3.3. Results of the Quantitative Comparison of Samples from Different Regions
Males were identified by the genitalia (manubrium structure). Females, collected at the same time with certain males or in a region from which only one species is known, were identified accordingly. Samples from populations are grouped as follows. Regions 1, 2, and 3 represent the area of T. hummelii starcki, 4—the area of T. armeniaca, 5—the area of T. hummelii hummelii, 6—the total area of T. armeniaca + T. hummelii hummelii, 7—the area of T. metallica, 8—the area of T. corinthia.
3.3.1. Total Body Length (in Lateral View)
According to Faldermann [
23],
T. hummelii is 10.16 mm long, and
T. armeniaca is 13.97 mm long. According to [
10],
T. corinthia is 11 mm long,
T. metallica is 7–10 mm long,
T. gibba is 8–9 mm long. According to Marseul [
17],
T. corinthia is “large”, and
T. metallica and
T. gibba, both are “small”. According to Weise [
16],
T. corinthia is 10–13,
T. metallica is 5–10, and
T. hummelii is 8–13 mm long. According to Bechyné [
26], females of
T. hummelii starcki are 12–13 mm long, longer than those of
T. hummelii hummelii. According to Medvedev, Shapiro [
20],
T. hummelii is 7.5–13, and
T. metallica is 5–10 mm long. According to Warchałowski [
3],
T. corinthia is 8.5–13 mm long, but the female is 10.5–15 mm long,
T. metallica is 6.0–8.5 mm long, but females up to 13 mm long,
T. gibba is 8–12 mm long, female of
T. hummelii up to 11 mm long.
Comparison of T. corinthia, T. metallica, and T. hummelii.
It was found (
Table 2 and
Table 3) that
T. corinthia is mostly larger than
T. metallica, but there is no clear interspecific difference (hiatus) by the length of all specimens, as well as, separately, males and females.
Timarcha hummelii is mostly larger than
T. metallica, but there is no species difference too.
Intraspecific variability of T. hummelii.
Comparison of regions 4 and 5.
The lengths of all specimens
Therefore, |M4 − M5| < 3.24σ4 + 0.68σ5 and |M4 − M8| < 3.24σ5 + 0.68σ4.
The difference does not reach the level of subspecies.
Therefore, |M4 − M5| < 3.24σ4 + 0.68σ5 and |M4 − M8| < 3.24σ5 + 0.68σ4.
The difference does not reach the level of subspecies.
Therefore, |M4 − M5| < 3.24σ4 + 0.68σ5 and |M4 − M8| < 3.24σ5 + 0.68σ4.
The difference does not reach the level of subspecies.
Comparison of regions 3 and 6
The length of all specimens.
Therefore, |M6 − M3| < 3.24σ6 + 0.68σ3 and |M9 − M3| < 3.24σ3 + 0.68σ6.
The difference does not reach the level of subspecies.
Therefore, |M6 − M3| < 3.24σ6 + 0.68σ3 and |M9 − M3| < 3.24σ3 + 0.68σ6.
The difference does not reach the level of subspecies.
Therefore, |M6 − M3| < 3.24σ6 + 0.68σ3 and |M9 − M3| < 3.24σ3 + 0.68σ6.
The difference does not reach the level of subspecies.
Bechyné [
26] noted that females of
T. hummelii starcki are larger than those of
T. hummelii hummelii. According to the available specimens, the largest female from the Western Caucasus is 12.24 mm long, and the largest female from Transcaucasia not smaller, but even slightly larger, 12.46 mm long. The populations from the WesternCaucasus and Transcaucasia do not correspond to Amadon’s criteria [
40] of the subspecies by this character.
3.3.2. Size of the Pronotum (a): Pronotal Length (in Dorsal View)/Elytral Length (in Lateral View)
According to Weise [
16], Medvedev, Shapiro [
20], the pronotum is “large” in
T. hummelii, and “small” in
T. metallica. However, the authors did not indicate which parameters were taken into account. Two parameters: relative length (present feature) and relative width (next feature) of the pronotum were selected in the present work (
Table 4).
Comparison of T. corinthia, T. metallica, and T. hummelii.
Timarcha corinthia has, on average, a slightly shorter pronotum, than T. metallica, but there is no clear interspecific difference (hiatus) by this character. Timarcha hummelii practically does not differ from T. metallica in the relative length of the pronotum.
