Silencing of Aquaporin Homologue Accumulates Uric Acid and Decreases the Lifespan of the Asian Citrus Psyllid, Diaphorina citri (Hemiptera: Liviidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diaphorina citri Colonies
2.2. In Silico Analysis
2.3. Double-Stranded DNA Synthesis
2.4. Administering of dsRNA
2.5. Gene Expression Using Semi-Quantitative RT-PCR
2.6. Gene Expression Using Quantitative PCR
2.7. Insect Bioassays
2.7.1. Mortality and Survival Assay
2.7.2. Body Length and Weight
2.8. Metabolomic Assay
2.8.1. Collection and Extraction of D. citri Metabolites
2.8.2. MSTFA Derivatizations
2.8.3. MCF Derivatizations
3. Results
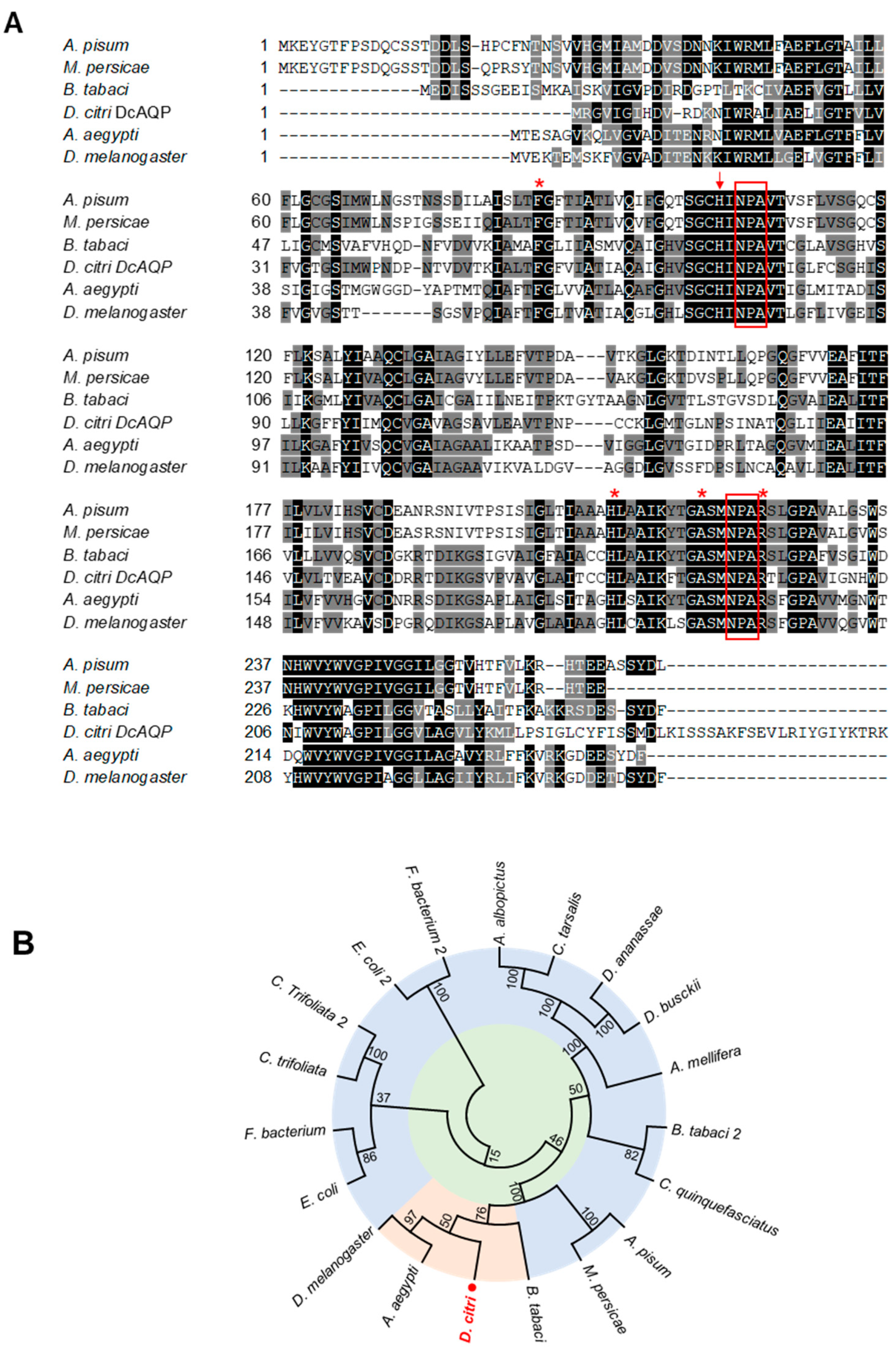
3.1. BLASTp and Phylogenitic Analysis of Candidate Aquaporin Homologs of D. citri
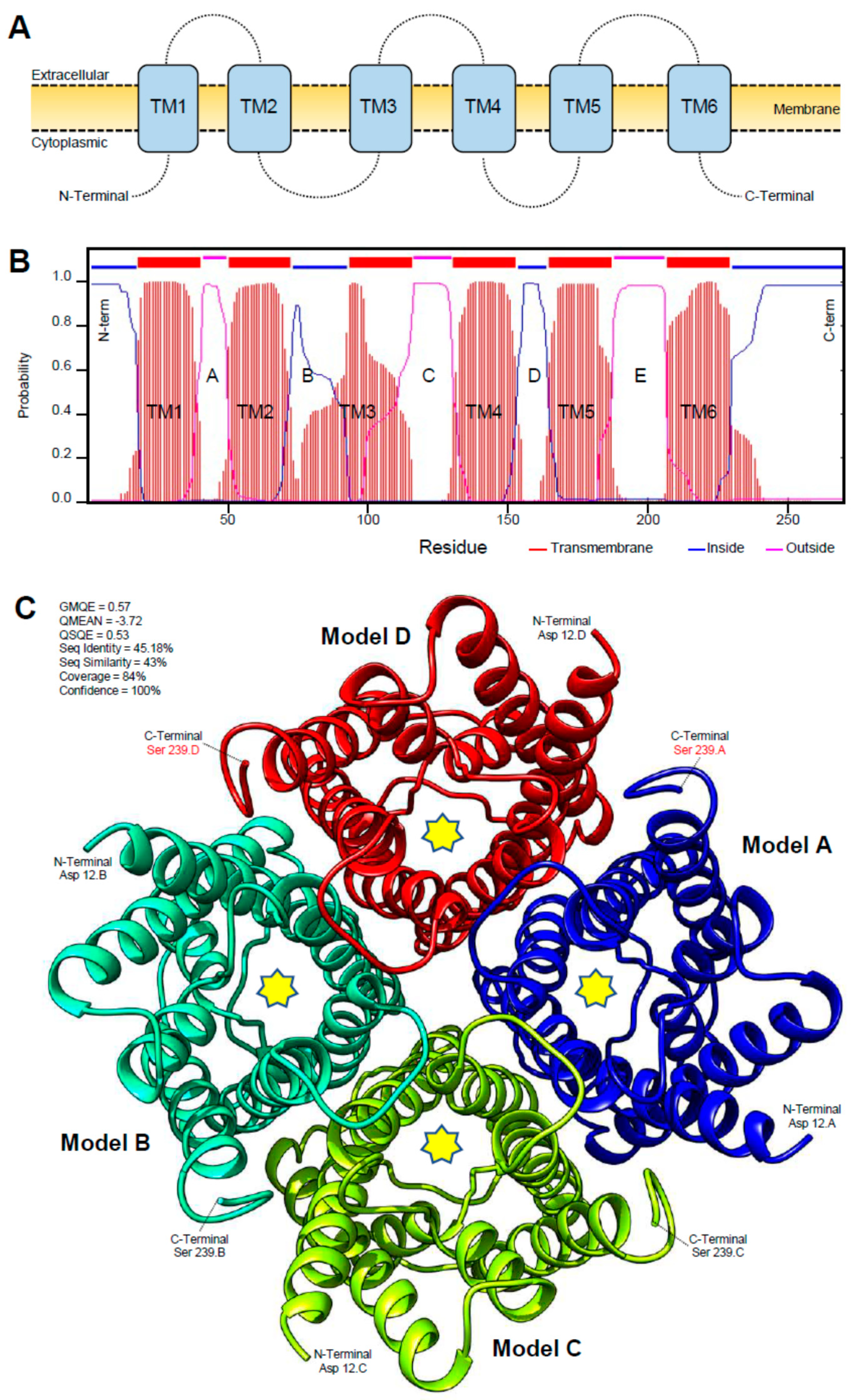
3.2. Protein Characterization of Candidate Aquaporin Homologs of D. citri
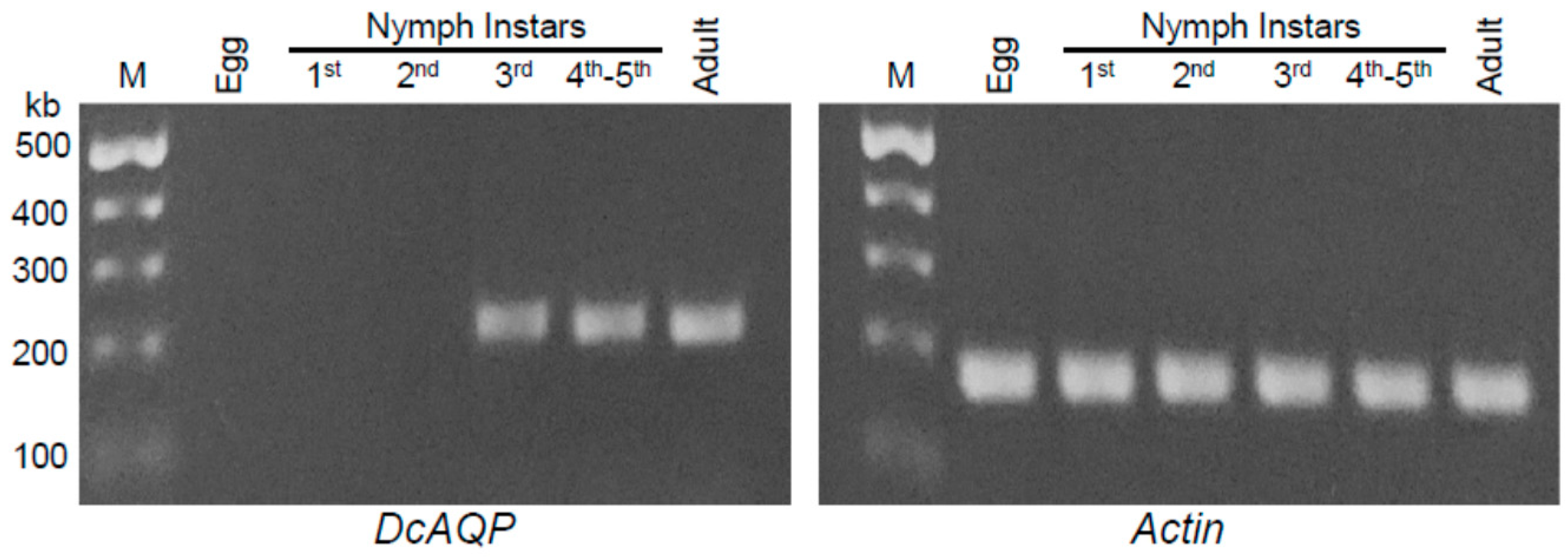
3.3. DcAQP Is Highly Expressed at Late Developmental Stages of D. citri
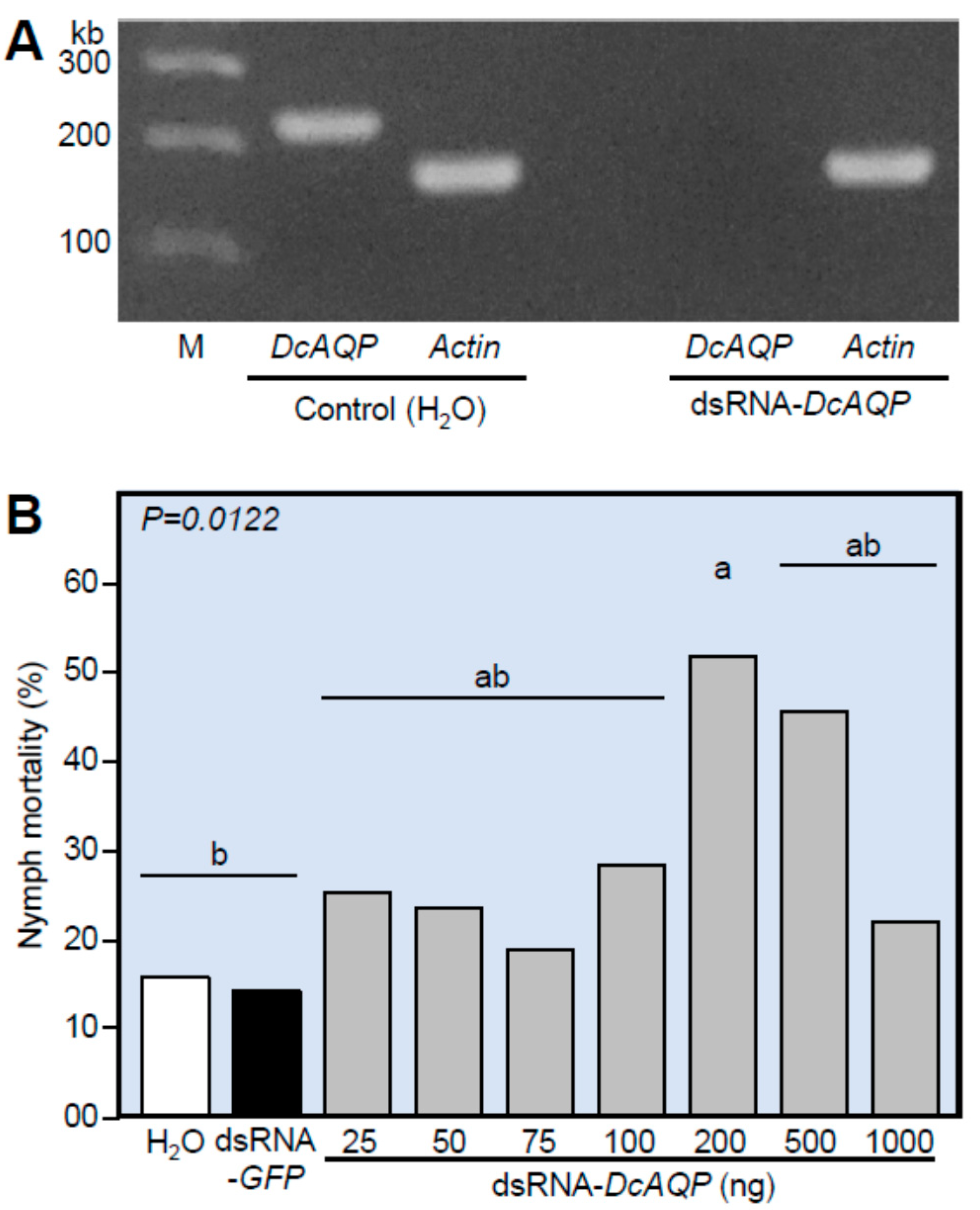
3.4. Application of dsRNA-DcAQP Reduces DcAQP Expression and Increases the Nymphal Mortality
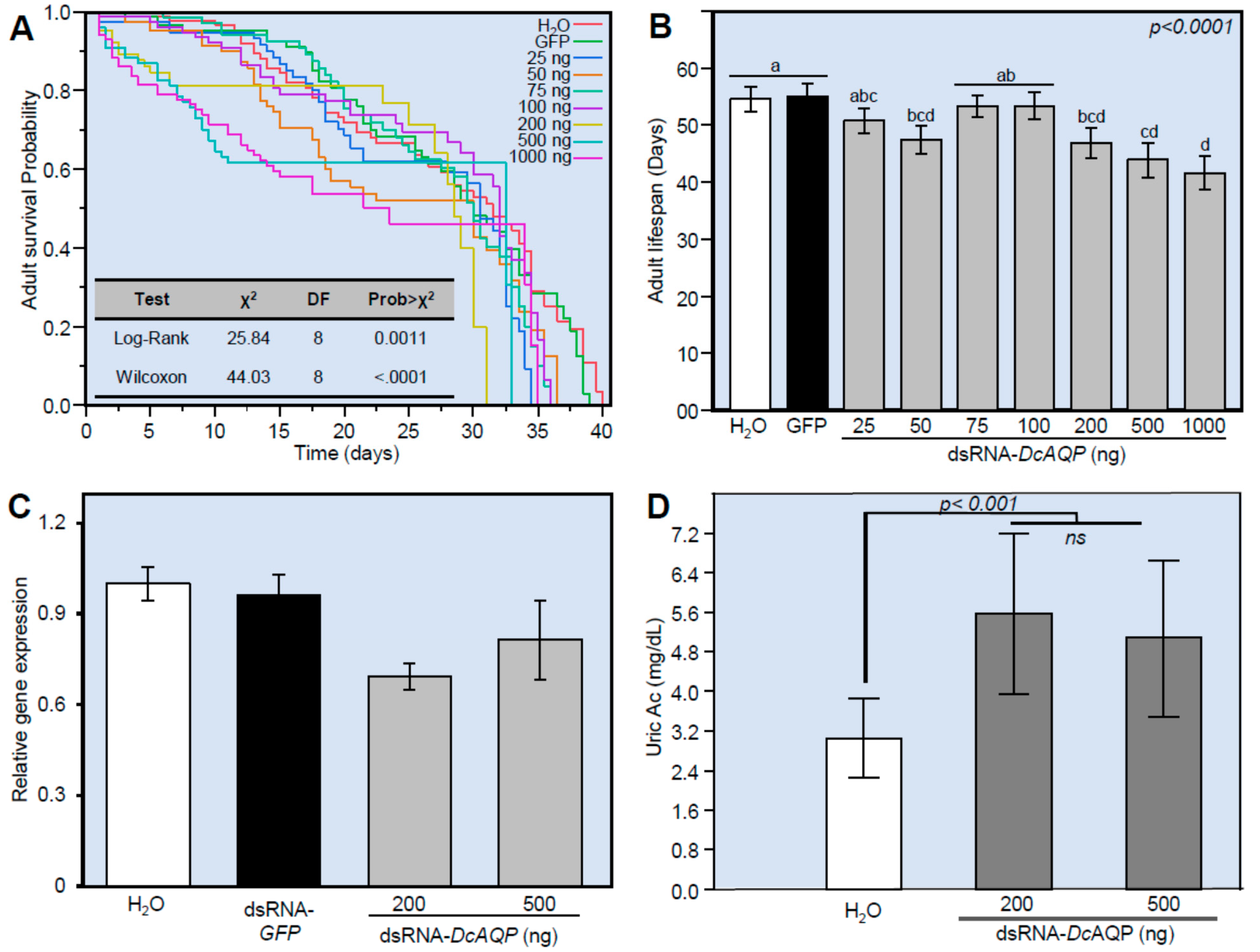
3.5. DcAQP Silencing Decreases the Survival, Down-Regulates DcAQP and Accumulates Uric Acid in Emerged Adult D. citri
3.6. DcAQP Silencing Alters the Body Weight and Length of Adults Emerged from Treated Nymphs
3.7. Application with dsRNA-DcAQP Alters the Metabolite Profile in Adults Emerged from Treated Nymphs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, E.M.; Ball, A.; Hoppler, S.; Bowman, A.S. Invertebrate aquaporins: A review. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2008, 178, 935–955. [Google Scholar] [CrossRef]
- Van Ekert, E.; Chauvigne, F.; Finn, R.N.; Mathew, L.G.; Hull, J.J.; Cerda, J.; Fabrick, J.A. Molecular and functional characterization of Bemisia tabaci aquaporins reveals the water channel diversity of hemipteran insects. Insect Biochem. Mol. Biol. 2016, 77, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Zardoya, R. Phylogeny and evolution of the major intrinsic protein family. Biol. Cell 2005, 97, 397–414. [Google Scholar] [CrossRef]
- Chrispeels, M.J.; Agre, P. Aquaporins: Water channel proteins of plant and animal cells. Trends Biochem. Sci. 1994, 19, 421–425. [Google Scholar] [CrossRef]
- Knepper, M.A. The Aquaporin family of water channels. Proc. Natl. Acad. Sci. USA 1994, 91, 6255–6258. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Mitsuoka, K.; Hirai, T.; Walz, T.; Agre, P.; Heymann, J.B.; Engel, A.; Fujiyoshi, Y. Structural determinants of water permeation through aquaporin-1. Nature 2000, 407, 599–605. [Google Scholar] [CrossRef]
