Bioactivity of Different Chemotypes of Oregano Essential Oil against the Blowfly Calliphora vomitoria Vector of Foodborne Pathogens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Essential Oils Extraction and Chemical Analysis
2.3. Calliphora vomitoria Rearing
2.4. Toxicity Bioassays
2.5. Oviposition Deterrence Bioassay
2.6. Data Analyses
3. Results
3.1. Chemical Analysis
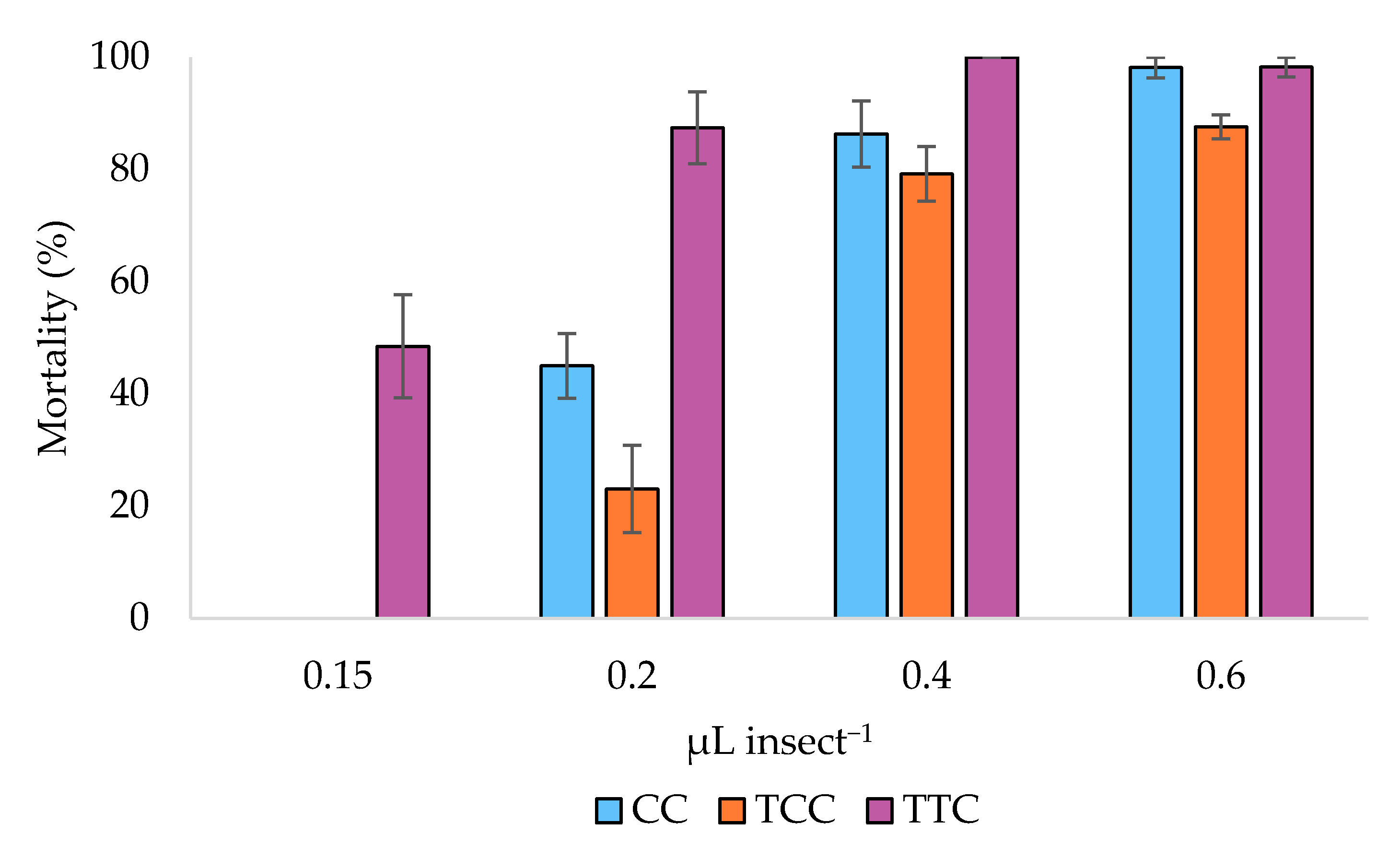
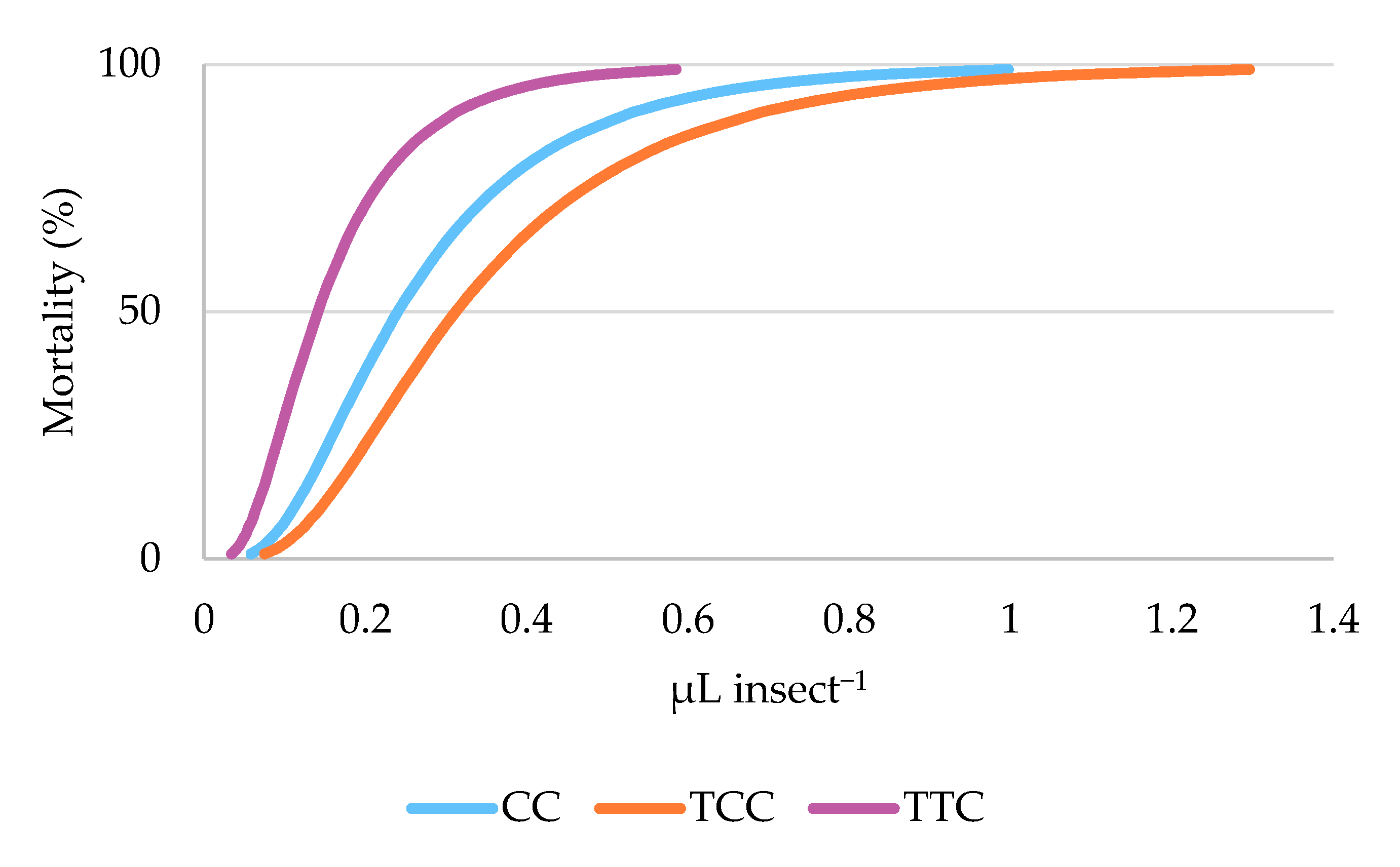
3.2. Toxicity Bioassays
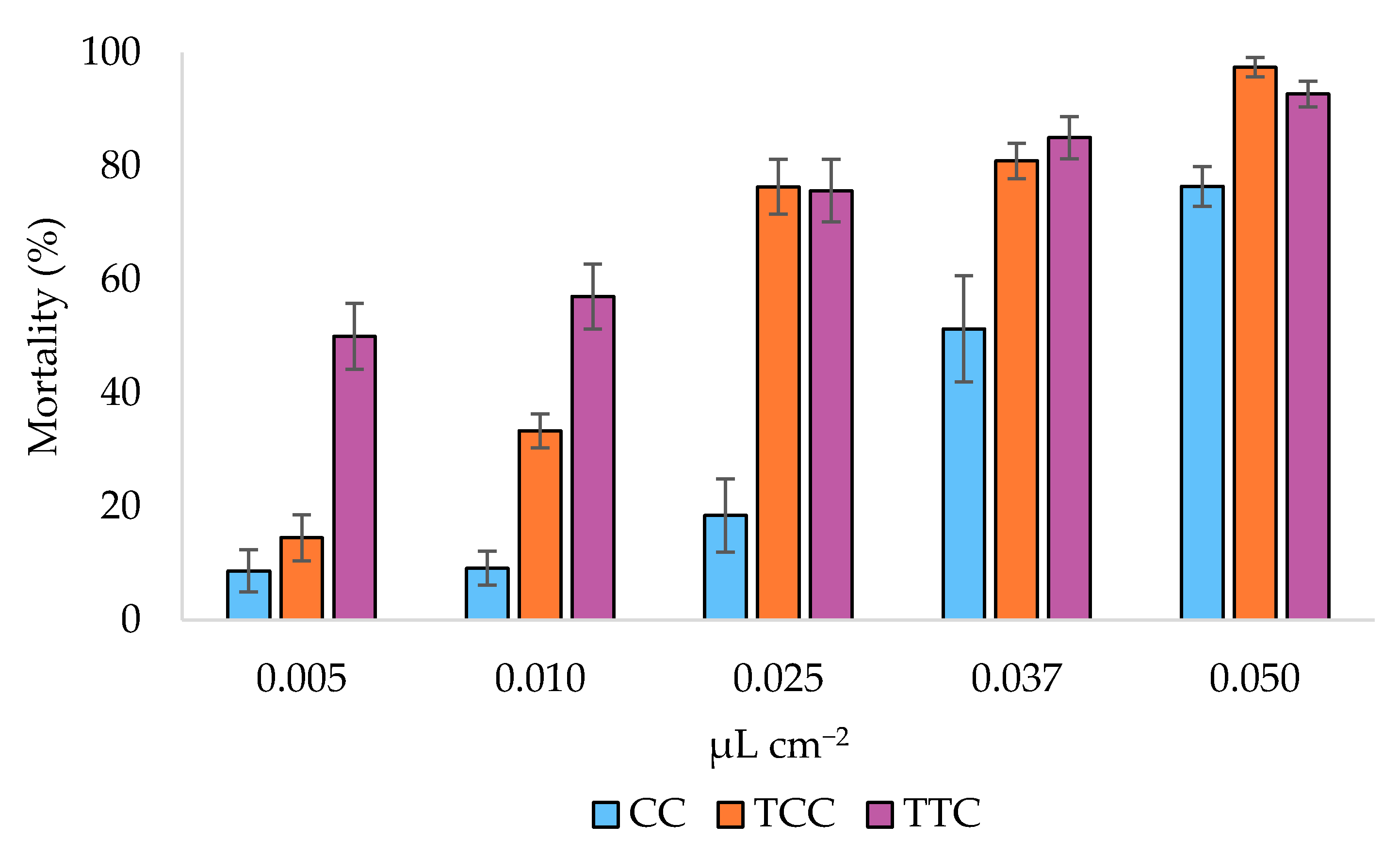
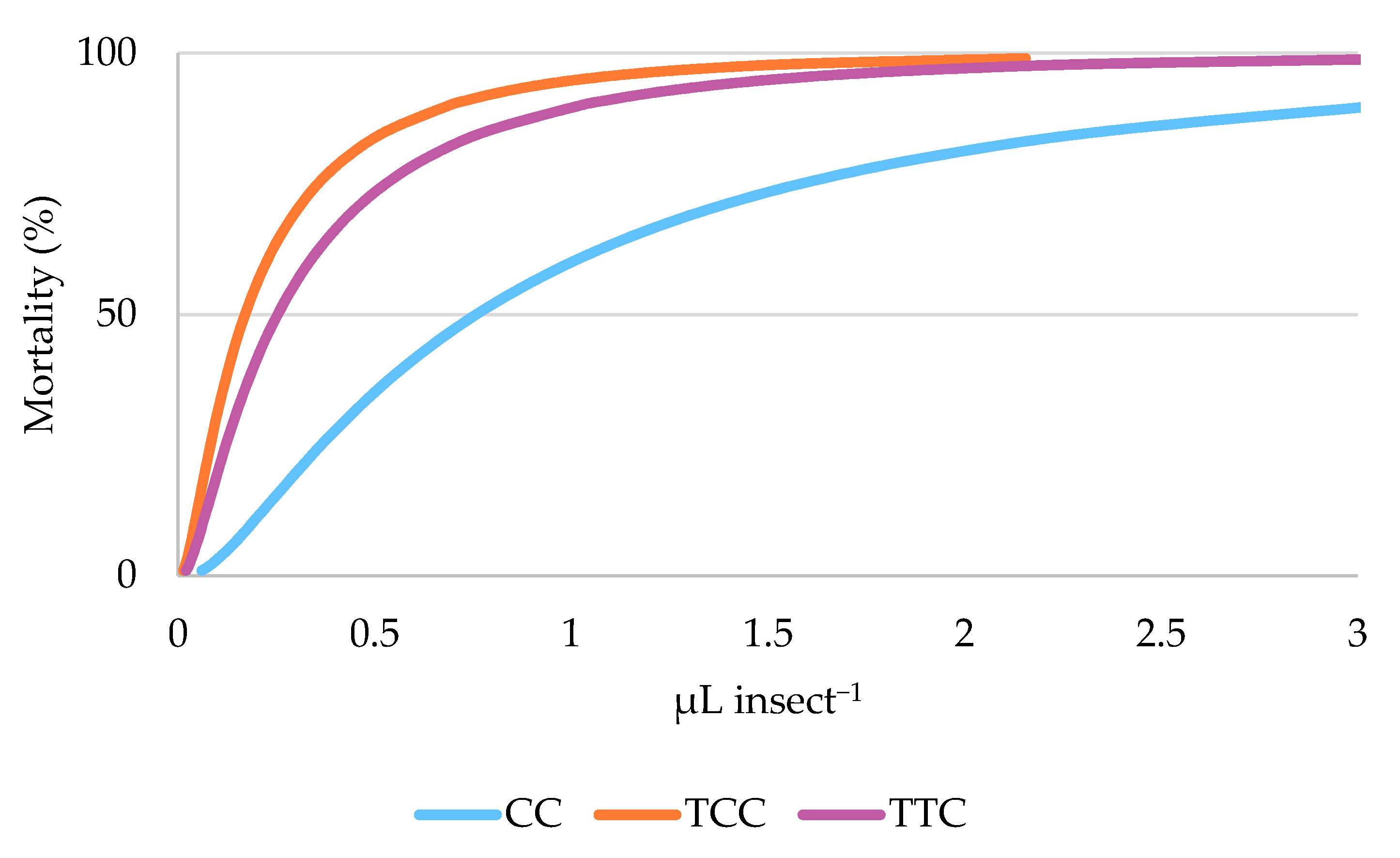
3.3. Oviposition Deterrence Bioassay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Blackburn, C.; McClure, P.J. Foodborne Pathogens: Hazards, Risk Analysis and Control, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2009; p. 1232. [Google Scholar]
- Förster, M.; Klimpel, S.; Mehlhorn, H.; Sievert, K.; Messler, S.; Pfeffer, K. Pilot study on synanthropic flies (e.g., Musca, Sarcophaga, Calliphora, Fannia, Lucilia, Stomoxys) as vectors of pathogenic microorganisms. Parasitol. Res. 2007, 101, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, A.C.M.; Ratan, A.; Acerbi, E.; Drautz-Moses, D.I.; Premkrishnan, B.N.V.; Costea, P.I.; Linz, B.; Purbojati, R.W.; Paulo, D.F.; Gaultier, N.E.; et al. The microbiomes of blowflies and houseflies as bacterial transmission reservoirs. Sci. Rep. 2017, 7, 16324. [Google Scholar] [CrossRef] [PubMed]