Intraspecific variability of T. hummelii.
Comparison of regions 4 and 5
Therefore, |M4 − M5| < 3.24σ4 + 0.68σ5 and |M4 − M8| < 3.24σ5 + 0.68σ4.
The difference does not reach the level of subspecies.
Comparison of regions 3 and 6
Therefore, |M6 − M3| < 3.24σ6 + 0.68σ3 and |M9 − M3| < 3.24σ3 + 0.68σ6.
The difference does not reach the level of subspecies.
3.3.3. Size of the Pronotum (b): Pronotal Width (in Dorsal View)/Elytral Length
Size of the pronotum (b): Pronotal Width (in Dorsal View)/Elytral Length (
Table 5).
Comparison of T. corinthia, T. metallica, and T. hummelii.
Timarcha corinthia has, on average, a narrower pronotum than T. metallica, but there is no clear interspecific difference (hiatus). Timarcha hummelii has, on average, a slightly narrower pronotum than T. metallica. There is no clear interspecific difference (hiatus).
Intraspecific variability of T. hummelii
Comparison of regions 4 and 5
Therefore, |M4 − M5| < 3.24σ4 + 0.68σ5 and |M4 − M8| < 3.24σ5 + 0.68σ4.
The difference does not reach the level of subspecies.
Comparison of regions 3 and 6
Therefore, |M6 − M3| < 3.24σ6 + 0.68σ3 and |M9 − M3| < 3.24σ3 + 0.68σ6.
The difference does not reach the level of subspecies.
3.3.4. Location of Maximal Width of the Pronotum: Distance from the Level of the Front Corners to the Level of the Greatest Width of the Pronotum/Total Length of the Pronotum (Both in Dorsal View)
According to Faldermann [
23], the maximal width of the pronotum is at mid-length in
T. hummelii, and before the mid-length in
T. armeniaca. According to [
10], the maximal width of the pronotum is at base in
T. gibba, and before the mid-length in
T. metallica. According to Weise [
16], the maximal width of the pronotum is at base in
T. gibba, pronotum narrowed anteriorly (slightly more) and posteriorly in
T. corinthia, T. metallica, and
T. hummelii. According to Marseul [
17], the maximal width of the pronotum is at mid-length in
T. armeniaca, and before the mid-length in
T. hummelii. According to Medvedev, Shapiro [
20], the maximal width of the pronotum is almost at the anterior margin in
T. hummelii, and before the mid-length in
T. metallica. According to Warchałowski [
3], the maximal width of the pronotum is at the base in
T. gibba, at mid-length in
T. corinthia, and before mid-length in
T. metallica and
T. hummelii (
Table 6).
Comparison of T. corinthia, T. metallica, and T. hummelii
Timarcha corinthia has the greatest width of the pronotum, on average, closer to the apex than T. metallica, but there is no clear interspecific difference (hiatus). In T. hummelii, the greatest width of the pronotum is on average closer to the apex than in T. metallica, but there is no clear interspecific difference (hiatus).
Intraspecific variability of T. hummelii
Comparison of regions 4 and 5
Therefore, |M4 − M5| < 3.24σ4 + 0.68σ5 and |M4 − M8| < 3.24σ5 + 0.68σ4.
The difference does not reach the level of subspecies.
Comparison of regions 3 and 6
Therefore, |M6 − M3| < 3.24σ6 + 0.68σ3 and |M9 − M3| < 3.24σ3 + 0.68σ6.
The difference does not reach the level of the subspecies.
3.3.5. Emargination of Pronotal Lateral Side before Base (Present or Absent)
According to Faldermann [
23], the lateral side is slightly emarginate before the base in
T. armeniaca, and almost without emargination in
T. hummelii. According to Marseul [
17], the lateral side is distinctly emarginate before the base in
T. corinthia and
T. armeniaca, slightly emarginate in
T. hummelii, and without emargination in
T. gibba and
T. metallica. According to Medvedev, Shapiro [
20], the lateral side is slightly emarginate in
T. hummelii, and more or less rounded in
T. metallica (
Table 7).
Comparison of T. corinthia, T. metallica, and T. hummelii.
Specimens of T. corinthia has emargination more often than T. metallica and T. hummelii, but there is no clear interspecific difference (hiatus).