- Benga, G. Water Channel Proteins (Later Called Aquaporins) and Relatives: Past, Present, and Future. Iubmb Life 2009, 61, 112–133. [Google Scholar] [CrossRef]
- Finn, R.N.; Cerda, J. Evolution and Functional Diversity of Aquaporins. Biol. Bull. 2015, 229, 6–23. [Google Scholar] [CrossRef]
- Kreida, S.; Tornroth-Horsefield, S. Structural insights into aquaporin selectivity and regulation. Curr. Opin. Struct. Biol. 2015, 33, 126–134. [Google Scholar] [CrossRef]
- Ibanez, F.; Hancock, J.; Tamborindeguy, C. Identification and Expression Analysis of Aquaporins in the Potato Psyllid, Bactericera cockerelli. PLoS ONE 2014, 9. [Google Scholar] [CrossRef][Green Version]
- Cohen, E. Water Homeostasis and Osmoregulation as Targets in the Control of Insect Pests. In Target Receptors in the Control of Insect Pests: Pt I; Cohen, E., Ed.; Elsevier: Oxford, UK, 2013; Volume 44, pp. 1–61. [Google Scholar]
- Spring, J.H.; Robichaux, S.R.; Hamlin, J.A. The role of aquaporins in excretion in insects. J. Exp. Biol. 2009, 212, 358–362. [Google Scholar] [CrossRef]
- Ashford, D.A.; Smith, W.A.; Douglas, A.E. Living on a high sugar diet: The fate of sucrose ingested by a phloem-feeding insect, the pea aphid Acyrthosiphon pisum. J. Insect Physiol. 2000, 46, 335–341. [Google Scholar] [CrossRef]
- Karley, A.J.; Ashford, D.A.; Minto, L.M.; Pritchard, J.; Douglas, A.E. The significance of gut sucrase activity for osmoregulation in the pea aphid, Acyrthosiphon pisum. J. Insect Physiol. 2005, 51, 1313–1319. [Google Scholar] [CrossRef]
- Douglas, A.E. Phloem-sap feeding by animals: Problems and solutions. J. Exp. Bot. 2006, 57, 747–754. [Google Scholar] [CrossRef]
- Shakesby, A.J.; Wallace, I.S.; Isaacs, H.V.; Pritchard, J.; Roberts, D.M.; Douglas, A.E. A water-specific aquaporin involved in aphid osmoregulation. Insect Biochem. Mol. Biol. 2009, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Santos-Ortega, Y.; Killiny, N. Silencing of sucrose hydrolase causes nymph mortality and disturbs adult osmotic homeostasis in Diaphorina citri (Hemiptera: Liviidae). Insect Biochem. Mol. Biol. 2018, 101, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Mathew, L.G.; Campbell, E.M.; Yool, A.J.; Fabrick, J.A. Identification and characterization of functional aquaporin water channel protein from alimentary tract of whitefly, Bemisia tabaci. Insect Biochem. Mol. Biol. 2011, 41, 178–190. [Google Scholar] [CrossRef]