- Tomberlin, J.K.; Crippen, T.L.; Tarone, A.M.; Chaudhury, M.F.B.; Singh, B.; Cammack, J.A.; Meisel, R.P. A review of bacterial interactions with blow flies (Diptera: Calliphoridae) of medical, veterinary, and forensic importance. Ann. Entomol. Soc. Am. 2017, 110, 19–36. [Google Scholar] [CrossRef]
- Blazar, J.; Allard, M.; Lienau, E.K. Insects as vectors of foodborne pathogenic bacteria. Terr. Arthropod Rev. 2011, 4, 5–16. [Google Scholar] [CrossRef]
- Szpila, K.; Pape, T.; Hall, M.J.R.; Madra, A. Morphology and identification of first instars of European and Mediterranean blowflies of forensic importance. Part III: Calliphorinae. Med. Vet. Entomol. 2014, 28, 133–142. [Google Scholar] [CrossRef]
- Alexander, J.O.D. Cutaneous Myiasis. In Arthropods and Human Skin; Springer: London, UK, 1984; pp. 87–113. [Google Scholar]
- Morris, O.S.; Titchener, R.N. Blowfly species composition in sheep myiasis in Scotland. Med. Vet. Entomol. 1997, 22, 253–256. [Google Scholar] [CrossRef]
- French, N.P.; Wall, R.; Cripps, P.J.; Morgan, K.L. Prevalence, regional distribution and control of blowfly strike in England and Wales. Vet. Rec. 1992, 131, 337–342. [Google Scholar] [CrossRef]
- Baker, K.E.; Rolfe, P.F.; George, A.G.; Vanhoof, K.J.; Kluver, P.F.; Bailey, J.N. Effective control of a suspected cyromazine-resistant strain of Lucilia cuprina using commercial spray-on formulations of cyromazine or dicyclanil. Aust. Vet. J. 2014, 92, 376–380. [Google Scholar] [CrossRef]