Intraspecific variability of T. hummelii.
Comparison of regions 4 and 5. Populations from these regions hardly differ in this character.
Comparison of regions 3 and 6. In individuals from the Western Caucasus, the emargination presents more often than in individuals from the Transcaucasia, but the difference does not reach the level of subspecies.
3.3.6. Dorsal Color
Different authors could designate the same colors in different ways. In the present work, a spectrum of colors that reflects variability is adopted. According to Faldermann [
23], the dorsal color is purple copper with an elytra dark copper in
T. hummelii, and copper green with an elytra dark greenish copper in
T. armeniaca. According to Weise [
16], dorsum is brassy in
T. corinthia, brown with strong brass shine in
T. metallica, brown, or violet, or greenish, with copper shine in
T. hummelii, piceous with violet or blue shine in
T. gibba. According to Marseul [
17], the dorsal color is golden bronze in
T. corinthia, black in
T. gibba, and bronze brown in
T. metallica. According to Medvedev, Shapiro [
20], the dorsal color is rusty red or purple in
T. hummelii, and darker, bronze or coppery in
T. metallica. According to Warchałowski [
3], the dorsal color is greenish bronze, copper, blue, or violet in
T. corinthia, and almost black with blue sheen in
T. metallica (
Table 8).
Comparison of T. corinthia, T. metallica, and T. hummelii.
In T. corinthia and T. metallica, colors 5 and 6 are sharply predominant, in T. hummelii, they are not found, but colors 1 and 4 are predominant. Due to the presence of color types 1–4 in the first two species, there is no clear interspecific difference (hiatus).
Intraspecific variability of T. hummelii.
Comparison of regions 4 and 5.
Populations from these regions are very similar in color types. The difference does not reach the level of subspecies.
Comparison of regions 3 and 6.
Populations from the Western Caucasus and from the Transcaucasia are very similar in color types. The difference between them does not reach the level of subspecies.
3.3.7. Color of Femora
Most authors described the coloration of the legs in general. However, since different parts of the leg can be colored differently, the coloring of the femora (present feature) and tarsi (the next feature) were considered separately in the present work.
According to Faldermann [
23], legs are brown, almost bronze, with tarsi light in
T. hummelii, and legs are pitch-brown, slightly copper, with tarsi brown in
T. armeniaca. According to Weise [
16], legs are violet in
T. corinthia; they are more or less red–brown in
T. hummelii and
T. metallica. According to Marseul [
17], legs are violet in
T. corinthia, and blue–black in
T. gibba. According to Warchałowski [
3,
13], legs are red–brown or red in
T. metallica and
T. hummelii, and black or almost black with metallic shine in
T. corinthia (
Table 9).
Comparison of T. corinthia, T. metallica, and T. hummelii.
Timarcha corinthia differs sharply from T. metallica and T. hummelii in the predominance of color 3, but there is no clear interspecific difference (hiatus). In T. hummelii and T. metallica, color 2 is predominant; there is no clear interspecific difference (hiatus).
Intraspecific variability of T. hummelii.
Comparison of regions 4 and 5: populations from these regions des not differ by the color.
Comparison of regions 3 and 6.
The proportion of color 2 is slightly higher in region 9 than in region 3, but the difference does not reach the level of subspecies.
3.3.8. Color of Tarsi
Comparison of T. corinthia, T. metallica, and T. hummelii.
Timarcha corinthia sharply differs from T. metallica and T. hummelii in the presence of color 3 in all studied individuals, but due to the presence of this color in a small proportion in T. hummelii and T. metallica, there is no clear interspecific difference (hiatus).
Intraspecific variability of T. hummelii.
Comparison of regions 4 and 5.
Populations from these regions differ slightly. The difference does not reach the level of subspecies.
Comparison of regions 3 and 6.
Populations from these regions differ slightly. The difference does not reach the level of subspecies.