- Cicero, J.M.; Brown, J.K.; Roberts, P.D.; Stansly, P.A. The Digestive System of Diaphorina citri and Bactericera cockerelli (Hemiptera: Psyllidae). Ann. Entomol. Soc. Am. 2009, 102, 650–665. [Google Scholar] [CrossRef]
- Guillam, M.T.; Beuron, F.; Grandin, N.; Hubert, J.F.; Boisseau, C.; Cavalier, A.; Couturier, A.; Gouranton, J.; Thomas, D. Expression of RNA isolated from the water-shunting comples of a sap-sucking insect increases the membrane permeability for water in Xenopus oocytes. Exp. Cell Res. 1992, 200, 301–305. [Google Scholar] [CrossRef]
- LeCaherec, F.; Guillam, M.T.; Beuron, F.; Cavalier, A.; Thomas, D.; Gouranton, J.; Hubert, J.F. Aquaporin-related proteins in the filter chamber of homopteran insects. Cell Tissue Res. 1997, 290, 143–151. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Sakamoto, J.M.; Rasgon, J.L. Functional characterization of Aquaporin-like genes in the human bed bug Cimex lectularius. Sci. Rep. 2017, 7, 3214. [Google Scholar] [CrossRef] [PubMed]
- Huvenne, H.; Smagghe, G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. J. Insect Physiol. 2010, 56, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.E.; Scoles, G.A.; Ueti, M.W.; Suarez, C.E.; Adham, F.K.; Guerrero, F.D.; Bastos, R.G. Targeted silencing of the Aquaporin 2 gene of Rhipicephalus (Boophilus) microplus reduces tick fitness. Parasites Vectors 2015, 8, 618. [Google Scholar] [CrossRef] [PubMed]
- Jagoueix, S.; Bové, J.M.; Garnier, M. The phloem-limited bacterium of greening disease of citrus is a member of the alpha-subdivision of the proteobacteria. Int. J. Syst. Bacteriol. 1994, 44, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Bové, J.M. Huanglongbing: A destructive, newly emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Boina, D.R.; Bloomquist, J.R. Chemical control of the Asian citrus psyllid and of huanglongbing disease in citrus. Pest Manag. Sci. 2015, 71, 808–823. [Google Scholar] [CrossRef]
- Vanaclocha, P.; Jones, M.M.; Tansey, J.A.; Monzo, C.; Chen, X.L.; Stansly, P.A. Residual toxicity of insecticides used against the Asian citrus psyllid and resistance management strategies with thiamethoxam and abamectin. J. Pest Sci. 2019, 92, 871–883. [Google Scholar] [CrossRef]
- El-Shesheny, I.; Hajeri, S.; El-Hawary, I.; Gowda, S.; Killiny, N. Silencing Abnormal Wing Disc Gene of the Asian Citrus Psyllid, Diaphorina citri Disrupts Adult Wing Development and Increases Nymph Mortality. PLoS ONE 2013, 8, e65392. [Google Scholar] [CrossRef]