- Deb, P. Chapter 14—Environmental pollution and the burden of food-borne diseases. In Foodborne Diseases; Academic Press: Cambridge, MA, USA, 2018; Volume 15, pp. 473–500. [Google Scholar] [CrossRef]
- Roldán-Tapia, L.; Parrón, T.; Sánchez-Santed, F. Neuropsychological effects of long-term exposure to organophosphate pesticides. Neurotoxicol. Teratol. 2005, 27, 259–266. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Borel, B. When the pesticides run out. Nature 2017, 543, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Naqqash, M.N.; Gokce, A.; Bakhsh, A.; Salim, M. Insecticide resistance and its molecular basis in urban insect pests. Parasitol. Res. 2016, 115, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, A.; Conti, B.; Mazzoni, V.; Meini, L.; Pistelli, L. Volatile chemical composition and bioactivity of six essential oils against the stored food insect Sitophilus zeamais Motsch. (Coleoptera Dryophthoridae). Nat. Prod. Res. 2012, 26, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Muniz, E.R.; Bedini, S.; Sarrocco, S.; Vannacci, G.; Mascarin, G.M.; Fernandes, É.K.; Conti, B. Carnauba wax enhances the insecticidal activity of entomopathogenic fungi against the blowfly Lucilia sericata (Diptera: Calliphoridae). J. Invertebr. Pathol. 2020, 174, 107391. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Echeverria, M.C.; Guidi, L.; Landi, M.; Lucchi, A.; Conti, B. Artemisia spp. essential oils against the disease-carrying blowfly Calliphora vomitoria. Parasites Vectors 2017, 10, 80. [Google Scholar] [CrossRef]