3.3.9. Punctures at the Elytral Disk (5 States from Fine to Large, i.e., Approximately 0.007, 0.009, 0.011. 0.013, 0.015 mm Wide)
According to Faldermann [
23], elytral punctures are large in
T. hummelii, and coarse in
T. armeniaca. According to Weise [
16], elytra are strongly punctate in
T. corinthia, and, rather densely, more or less strongly punctate in
T. metallica. According to Marseul [
17], elytral punctures are more dense and coarse in
T. gibba, and more sparse and small in
T. metallica. According to Bechyné [
26], elytral punctures are denser in
T. hummelii starcki than in
T. hummelii hummelii. According to Warchałowski [
3], elytral punctures are dense in
T. hummelii starcki, and moderately dense in
T. hummelii hummelii (
Table 11).
During the present study, it was found that the size of the puncture is a more variable parameter than the density.
Comparison of T. corinthia, T. metallica, and T. hummelii.
Timarcha corinthia sharply differs from T. metallica and T. hummelii due to the predominance of puncture state 6; due to the presence of this state in a small proportion in T. hummelii and T. metallica, there is no clear interspecific difference (hiatus).
Intraspecific variability of T. hummelii.
Comparison of regions 4 and 5.
Populations from these regions slightly differ; the difference does not reach the level of the subspecies.
Comparison of regions 3 and 6.
Populations from region 3 are distinguished by the predominance of punctation state 1, which is rare in region 9, but the difference does not reach the level of the subspecies.
3.3.10. Border at Upper Margin of Elytral Epipleura near Base (Present, Absent)
According to Marseul [
17], the border is present in
T. corinthia, T. gibba, T. metallica, and absent in
T. armeniaca and
T. hummelii (
Table 12).
Comparison of T. corinthia, T. metallica, and T. hummelii.
Timarcha corinthia and T. metallica differ from T. hummelii by the presence of a border in most specimens, but there is no clear interspecific difference (hiatus).
Intraspecific variability of T. hummelii.
Comparison of regions 4 and 5.
Populations from these regions does not differ.
Comparison of regions 3 and 6.
About a third of individuals from region 3 have borders, and no individuals from region 6 have borders. Thus, the difference does not reach the level of subspecies.
3.3.11. Shine of Elytron (Shining, Dull)
According to [
10], the shine of elytron is less in
T. gibba than in
T. metallica. According to Bechyne [
26], elytra shines in both sexes in