- Galdeano, D.M.; Breton, M.C.; Lopes, J.R.S.; Falk, B.W.; Machado, M.A. Oral delivery of double-stranded RNAs induces mortality in nymphs and adults of the Asian citrus psyllid, Diaphorina citri. PLoS ONE 2017, 12, e0171847. [Google Scholar] [CrossRef] [PubMed]
- Killiny, N.; Kishk, A. Delivery of dsRNA through topical feeding for RNA interference in the citrus sap piercing-sucking hemipteran, Diaphorina citri. Arch. Insect Biochem. Physiol. 2017, 95, e21394. [Google Scholar] [CrossRef]
- Kishk, A.; Anber, H.A.I.; AbdEl-Raof, T.K.; El-Sherbeni, A.H.D.; Hamed, S.; Gowda, S.; Killiny, N. RNA interference of carboxyesterases causes nymph mortality in the Asian citrus psyllid, Diaphorina citri. Arch. Insect Biochem. Physiol. 2017, 94, e21377. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Gowda, S.; Killiny, N. Double-stranded RNA delivery through soaking mediates silencing of the muscle protein 20 and increases mortality to the Asian citrus psyllid, Diaphorina citri. Pest Manag. Sci. 2017, 73, 1846–1853. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Gondhalekar, A.D.; Mann, R.S.; Scharf, M.E.; Stelinski, L.L. Characterization of five CYP4 genes from Asian citrus psyllid and their expression levels in Candidatus Liberibacter asiaticus- infected and uninfected psyllids. Insect Mol. Biol. 2011, 20, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Liu, Y.H. Biology of Diaphorina citri (Homoptera: Psyllidae) on Four Host Plants. J. Econ. Entomol. 2000, 93, 1721–1725. [Google Scholar] [CrossRef] [PubMed]
- Killiny, N.; Nehela, Y. Metabolomic Response to Huanglongbing: Role of Carboxylic Compounds in Citrus sinensis Response to ’Candidatus Liberibacter asiaticus’ and Its Vector, Diaphorina citri. Mol. Plant Microbe Interact. 2017, 30, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Hijaz, F.; Killiny, N. Collection and chemical composition of phloem sap from Citrus sinensis L. Osbeck (Sweet Orange). PLoS ONE 2014, 9, e101830. [Google Scholar] [CrossRef]
- Killiny, N. Generous hosts: What makes Madagascar periwinkle (Catharanthus roseus) the perfect experimental host plant for fastidious bacteria? Plant Physiol. Biochem. 2016, 109, 28–35. [Google Scholar] [CrossRef]
- Yin, C.L.; Shen, G.Y.; Guo, D.H.; Wang, S.P.; Ma, X.Z.; Xiao, H.M.; Liu, J.D.; Zhang, Z.; Liu, Y.; Zhang, Y.Q.; et al. InsectBase: A resource for insect genomes and transcriptomes. Nucleic Acids Res. 2016, 44, D801–D807. [Google Scholar] [CrossRef]
