Influence of Age and Mating Status on Pheromone Production in a Powderpost Beetle Lyctus africanus (Coleoptera: Lyctinae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Source
2.2. Life Span of Adult Male L. africanus in Starved and Unstarved Conditions
2.3. Effects of Mating Status on Pheromone Production
2.4. Effects of Aging on Pheromone Production
2.5. Chemical Analysis
2.5.1. Collection of Chemical Compounds
2.5.2. GC–MS Analysis
2.5.3. Quantitative Determination of Three Ester Compounds
2.6. Data Analysis
3. Results
3.1. Life Span of Adult Male L. africanus in Starved and Unstarved Conditions
3.2. Effects of Mating Status on Pheromone Production
3.3. Effects of Aging on Pheromone Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebeling, W. Wood-Destroying Insects and Fungi. In Urban Entomology; Division of Agricultural Sciences, University of California: San Diego, CA, USA, 1978; pp. 167–216. [Google Scholar]
- Kliejunas, J.T.; Burdsall, H.H., Jr.; DeNitto, G.A.; Eglitis, A.; Haugen, D.A.; Haverty, M.I.; Micales, J.A.; Tkacz, B.M.; Powell, M.R. Pest Risk Assessment of the Importation into the United States of Unprocessed Logs and Chips of Eighteen Eucalypt Species from Australia. True Powder Post Beetle; USDA Forest Service General Technical Report FPL-GTR-137; Forest Products Laboratory, United States Department of Agriculture: Washington, DC, USA, 2003.
- Liu, L.-Y. New records of Bostrichidae (Insecta: Coleoptera, Bostrichidae, Bostrichinae, Lyctinae, Polycaoninae, Dinoderinae, Apatinae). Mitt. Munch. Ent. Ges. 2010, 100, 103–117. [Google Scholar]
- Liu, L.-Y.; Beaver, R.A. A synopsis of the powderpost beetles of the Himalayas with a key to the genera (Insecta: Coleoptera: Bostrichidae). In Biodiversität und Naturausstattung im Himalaya VI; Hartmann, Barclay & Weipert: Erfurt, Germany, 2018; pp. 407–422. [Google Scholar]
- Liu, L.-Y.; Schönitzer, K. Phylogenetic analysis of the family Bostrichidae auct. at suprageneric levels (Coleoptera: Bostrichidae). Mitt. Munch. Ent. Ges. 2011, 101, 99–132. [Google Scholar]
- Gerberg, E.J. A revision of the new world species of powder-post beetles belonging to the family Lyctidae. Tech. Bull. 1957, 1157. [Google Scholar]
- Halperin, J.; Geis, K.U. Lyctidae (Coleoptera) of Israel, their damage and its prevention. Phytoparasitica 1999, 27, 257–262. [Google Scholar] [CrossRef]
- Furukawa, N.; Yoshimura, T.; Imamura, Y. Survey of lyctine damages on houses in Japan: Identification of species and infested area. Wood Preserv. 2009, 35, 260–264. (In Japanese) [Google Scholar] [CrossRef]
- Khalsa, H.; Nigam, B.; Agarwal, P. Breeding of powder post beetle, Lyctus africanus Lense (Coleoptera) in an artificial medium. Indian J. Entomol. 1962, 24, 139–142. [Google Scholar]
- Beroza, M. Microanalytical Methodology Relating to the Identification of Insect Sex Pheromones and Related Behavior-Control Chemicals. J. Chromatogr. Sci. 1975, 13, 314–321. [Google Scholar] [CrossRef]
- Wertheim, B. Ecology of Drosophila Aggregation Pheromone: A Multitrophic Approach; Laboratory of Entomology, Wageningen University: Wageningen, The Netherlands, 2001; p. 200. [Google Scholar]