- Bedini, S.; Guarino, S.; Echeverria, M.C.; Flamini, G.; Ascrizzi, R.; Loni, A.; Conti, B. Allium sativum, Rosmarinus officinalis, and Salvia officinalis essential oils: A spiced shield against blowflies. Insects 2020, 11, 143. [Google Scholar] [CrossRef]
- Bedini, S.; Cosci, F.; Tani, C.; Pierattini, E.C.; Venturi, F.; Lucchi, A.; Ioriatti, C.; Ascrizzi, R.; Flamini, G.; Ferroni, G.; et al. Essential oils as post-harvest crop protectants against the Fruit Fly Drosophila suzukii: Bioactivity and organoleptic profile. Insects 2020, 11, 508. [Google Scholar] [CrossRef]
- Pierattini, E.C.; Bedini, S.; Venturi, F.; Ascrizzi, R.; Flamini, G.; Bocchino, R.; Girardi, J.; Giannotti, P.; Ferroni, G.; Conti, B. Sensory quality of essential oils and their synergistic effect with diatomaceous earth, for the control of stored grain insects. Insects 2019, 10, 114. [Google Scholar] [CrossRef]
- Conti, B.; Bocchino, R.; Cosci, F.; Ascrizzi, R.; Flamini, G.; Bedini, S. Essential oils against Varroa destructor: A soft way to fight the parasitic mite of Apis mellifera. J. Apic. Res. 2020, 59, 774–782. [Google Scholar] [CrossRef]
- Ietswaart, J.H. A Taxonomic Revision of the Genus Origanum (Labiatae); Leiden University Press: Leiden, The Netherlands, 1980; Volume 4, p. 160. [Google Scholar]
- FDA, Food and Drug Administration. Code of Federal Regulations Title 21, Volume 3, 21CFR182.20. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.20 (accessed on 4 January 2021).