- Yavuz, C.; Öztürk, Z.N. Working with Proteins in silico: A Review of Online Available Tools for Basic Identification of Proteins. Turk. J. Agric. Food Sci. Tecnol. 2017, 5, 65. [Google Scholar] [CrossRef][Green Version]
- Ishibashi, K.; Kondo, S.; Hara, S.; Morishita, Y. The evolutionary aspects of aquaporin family. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R566–R576. [Google Scholar] [CrossRef]
- Maruyama, M.; Kambara, K.; Naka, H.; Azuma, M. Insect Water-Specific Aquaporins in Developing Ovarian Follicles of the Silk Moth Bombyx mori: Role in Hydration during Egg Maturation. Biol. Bull. 2015, 229, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.A.; Chen, Q.G.; Luan, J.B.; Chung, S.H.; Van Eck, J.; Turgeon, R.; Douglas, A.E. Towards an understanding of the molecular basis of effective RNAi against a global insect pest, the whitefly Bemisia tabaci. Insect Biochem. Mol. Biol. 2017, 88, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Meyering-Vos, M.; Muller, A. RNA interference suggests sulfakinins as satiety effectors in the cricket Gryllus bimaculatus. J. Insect Physiol. 2007, 53, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Taning, C.N.T.; Andrade, E.C.; Hunter, W.B.; Christiaens, O.; Smagghe, G. Asian Citrus Psyllid RNAi Pathway—RNAi evidence. Sci. Rep. 2016, 6, 38082. [Google Scholar] [CrossRef] [PubMed]
- Zotti, M.; dos Santos, E.A.; Cagliari, D.; Christiaens, O.; Taning, C.N.T.; Smagghe, G. RNA interference technology in crop protection against arthropod pests, pathogens and nematodes. Pest Manag. Sci. 2018, 74, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.D.; Killiny, N. Effect of silencing a boule homologue on the survival and reproduction of Asian citrus psyllid Diaphorina citri. Physiol. Entomol. 2018, 43, 268–275. [Google Scholar] [CrossRef]
- Jaubert-Possamai, S.; Le Trionnaire, G.; Bonhomme, J.; Christophides, G.K.; Rispe, C.; Tagu, D. Gene knockdown by RNAi in the pea aphid Acyrthosiphon pisum. BMC Biotechnol. 2007, 7, 1–8. [Google Scholar] [CrossRef]
- Shukla, J.N.; Kalsi, M.; Sethi, A.; Narva, K.E.; Fishilevich, E.; Singh, S.; Mogilicherla, K.; Palli, S.R. Reduced stability and intracellular transport of dsRNA contribute to poor RNAi response in lepidopteran insects. RNA Biol. 2016, 13, 656–669. [Google Scholar] [CrossRef]
- Killiny, N.; Hajeri, S.; Tiwari, S.; Gowda, S.; Stelinski, L.L. Double-Stranded RNA Uptake through Topical Application, Mediates Silencing of Five CYP4 Genes and Suppresses Insecticide Resistance in Diaphorina citri. PLoS ONE 2014, 9, e110536. [Google Scholar] [CrossRef]