- Wertheim, B.; van Baalen, E.J.A.; Vet, L.E.M. Pheromone-mediated aggregation in non-social arthropods: An evolutionary ecological perspective. Annu. Rev. Entomol. 2005, 50, 321–346. [Google Scholar] [CrossRef]
- Shorey, H.H. Behavioral Responses to Insect Pheromones. Annu. Rev. Ѐntomol. 1973, 18, 349–380. [Google Scholar] [CrossRef]
- Borden, J.H. Aggregation Pheromones. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; pp. 257–285. [Google Scholar]
- Kartika, T.; Shimizu, N.; Yoshimura, T. Identification of Esters as Novel Aggregation Pheromone Components Produced by the Male Powder-Post Beetle, Lyctus africanus Lesne (Coleoptera: Lyctinae). PLoS ONE 2015, 10, e0141799. [Google Scholar] [CrossRef]
- Papadopoulos, N.T.; Katsoyannos, B.L.; Kouloussis, N.A.; Carey, J.R.; Muller, H.-G.; Zhang, Y. High sexual signaling rates of young individuals predict extended life span in male Mediterranean fruit flies. Oecologia 2004, 138, 127–134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cymorex, V.S.; Schmidt, H. On the gnawing and feeding behavior of powder-post beetles (Lyctidae) with observations on gut anatomy and gut parasites. Mater. Organ. Beih. 1976, 3, 429–440. [Google Scholar]
- Iwata, R.; Nishimoto, K. Studies on the autecology of Lyctus brunneus (Stephens): Artificial diets in relationship to beetle supply. Mokuzai Gakkaishi 1983, 29, 336–343. (In Japanese) [Google Scholar]
- Kartika, T.; Yoshimura, T. Evaluation of Wood and Cellulosic Materials as Fillers in Artificial Diets for Lyctus africanus Lesne (Coleoptera: Bostrichidae). Insects 2015, 6, 696–703. [Google Scholar] [CrossRef]
- Ito, T. Tasting Behavior of Lyctus brunneus Stephens (Coleoptera, Lyctidae). Appl. Entomol. Zool. 1983, 18, 289–292. [Google Scholar] [CrossRef]
- Yinon, U.; Shulov, A. New findings concerning pheromones produced by Trogoderma granarium (Everts), (Coleoptera, Dermestidae). J. Stored Prod. Res. 1967, 3, 251–254. [Google Scholar] [CrossRef]
- Korada, R.R.; Griepink, F.C. Aggregation pheromone compounds of the black larder beetle Dermestes haemorrhoidalis Kuster (Coleoptera: Dermestidae). Chemoecology 2009, 19, 177–184. [Google Scholar] [CrossRef]
- Shimizu, N.; Kuwahara, Y.; Yakumaru, R.; Tanabe, T. n-Hexyl Laurate and Fourteen Related Fatty Acid Esters: New Secretory Compounds from the Julid Millipede, Anaulaciulus sp. J. Chem. Ecol. 2012, 38, 23–28. [Google Scholar] [CrossRef]
- Bong, L.-J.; Neoh, K.-B.; Jaal, Z.; Lee, C.-Y. Life table of Paederus fuscipes (Coleoptera: Staphylinidae). J. Med. Ѐntomol. 2012, 49, 451–460. [Google Scholar] [CrossRef]
- Burkholder, W. Stored-product insect behavior and pheromone studies: Keys to successful monitoring and trapping. In Proceedings of the 3rd International Working Conference of Stored-Product Entomology, Manhattan, KS, YSA, 23–28 October 1983; pp. 20–33. [Google Scholar]
- Gay, F.J. Observation on biology of Lyctus brunneus (Steph). Aust. J. Zool. 1953, 1, 102–110. [Google Scholar] [CrossRef]