- Kokkini, S.; Karousou, R.; Hanlidou, E. Herbs of the Labiatae. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 3082–3090. [Google Scholar] [CrossRef]
- Kintzios, S.E. Oregano. In Handbook of Herbs and Spices; Woodhead Publishing: Cambridge, UK, 2004; Volume 2, pp. 215–229. [Google Scholar] [CrossRef]
- Kokkini, S.; Karousou, R.; Vokou, S. Pattern of geographic variation of Origanum vulgare trichomes and essential oil content in Greece. Biochem. Syst. Ecol. 1994, 22, 517–528. [Google Scholar] [CrossRef]
- Napoli, E.; Siracusa, L.; Ruberto, G. New tricks for old guys: Recent development in the chemistry, biochemistry, applications and exploitation of selected species from the Lamiaceae family. Chem. Biodivers. 2020, 17, e1900677. [Google Scholar] [CrossRef] [PubMed]
- Sivropoulou, A.; Papanikolaou, E.; Nikolaou, C.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antimicrobial and cytotoxic activities of Origanum essential oils. J. Agric. Food Chem. 1996, 44, 1202–1205. [Google Scholar] [CrossRef]
- Şahin, F.; Güllüce, M.; Daferera, D.; Sökmen, A.; Sökmen, M.; Polissiou, M.; Agar, G.; Özerg, H. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control 2004, 15, 549–557. [Google Scholar] [CrossRef]
- Souza, E.L.; Stamford, T.L.M.; Lima, E.O.; Trajano, V.N. Effectiveness of Origanum vulgare L. essential oil to inhibit the growth of food spoiling yeasts. Food Control 2007, 18, 409–413. [Google Scholar] [CrossRef]
- Coelho da Costa, A.; Cavalcanti dos Santos, B.E.; Santos, F.L.; Lima, E.O. Antibacterial activity of the essential oil of Origanum vulgare L. (Lamiaceae) against bacterial multiresistant strains isolated from nosocomial patients. Rev. Bras. Farmacogn. 2009, 19, 236–241. [Google Scholar] [CrossRef]
- De Martino, L.; De Feo, V.; Formisano, C.; Mignola, E.; Senatore, F. Chemical composition and antimicrobial activity of the essential oils from three chemotypes of Origanum vulgare L. ssp. hirtum (Link) Ietswaart growing wild in Campania (Southern Italy). Molecules 2009, 14, 2735–2746. [Google Scholar] [CrossRef]
- Saeed, S.; Tariq, P.; Pak, J. Antibacterial activity of oregano (Origanum vulgare Linn.) against Gram positive bacteria. Pharm. Sci. 2009, 22, 421–424. [Google Scholar]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C. Decoction, infusion and hydroalcoholic extract of Origanum vulgare L.: Different performances regarding bioactivity and phenolic compounds. Food Chem. 2014, 158, 73–80. [Google Scholar] [CrossRef]
- La Pergola, A.; Restuccia, C.; Napoli, E.; Bella, S.; Brighina, S.; Russo, A.; Suma, P. Commercial and wild Sicilian Origanum vulgare essential oils: Chemical composition, antimicrobial activity and repellent effects. J. Essent. Oil Res. 2017, 29, 451–460. [Google Scholar] [CrossRef]
- Aslan, İ.; Çalmaşur, Ö.; Şahin, F.; Çaglar, Ö. Insecticidal effects of essential plant oils against Ephestia kuehniella (Zell.), Lasioderma serricorne (F.) and Sitophilus granarius (L.). J. Plant Dis. Prot. 2009, 112, 257–267. [Google Scholar]
- Nasr, M.; Sendi, J.J.; Moharramipourb, S.; Zibaee, A. Evaluation of Origanum vulgare L. essential oil as a source of toxicant and an inhibitor of physiological parameters in diamondback moth, Plutella xylustella L. (Lepidoptera: Pyralidae). J. Saudi Soc. Agric. Sci. 2017, 16, 184–190. [Google Scholar] [CrossRef]
