google Scholar]
- Plath, O.E. Bumblebees and Their Ways; Macmillan Co.: New York, NY, USA, 1934. [Google Scholar]
- Matteson, K.C.; Ascher, J.S.; Lancellotto, G.A. Bee richness and abundance in New York City urban gardens. Ann. Entomol. Soc. Am. 2008, 101, 140–150. [Google Scholar] [CrossRef]
- Ascher, J.S.; Kornbluth, S.; Geolet, R.G. Bees (Hymenoptera: Apoidea: Anthophila) of Gardiners Island, Suffolk County, New York. Northeast. Nat. 2014, 21, 47–71. [Google Scholar] [CrossRef]
- Whitehorn, P.R.; Tinsley, M.C.; Brown, M.J.F.; Darvill, B.; Goulson, D. Genetic diversity, parasite prevalence and immunity in wild bumblebees. Proc. R. Soc. B 2011, 278, 1195–1202. [Google Scholar] [CrossRef]
- Woodward, S.H.; Lozier, J.D.; Goulson, D.; Williams, P.H.; Strange, J.P.; Jha, S. Molecular tools and bumble bees: Revealing hidden details of ecology and evolution in a model system. Mol. Biol. 2015, 24, 2916–2936. [Google Scholar] [CrossRef]
- Bushman, S.L.; Drummond, F.A.; Beers, L.A.; Groden, E. Wild bumblebee (Bombus) diversity and Nosema (Microsporidia: Nosematidae) infection levels associated with lowbush blueberry (Vaccinium angustifloium) production and commercial bumblebee pollinators. Psyche J. Entomol. 2012, 2012, 429398. [Google Scholar]
- Mockler, B.K.; Kwong, W.K.; Moran, N.A.; Koch, H. Microbiome structure influences infection by the parasite Crithidia bombi in bumble bees. Appl. Environ. Microbiol. 2018, 84, e02335-17. [Google Scholar] [CrossRef]
- Cariveau, D.P.; Powell, J.E.; Koch, H.; Winfree, R.; Moran, N.A. Variation in gut microbial communities and its association with pathogen infection in wild bumble bees (Bombus). ISME J. 2014, 8, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Ayres, J.S.; Schneider, D.S. Two ways to survive an infection: What resistance and tolerance can teach us about treatments for infectious diseases. Nat. Rev. Immunol. 2008, 8, 88–895. [Google Scholar]
- McFrederick, Q.S.; LeBuhn, G. Are urban parks refuges for bumble bees Bombus spp. (Hymenoptera:Apidae)? Biol. Conserv. 2005, 129, 372–382. [Google Scholar] [CrossRef]
- Blaker, E.A.; Strange, J.P.; James, R.R.; Monroy, F.P.; Cobb, N.S. PCR reveals high prevalence of non/low sporulating Nosema bombi (microsporidia) infections in bumble bees (Bombus) in Northern Arizona. J. Invert. Pathol. 2014, 123, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Barolome, C.; Buendia, M.; Benito, M.; De la Rua, P.; Ornosa, C.; Martin-Hernandez, R.; Higes, M.; Maside, X. A new multiplex PCR protocol to detect mixed trypanosomatid infections in species of Apis and Bombus. J. Invert. Pathol. 2018, 154, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.D.; Szalanski, A.L.; Strange, J.P. Novel multiplex PCR reveals multiple trypanosomatid species infecting North American bumble bees (Hymenoptera: Apidae: Bombus). J. Invert. Pathol. 2018, 153, 147–155. [Google Scholar] [CrossRef]
- Cameron, S.A.; Lozier, J.D.; Strange, J.P.; Koch, J.B.; Cordes, N.; Solter, L.F.; Griswold, T.L. Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. USA 2011, 108, 662–667. [Google Scholar] [CrossRef]
| Species | Percent Change | Change in Relative Abundance | Percent of Sites | N | Percent Microparasites | Percent High Intensity | Percent Mixed |
|---|---|---|---|---|---|---|---|
| Bombus griseocollis | +60 | +3.5 | 10.4 | 20 | 10.5 | 0 | 0 |
| Bombus impatiens | +20 | +42.1 | 87.0 | 902 | 13.2 | 2.7 | 2.9 |
| Bombus bimaculatus | +10 | −9.3 | 46.1 | 120 | 55.2 | 11.2 | 12.6 |
| Bombus perplexus | −50 | −7.3 | 40.9 | 99 | 56.6 | 17.1 | 9.8 |
| Bombus vagans | −60 | −10.4 | 21.7 | 61 | 52.5 | 17.0 | 14.5 |
| Species | Caste | N | Percent trypanosomatid | Percent neogregarine | Percent Nosema | Percent Conopid | Percent Nematode |
|---|---|---|---|---|---|---|---|
| Pyrobombus subgenus | |||||||
| Bombus impatiens | Worker | 710 | 11.0 (1.9) | 1.8 (0.3) | 4.7 (0) | 16.5 | 0.9 |
| Bombus impatiens | Male | 192 | 2.1 (1.0) | 0 (0) | 1.1 (0) | 2.6 | 0 |
| Bombus bimaculatus | Worker | 107 | 48.6 (9.1) | 9.4 (0.9) | 0 (0) | 6.5 | 4.7 |
| Bombus bimaculatus | Male | 13 | 21.4 (15.4) | 30.8 (0) | 0 (0) | 14.3 | 15.4 |
| Bombus perplexus | Worker | 99 | 49.5 (10.5) | 4.0 (0) | 11.1 (6.0) | 7.1 | 0 |
| Bombus perplexus | Male | 0 | -- | -- | -- | -- | -- |
| Bombus vagans | Worker | 59 | 32.2 (5.3) | 5.1 (1.7) | 20.3 (15.0) | 13.6 | 1.7 |
| Bombus vagans | Male | 2 | 0 (0) | 50.0 (0) | 50.0 (50) | 0 | 0 |
| Cullumanobombus subgenus | |||||||
| Bombus griseocollis | Worker | 19 | 10.5 (0) | 0 (0) | 5.3 (0) | 15.8 | 0 |
| Bombus griseocollis | Male | 1 | -- | -- | -- | -- | -- |
| Thoracobombus subgenus | |||||||
| Bombus fervidus | Worker | 3 | 0 (0) | 0 (0) | 66.7 (0) | 0 | 0 |
| Bombus fervidus | Male | 0 | -- | -- | -- | -- | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Averill, A.L.; Couto, A.V.; Andersen, J.C.; Elkinton, J.S. Parasite Prevalence May Drive the Biotic Impoverishment of New England (USA) Bumble Bee Communities. Insects 2021, 12, 941. https://doi.org/10.3390/insects12100941
Averill AL, Couto AV, Andersen JC, Elkinton JS. Parasite Prevalence May Drive the Biotic Impoverishment of New England (USA) Bumble Bee Communities. Insects. 2021; 12(10):941. https://doi.org/10.3390/insects12100941
Chicago/Turabian StyleAverill, Anne L., Andrea V. Couto, Jeremy C. Andersen, and Joseph S. Elkinton. 2021. "Parasite Prevalence May Drive the Biotic Impoverishment of New England (USA) Bumble Bee Communities" Insects 12, no. 10: 941. https://doi.org/10.3390/insects12100941
APA StyleAverill, A. L., Couto, A. V., Andersen, J. C., & Elkinton, J. S. (2021). Parasite Prevalence May Drive the Biotic Impoverishment of New England (USA) Bumble Bee Communities. Insects, 12(10), 941. https://doi.org/10.3390/insects12100941