T. hummelii starcki, and elytra is dull in females of
T. hummelii hummelii. According to Warchałowski [
3], elytra shines or is dull in
T. metallica, dull in
T. hummelii hummelii, and shines in
T. hummelii starcki (
Table 13).
Comparison of T. corinthia, T. metallica, and T. hummelii.
Shining males predominate in all species, shining females—in T. corinthia and T. metallica, but there is no clear interspecific difference (hiatus).
Intraspecific variability of T. hummelii.
Comparison of regions 4 and 5.
Region 5 has more dull females and males than region 4, but the difference does not reach the level of subspecies.
Comparison of regions 3 and 6.
Region 6 has more dull females and males than region 3, but the difference does not reach the level of subspecies.
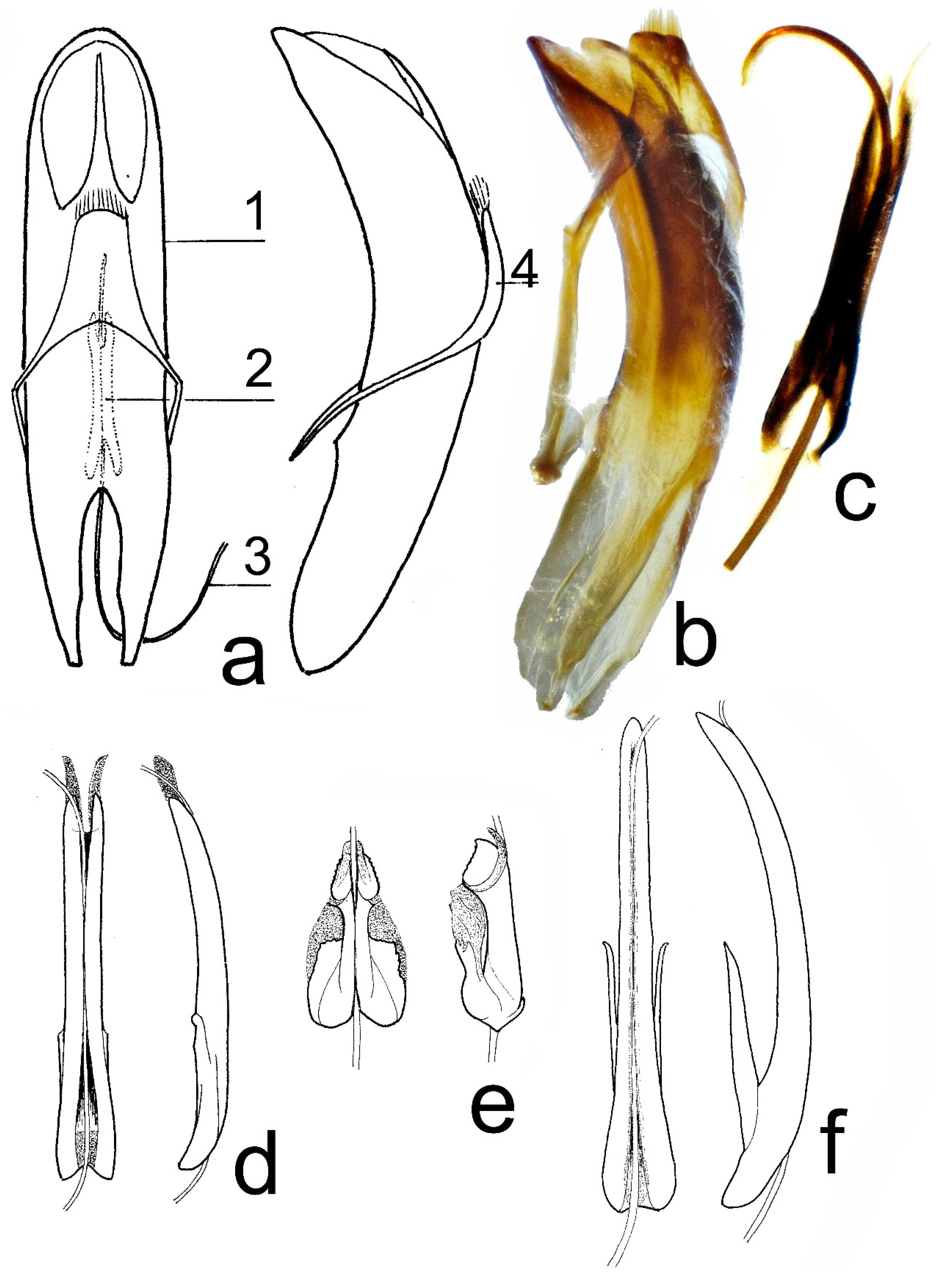
3.3.12. Shape of Aedeagus Apex in Lateral View (Recurved Dorsally, Evenly Curved)
Shape of Aedeagus Apex in Lateral View (Recurved Dorsally, Evenly Curved) (
Table 14).
Comparison of T. corinthia, T. metallica, and T. hummelii.
There is no clear interspecific difference (hiatus) by this character. All available males of T. corinthia have state 2, as most of T. hummelii males, while the majority of T. metallica males has state 1.
3.5. Key to Species (T. gibba Is Not Included There Because Its Taxonomical Position Is Unclear)
1. Species from Western Europe. Border at upper margin of elytral epipleura near the base mostly present...2.
–Species from the Caucasus and Asia Minor. Border at upper margin of elytral epipleura near the base is usually absent, rarely present. Male genitalia: manubrium 1.22–1.42 mm long, without distinct wings, narrow, elongate. Body 6.86–12.46 mm long. Dorsum usually violet or golden coppery; femora usually piceous, rarely rufous or black, tarsi usually rufous or piceous. The apex of aedeagus is mostly evenly curved...T. hummelii.
2. Male genitalia: manubrium 1.71–1.94 mm long, with long paired basal wings, narrow, elongate, broadest at the base, and gradually narrowed from the base to apex. Body 9.08–12.77 mm long. The dorsum is usually bronze, the femora is mostly black, and the tarsi black. The apex of aedeagus is evenly curved...T. corinthia.
–Male genitalia: manubrium 0.74–0.86 mm long, without distinct wings, broadest and quadrangular (in dorsal view) in basal ½. Body 6.86–10.50 mm long. The dorsum is usually bronze or blackish bronze, the femora is usually piceous, rarely rufous or black, the tarsi is usually piceous or black. The apex of aedeagus mostly recurved dorsally...T. metallica.