- Campolo, O.; Giunti, G.; Russo, A.; Palmeri, V.; Zappalà, L. Essential oils in stored product insect pest control. J. Food Qual. 2018, 2018, 6906105. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Q.; Rao, Y.; Hong, L.; Zhang, D. Efficacy of Origanum vulgare essential oil and carvacrol against the housefly, Musca domestica L. (Diptera: Muscidae). Environ. Sci. Pollut. Res. 2019, 26, 23824–23831. [Google Scholar] [CrossRef] [PubMed]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Echeverria, M.C.; Gomez, E.V.; Guidi, L.; Landi, M.; Lucchi, A.; Conti, B. Toxicity and oviposition deterrence of essential oils of Clinopodium nubigenum and Lavandula angustifolia against the myiasis inducing blowfly Lucilia sericata. PLoS ONE 2019, 14, e0212576. [Google Scholar] [CrossRef]
- European Pharmacopoeia 6.0. Determination of Essential Oils in Herbal Drugs; The Stationery Office: London, UK, 2008; pp. 251–252. [Google Scholar]
- Giuliani, C.; Ascrizzi, R.; Tani, C.; Bottoni, M.; Maleci Bini, L.; Flamini, G.; Fico, G. Salvia uliginosa Benth.: Glandular trichomes as bio-factories of volatiles and essential oil. Flora 2017, 233, 12–21. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. NIST/EPA/NIH Mass Spectral Library, NIST Standard Reference Database Number 69; The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2014. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; p. 804. [Google Scholar]
- Ujvari, B.; Wallman, J.F.; Madsen, T.; Whelan, M.; Hulbert, A.J. Experimental studies of blowfly (Calliphora stygia) longevity: A little dietary fat is beneficial but too much is detrimental. Comp. Biochem. Phys. A 2009, 154, 383–388. [Google Scholar] [CrossRef]
- Kelly, M.A.; Zieba, A.P.; Buttemer, W.A.; Hulbert, A.J. Effect of temperature on the rate of ageing: An experimental study of the blowfly Calliphora stygia. PLoS ONE 2013, 8, e73781. [Google Scholar] [CrossRef]
- Abbott, W.J. A method of computing effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 256–267. [Google Scholar] [CrossRef]
- Cheah, S.X.; Tay, J.W.; Chan, L.K.; Jaal, Z. Larvicidal, oviposition, and ovicidal effects of Artemisia annua (Asterales: Asteraceae) against Aedes aegypti, Anopheles sinensis, and Culex quinquefasciatus (Diptera: Culicidae). Parasitol. Res. 2013, 112, 3275–3282. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, NY, USA, 1971; p. 333. [Google Scholar] [CrossRef]
- Bedini, S.; Muniz, E.R.; Tani, C.; Conti, B.; Ruiu, L. Insecticidal potential of Brevibacillus laterosporus against dipteran pest species in a wide ecological range. J. Invertebr. Pathol. 2020, 177, 107493. [Google Scholar] [CrossRef] [PubMed]
- Politi, M.; Menghini, L.; Conti, B.; Bedini, S.; Farina, P.; Cioni, P.L.; Braca, A.; De Leo, M. Reconsidering hydrosols as main products of aromatic plants manufactory: The Lavandin (Lavandula × intermedia) case study in Tuscany. Molecules 2020, 25, 2225. [Google Scholar] [CrossRef] [PubMed]
- Belhamel, C.; Boulekbache-Makhlouf, L.; Bedini, S.; Tani, C.; Lombardi, T.; Giannotti, P.; Madani, K.; Belhamel, K.; Conti, B. Nanostructured alumina as seed protectant against three stored-product insect pests. J. Stored Prod. Res. 2020, 87, 101607. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Ascrizzi, R.; Venturi, F.; Ferroni, G.; Bader, A.; Girardi, J.; Conti, B. Essential oils sensory quality and their bioactivity against the mosquito Aedes albopictus. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Gottardi, D.; Bukvicki, D.; Prasad, S.; Tyagi, A.K. Beneficial effects of spices in food preservation and safety. Front. Microbiol. 2016, 7, 1394. [Google Scholar] [CrossRef] [PubMed]