- Lu, M.X.; Pan, D.D.; Xu, J.; Liu, Y.; Wang, G.R.; Du, Y.Z. Identification and Functional Analysis of the First Aquaporin from Striped Stem Borer, Chilo suppressalis. Front. Physiol. 2018, 9, 57. [Google Scholar] [CrossRef]
- George, J.; Ammar, E.; Hall, D.G.; Shatters, R.G.; Lapointe, S.L. Prolonged phloem ingestion by Diaphorina citri nymphs compared to adults is correlated with increased acquisition of citrus greening pathogen. Sci. Rep. 2018, 8, 10352. [Google Scholar] [CrossRef]
- Jungreis, A.M. Physiology and composition of molting fluid and midgut lumenal contents in silkmoth Hyalophora cecropia. J. Comp. Physiol. 1974, 88, 113–127. [Google Scholar] [CrossRef]
- Lee, J.; Kiuchi, T.; Kawamoto, M.; Shimada, T.; Katsuma, S. Accumulation of uric acid in the epidermis forms the white integument of Samia ricini larvae. PLoS ONE 2018, 13, e0205758. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.J.; Maddrell, S.H. Paracellular and transcellular routes for water and solute movements across insect epithelia. J Exp Biol 1983, 106, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Pant, R. Nitrogen excretion in insects. Proc. Anim. Sci. 1988, 97, 379–415. [Google Scholar] [CrossRef]
- Spring, J.H.; Hazelton, S.R. Excretion in the house cricket (Acheta domesticus) Stimulation of diuresis by tissue homogenates. J. Exp. Biol. 1987, 129, 63–81. [Google Scholar] [CrossRef]

| Gene | Primer Name | Primer Orination | Primer SEQUENCE | Amplicon Size | Application |
|---|---|---|---|---|---|
| DcAQP | DcAQP | F: | TTGGTCACGTGAGTGGATGT | 306 | cDNA fragment amplification |
| R: | CTGGCACCGAACCCTTAATA | ||||
| DcAQP-T7 b | F: | *TTGGTCACGTGAGTGGATGT | 346 | dsRNA synthesis | |
| R: | *CTGGCACCGAACCCTTAATA | ||||
| DcAQPSRT- | F: | CCTGCTGTCACCATTGGTCT | 234 | Semi RT-PCR | |
| R: | GGCTTCCACCGTTAGAACCA | ||||
| DcAQP-RT | F: | CTCGAAGCTGTCACACCAAA | 129 | q RT-PCR | |
| R: | GGCTTCCACCGTTAGAACCA | ||||
| gfp | gfp-T7 b | F: | *TTTTCAAGAGTGCCATGCCC | 341 | dsRNA synthesis |
| R: | *TGGTAAAAGGACAGGGCCAT | ||||
| gfp-RT | F: | GACGGGACCTACAAGACACG | 165 | q RT-PCR | |
| R: | TTGTTTGTCTGCCGTGATGT | ||||
| Actinc | Act-RT | F: | CCCTGGACTTTGAACAGGAA | 170 | q RT-PCR |
| R: | AATCTTGCGGTATCCACGAG |
| Component | RT a (min) | Concentration (Means ± std) | p-Value b | |
|---|---|---|---|---|
| Control (n = 8) | dsRNA-AQP (n = 8) | |||
| Pyruvic Acid d | 4.30 | 0.732 ± 0.006 | 0.739 ± 0.007 | 0.159 |
| L-Alanine d | 5.02 | 0.872 ± 0.320 | 2.598 ± 0.322 | 0.003 |