- Walgenbach, C.A.; Phillips, J.K.; Faustini, D.L.; Burkholder, W.E. Male-produced aggregation pheromone of the maize weevil, Sitophilus zeamais, and interspecific attraction between three Sitophilus species. J. Chem. Ecol. 1983, 9, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Oppelt, A.; Heinze, J. Mating is associated with immediate changes of the hydrocarbon profile of Leptothorax gredleri ant queens. J. Insect Physiol. 2009, 55, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Happ, G.M.; Wheeler, J. Bioassay, Preliminary Purification, and Effect of Age, Crowding, and Mating on the Release of Sex Pheromone by Female Tenebrio molitor1. Ann. Ѐntomol. Soc. Am. 1969, 62, 846–851. [Google Scholar] [CrossRef]
- Scott, D.; Richmond, R.C.; Carlson, D.A. Pheromones exchanged during mating: A mechanism for mate assessment in Drosophila. Anim. Behav. 1988, 36, 1164–1173. [Google Scholar] [CrossRef]
- Thomas, M.L. Detection of female mating status using chemical signals and cues. Biol. Rev. 2010, 86, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Edde, P.A.; Phillips, T. Longevity and pheromone output in stored-product Bostrichidae. Bull. Ѐntomol. Res. 2006, 96, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.-H.; Yew, J.Y.; Fedina, T.Y.; Dreisewerd, K.; Dierick, H.A.; Pletcher, S.D. Aging modulates cuticular hydrocarbons and sexual attractiveness in Drosophila melanogaster. J. Exp. Biol. 2012, 215, 814–821. [Google Scholar] [CrossRef] [PubMed]
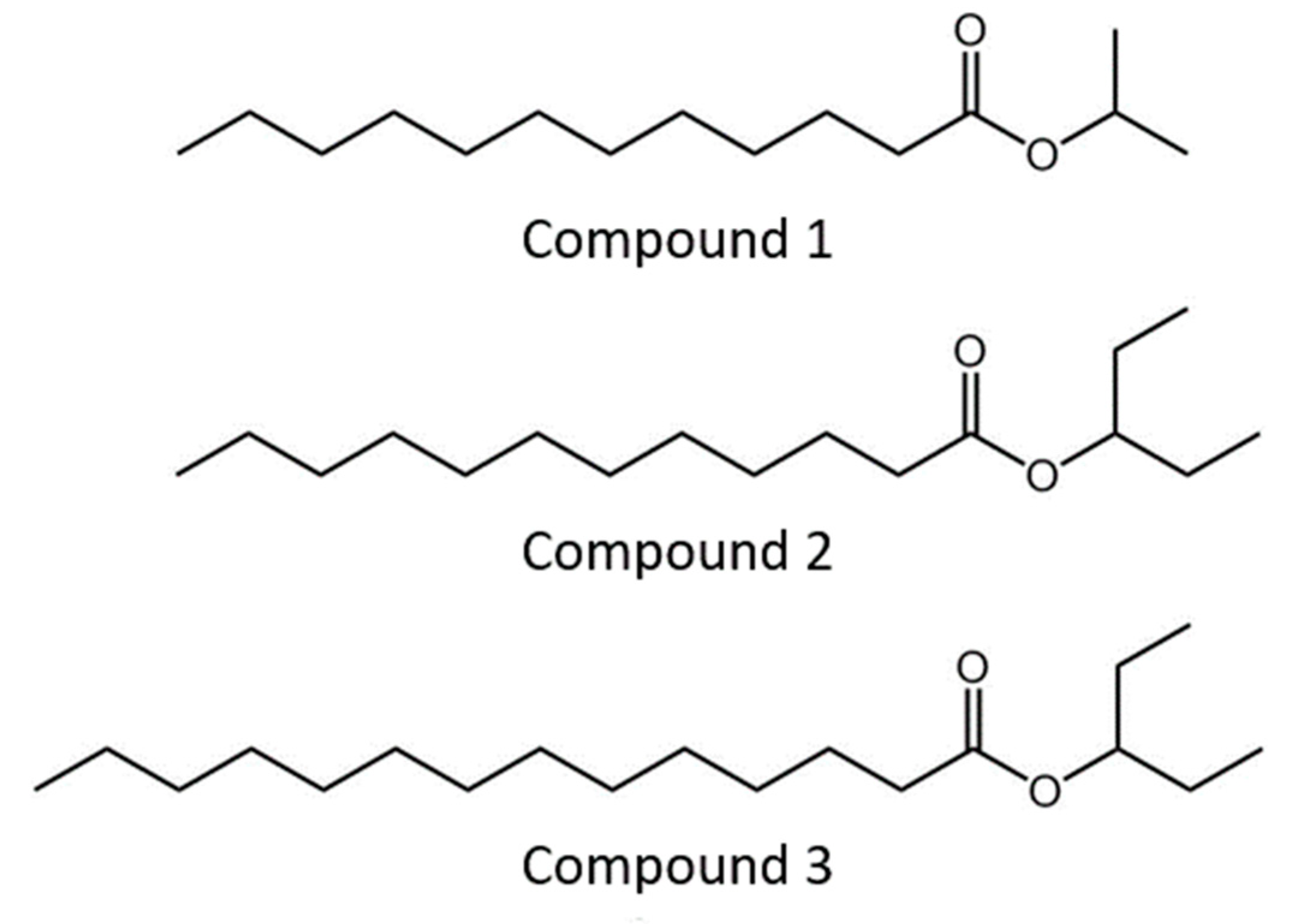


| Time (Weeks) | Pheromone Quantity (ng) | ||
|---|---|---|---|
| Compound 1 | Compound 2 | Compound 3 | |
| 1 | 0.00 ± 0.00 a | 93.14 ± 19.15 b | 2.22 ± 1.93 c |
| 3 | 8.06 ± 2.31 a,* | 142.73 ± 45.31 b | 14.84 ± 6.87 b,c |
| 5 | 21.67 ± 15.04 a | 1185.15 ± 412.60 a | 186.73 ± 56.36 a |
| 9 | 5.84 ± 3.60 a | 376.34 ± 154.50 a,b | 65.07 ± 20.31 a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kartika, T.; Shimizu, N.; Himmi, S.K.; Guswenrivo, I.; Tarmadi, D.; Yusuf, S.; Yoshimura, T. Influence of Age and Mating Status on Pheromone Production in a Powderpost Beetle Lyctus africanus (Coleoptera: Lyctinae). Insects 2021, 12, 8. https://doi.org/10.3390/insects12010008
Kartika T, Shimizu N, Himmi SK, Guswenrivo I, Tarmadi D, Yusuf S, Yoshimura T. Influence of Age and Mating Status on Pheromone Production in a Powderpost Beetle Lyctus africanus (Coleoptera: Lyctinae). Insects. 2021; 12(1):8. https://doi.org/10.3390/insects12010008
Chicago/Turabian StyleKartika, Titik, Nobuhiro Shimizu, Setiawan Khoirul Himmi, Ikhsan Guswenrivo, Didi Tarmadi, Sulaeman Yusuf, and Tsuyoshi Yoshimura. 2021. "Influence of Age and Mating Status on Pheromone Production in a Powderpost Beetle Lyctus africanus (Coleoptera: Lyctinae)" Insects 12, no. 1: 8. https://doi.org/10.3390/insects12010008
APA StyleKartika, T., Shimizu, N., Himmi, S. K., Guswenrivo, I., Tarmadi, D., Yusuf, S., & Yoshimura, T. (2021). Influence of Age and Mating Status on Pheromone Production in a Powderpost Beetle Lyctus africanus (Coleoptera: Lyctinae). Insects, 12(1), 8. https://doi.org/10.3390/insects12010008