- Bedini, S.; Bougherra, H.H.; Flamini, G.; Cosci, F.; Belhamel, K.; Ascrizzi, R.; Conti, B. Repellency of anethole- and estragole-type fennel essential oils against stored grain pests: The different twins. Bull. Insectol. 2016, 69, 149–157. [Google Scholar]
- Napoli, E.M.; Ruberto, G. Sicilian Aromatic Plants: From Traditional Heritage to a New Agro-Industrial Exploitation. In Spices: Types, Uses and Health Benefits; Kralis, J.F., Ed.; NovaPublishers: Hauppauge, NY, USA, 2012; pp. 1–56. [Google Scholar]
- D’Antuono, L.F.; Galleti, G.C.; Bocchini, P. Variability of essential oil content and composition of Origanum vulgare L. populations from a North Mediterranean Area (Liguria region, Northern Italy). Ann. Bot. 2008, 6, 471–478. [Google Scholar] [CrossRef]
- Skoula, M.; Harborne, J.B. Taxonomy and Chemistry. In Oregano: The Genera Origanum and Lippia. Medicinal and Aromatic Plants—Industrial Profiles; Kintzios, S.E., Ed.; Taylor and Francis CRC Press: Boca Raton, FL, USA, 2002; Volume 25, pp. 67–108. [Google Scholar]
- Napoli, E.; Giovino, A.; Carrubba, A.; How Yuen Siong, V.; Rinoldo, C.; Nina, O.; Ruberto, G. Variations on essential oils constituents in Oregano (Origanum vulgare subsp. viridulum (= O. heracleoticum) over cultivation cycles. Plants 2020, 9, 1174. [Google Scholar] [CrossRef]
- Vokou, D.; Kokkini, S.; Bessiere, J.M. Geographic variation of Greek oregano (Origanum vulgare ssp. hirtum) essential oils. Biochem. Syst. Ecol. 1993, 21, 287–295. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Özek, T.; Tümen, G.; Sezik, E. Composition of the essential oils of Turkish Origanum species with commercial importance. J. Essent. Oil Res. 1993, 5, 619–623. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Özek, T.; Kurkcuoglu, M.; Tümen, G. The essential oil of Origanum vulgare subsp. hirtum of Turkish origin. J. Essent. Oil Res. 1994, 6, 31–36. [Google Scholar] [CrossRef]
- Russo, M.; Galletti, G.C.; Bocchini, P.; Carnacini, A. Essential oil chemical composition of wild populations of Italian Oregano spice (Origanum vulgare ssp. hirtum (Link) Ietswaart): A preliminary evaluation of their use in chemotaxonomy by cluster analysis. 1. Inflorescences. J. Agric. Food Chem. 1998, 46, 3741–3746. [Google Scholar] [CrossRef]
- Kokkini, S. Taxonomy, diversity and distribution of Origanum species. In Oregano. Promoting the Conservation and Use of Underutilized and Neglected Crops, Proceedings of the IPGRI International Workshop on Oregano, Bari, Italy, 8–12 May 1996; Padulosi, S., Ed.; IPGRI: Rome, Italy, 1997; pp. 2–12. [Google Scholar]
- Xhuvel, L.; Lipe, Q. Oregano (Origanum vulgare L.) in Albania. In Oregano. Promoting the Conservation and Use of Underutilized and Neglected Crops, Proceedings of the IPGRI International Workshop on Oregano, Bari, Italy, 8–12 May 1996; Padulosi, S., Ed.; IPGRI: Rome, Italy, 1997; pp. 132–136. [Google Scholar]
- Karpouhtsis, I.; Pardali, E.; Feggou, E.; Kokkini, S.; Scouras, Z.G.; Mavragani-Tsipidou, P. Insecticidal and genotoxic activities of oregano essential oils. J. Agric. Food Chem. 1998, 46, 1111–1115. [Google Scholar] [CrossRef]