| Oxalic acid d | 5.13 | 0.761 ± 0.050 | 0.916 ± 0.030 | 0.002 |
| Phosphoric acid d | 7.13 | 6.673 ± 3.272 | 16.214 ± 6.202 | 0.028 |
| L-Isoleucine d | 7.43 | 0.731 ± 0.040 | 0.722 ± 0.004 | 0.684 |
| L-Glycine d | 7.53 | 0.838 ± 0.094 | 0.930 ± 0.217 | 0.456 |
| L-Threonine d | 7.57 | 0.782 ± 0.063 | 1.220 ± 0.114 | 0.001 |
| Threose d | 10.02 | 0.728 ± 0.023 | 0.735 ± 0.025 | 0.673 |
| 2-Piperidinecarboxylic acid d | 10.25 | 3.787 ± 2.064 | 10.923 ± 1.657 | 0.002 |
| β-Alanine d | 10.43 | 0.896 ± 0.154 | 1.245 ± 0.071 | 0.009 |
| 2-Ketoglutaric acid d | 10.97 | 0.801 ± 0.001 | 0.820 ± 0.018 | 0.137 |
| Arabinopyranose d | 11.63 | 0.704 ± 0.008 | 0.698 ± 0.004 | 0.236 |
| Xylose d | 11.99 | 0.734 ± 0.003 | 0.814 ± 0.016 | 0.001 |
| Arabinose d | 12.09 | 0.695 ± 0.003 | 0.715 ± 0.005 | 0.001 |
| Xylose 2 d | 12.19 | 0.692 ± 0.001 | 0.685 ± 0.001 | 0.718 |
| Unknown sugar alcohol-1 c | 12.25 | 0.712 ± 0.014 | 0.713 ± 0.012 | 0.949 |
| β-L-Arabinopyranose d | 12.44 | 0.715 ± 0.015 | 0.722 ± 0.016 | 0.551 |
| Arabitol/Xylitol e | 12.50 | 0.714 ± 0.015 | 0.719 ± 0.015 | 0.586 |
| Putrescine d | 12.64 | 0.888 ± 0.016 | 1.123 ± 0.062 | 0.002 |
| Sorbose-1 d | 12.91 | 0.817 ± 0.076 | 0.804 ± 0.060 | 0.785 |
| β-Glycerophosphate d | 12.95 | 0.785 ± 0.059 | 1.559 ± 0.081 | 0.001 |
| α-D-Mannopyranoside d | 13.09 | 0.708 ± 0.011 | 0.704 ± 0.007 | 0.544 |
| Fructofuranose-1 d | 13.23 | 0.713 ± 0.014 | 0.724 ± 0.019 | 0.383 |
| Citric acid d | 13.53 | 3.649 ± 0.020 | 6.388 ± 0.809 | 0.002 |
| Unknown sugar c | 14.05 | 0.902 ± 0.127 | 1.079 ± 0.047 | 0.039 |
| Unknown sugar c | 14.15 | 0.925 ± 0.021 | 0.816 ± 0.029 | 0.003 |
| Glucose d | 14.37 | 50.404 ± 1.517 | 146.530 ± 1.805 | 0.001 |
| chiro-Inositol d | 14.52 | 10.980 ± 0.695 | 30.402 ± 1.805 | 0.001 |
| Galactitol d | 14.68 | 0.725 ± 0.021 | 0.731 ± 0.066 | 0.854 |
| Glucose-2 d | 14.71 | 1.542 ± 0.058 | 2.808 ± 0.269 | 0.001 |
| Mannitol d | 14.79 | 1.245 ± 0.062 | 1.276 ± 0.073 | 0.523 |
| scyllo-Inositol d | 14.86 | 0.761 ± 0.042 | 0.793 ± 0.055 | 0.373 |
| Unknown sugar alcohol-2 c | 15.58 | 0.756 ± 0.037 | 0.994 ± 0.047 | 0.001 |
| Glucuronic Acid d | 15.63 | 0.721 ± 0.020 | 1.455 ± 0.108 | 0.001 |
| Palmitoleic Acid d | 15.67 | 0.791 ± 0.061 | 0.796 ± 0.060 | 0.914 |
| myo-Inositol d | 16.24 | 15.543 ± 0.953 | 27.154 ± 1.288 | 0.001 |
| Oleic Acid d | 17.69 | 1.796 ± 0.118 | 6.300 ± 0.647 | 0.001 |
| Stearic Acid d | 17.99 | 0.787 ± 0.095 | 0.828 ± 0.080 | 0.535 |
| Unknown sugar alcohol-3 c | 20.83 | 1.022 ± 0.188 | 0.993 ± 0.145 | 0.802 |
| Sucrose d | 21.58 | 1.715 ± 0.162 | 1.881 ± 0.259 | 0.305 |
| Trehalose d | 22.54 | 109.770 ± 5.943 | 103.000 ± 3.623 | 0.167 |
| Component a | RT b (min) | Concentration (Means ± std) | p-Value c | |
|---|---|---|---|---|
| Control (n= 5) | dsRNA-AQP (n= 12) | |||
| L-Alanine | 8.96 | 4.195 ± 0.939 | 3.871 ± 0.376 | 0.312 |
| γ-Aminobutyric acid | 9.38 | 6.081 ± 0.972 | 6.008 ± 0.824 | 0.877 |
| L-Iso-leucine | 12.18 | 0.616 ± 0.231 | 0.595 ± 0.179 | 0.853 |
| L-Threonine | 12.36 | 0.590 ± 0.184 | 0.630 ± 0.068 | 0.510 |
| L-Proline | 12.84 | 2.034 ± 0.193 | 1.599 ± 0.120 | 0.001 |
| L-Glutamic Acid | 15.37 | 23.169 ± 5.622 | 16.735 ± 3.431 | 0.015 |
| L-Methionine | 15.55 | 0.368 ± 0.110 | 0.403 ± 0.078 | 0.492 |
| L-Cysteine | 15.61 | 1.219 ± 0.494 | 1.809 ± 1.479 | 0.456 |
| L-Phenylalanine | 16.89 | 0.682 ± 0.250 | 0.991 ± 0.179 | 0.011 |
| L-Histidine | 17.67 | 5.014 ± 0.931 | 2.618 ± 1.477 | 0.009 |
| L-Tyrosine | 18.96 | 10.354 ± 1.214 | 10.694 ± 2.160 | 0.749 |
| Palmitic acid (C16:0) | 19.15 | 0.135 ± 0.020 | 0.153 ± 0.025 | 0.174 |
| Heptadecanoic acid (C17:0) | 20.14 | 0.008 ± 0.002 | 0.0168 ± 0.006 | 0.019 |
| Linoleic acid (C18:2) | 20.83 | 1.042 ± 0.091 | 1.035 ± 0.230 | 0.952 |
| Oleic Acid (C18:1) | 20.91 | 1.789 ± 0.164 | 1.789 ± 0.164 | 0.676 |
| Stearic acid (C18:0) | 21.11 | 0.148 ± 0.013 | 0.138 ± 0.015 | 0.203 |
| Maleic acid | 23.45 | 245.810 ± 66.875 | 190.551 ± 11.715 | 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Ortega, Y.; Killiny, N. Silencing of Aquaporin Homologue Accumulates Uric Acid and Decreases the Lifespan of the Asian Citrus Psyllid, Diaphorina citri (Hemiptera: Liviidae). Insects 2021, 12, 931. https://doi.org/10.3390/insects12100931
Santos-Ortega Y, Killiny N. Silencing of Aquaporin Homologue Accumulates Uric Acid and Decreases the Lifespan of the Asian Citrus Psyllid, Diaphorina citri (Hemiptera: Liviidae). Insects. 2021; 12(10):931. https://doi.org/10.3390/insects12100931
Chicago/Turabian StyleSantos-Ortega, Yulica, and Nabil Killiny. 2021. "Silencing of Aquaporin Homologue Accumulates Uric Acid and Decreases the Lifespan of the Asian Citrus Psyllid, Diaphorina citri (Hemiptera: Liviidae)" Insects 12, no. 10: 931. https://doi.org/10.3390/insects12100931
APA StyleSantos-Ortega, Y., & Killiny, N. (2021). Silencing of Aquaporin Homologue Accumulates Uric Acid and Decreases the Lifespan of the Asian Citrus Psyllid, Diaphorina citri (Hemiptera: Liviidae). Insects, 12(10), 931. https://doi.org/10.3390/insects12100931