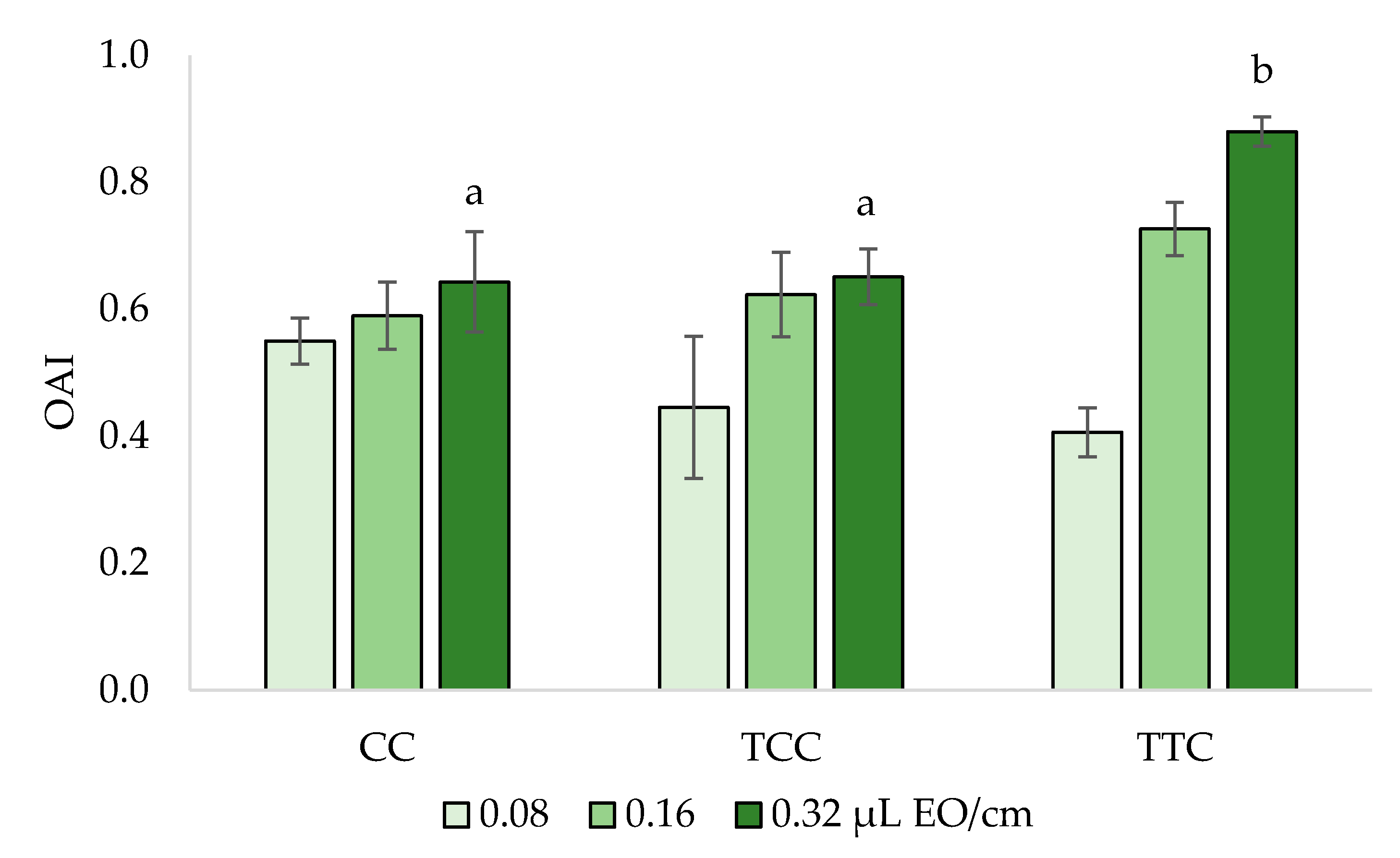
| Compounds | l.r.i. a | Relative Abundance (%) | ||
|---|---|---|---|---|
| TCC | TTC | CC | ||
| (E)-Hexenal | 855 | - b | 0.1 | - |
| Tricyclene | 928 | 1.1 | 1.8 | - |
| Cumene | 929 | 0.8 | 0.8 | - |
| α-Thujene | 931 | - | - | 0.4 |
| α-Pinene | 941 | 0.1 | 0.1 | 0.1 |
| 1-Octen-3-ol | 980 | 0.2 | 0.2 | 1.8 |
| 3-Octanone | 987 | 0.1 | - | 0.8 |
| β-Myrcene | 993 | 2.1 | 2.6 | 1.1 |
| α-Phellandrene | 1005 | 0.2 | 0.4 | - |
| iso-Sylvestrene | 1019 | 0.1 | 0.1 | - |
| α-Terpinene | 1020 | 2.1 | 3.3 | 0.8 |
| p-Cymene | 1027 | 18.4 | 9.3 | 8.0 |
| Limonene | 1032 | 0.7 | 0.6 | - |
| Eucalyptol | 1034 | - | - | 0.1 |
| (Z)-β-Ocimene | 1042 | 1.5 | 3.2 | - |
| (E)-β-Ocimene | 1052 | 0.3 | 0.7 | - |
| γ-Terpinene | 1062 | 11.4 | 19.7 | 2.4 |
| cis-Sabinene hydrate | 1070 | - | 0.2 | - |
| Terpinolene | 1088 | 0.2 | 0.1 | - |
| Linalool | 1101 | 0.6 | 1.2 | 0.3 |
| Borneol | 1165 | 0.2 | 0.2 | - |
| Terpinen-4-ol | 1178 | 0.7 | 1.0 | 0.2 |
| α-Terpineol | 1189 | 0.1 | 0.2 | - |
| Thymol methyl ether | 1235 | 1.9 | 3.8 | - |
| Carvacrol methyl ether | 1239 | 3.1 | 3.8 | 0.2 |
| Thymol | 1292 | 43.8 | 36.7 | - |
| Carvacrol | 1298 | 6.0 | 0.9 | 81.5 |
| α-Copaene | 1376 | 0.1 | 0.2 | - |
| β-Caryophyllene | 1420 | 1.0 | 1.9 | 1.7 |
| β-Copaene | 1429 | - | 0.1 | - |
| α-Humulene | 1456 | 0.1 | 0.2 | - |
| allo-Aromadendrene | 1461 | - | 0.1 | - |
| γ-Muurolene | 1477 | 0.3 | 0.3 | - |
| Germacrene D | 1478 | - | 1.0 | - |
| Bicyclogermacrene | 1496 | - | 0.2 | - |
| α-Muurolene | 1498 | 0.1 | 0.1 | - |
| β-Bisabolene | 1509 | 1.1 | 2.0 | - |
| γ-Cadinene | 1513 | 0.3 | 0.3 | - |
| δ-Cadinene | 1525 | 0.6 | 0.7 | - |
| Spathulenol | 1576 | - | 0.2 | - |
| Caryophyllene oxide | 1581 | 0.3 | 0.2 | 0.5 |
| 14-Hydroxy-9-epi-caryophyllene | 1664 | - | 0.2 | - |
| Monoterpene hydrocarbons | 37.0 | 40.0 | 12.8 | |
| Oxygenated monoterpenes | 56.5 | 47.8 | 82.4 | |
| Sesquiterpene hydrocarbons | 3.6 | 7.1 | 1.7 | |
| Oxygenated sesquiterpenes | 0.3 | 0.6 | 0.5 | |
| Non-terpene derivatives | 2.2 | 2.9 | 2.6 | |
| Total identified (%) | 99.5 | 98.4 | 100.0 | |
| Chemotype | Mean a ± SE | 95% Confidence Interval | |
|---|---|---|---|
| Lower Bound | Upper Bound | ||
| CC | 73.447 ± 5.314 a | 62.554 | 84.400 |
| TCC | 60.267 ± 5.314 b | 49.334 | 71.190 |
| TTC | 88.041 ± 4.632 a | 78.520 | 97.562 |
| EO | LD50 a | 95% CI b | LD95 c | 95% CI | Intercept ± SE | p |
|---|---|---|---|---|---|---|
| CC | 0.240 | 0.207–0.274 | 0.657 | 0.567 ± 0.787 | 2.331 ± 0.174 | <0.001 |
| TCC | 0.312 | 0.274–0.351 | 0.854 | 0.736 ± 1.029 | 1.903 ± 0.152 | <0.001 |
| TTC | 0.141 | 0.121–0.161 | 0.386 | 0.330 ± 0.465 | 3.203 ± 0.238 | <0.001 |
| Chemotype | Mean a ± SE | 95% Confidence Interval | |
|---|---|---|---|
| Lower Bound | Upper Bound | ||
| CC | 32.793 ± 2.769 a | 27.271 | 38.314 |
| TCC | 60.525 ± 2.769 b | 55.003 | 66.046 |
| TTC | 82.091 ± 2.769 c | 76.569 | 87.612 |
| EO | LC50 a | 95% CI b | LC95 c | 95% CI b | Intercept ± SE | p |
|---|---|---|---|---|---|---|
| CC | 0.038 | 0.023–0.063 | 0.235 | 0.122 ± 0.771 | 2.947 ± 0.128 | <0.001 |
| TCC | 0.008 | 0.005–0.013 | 0.052 | 0.031 ± 0.125 | 4.300 ± 0.157 | <0.001 |
| TTC | 0.013 | 0.008–0.020 | 0.080 | 0.046 ± 0.218 | 3.909 ± 0.151 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedini, S.; Farina, P.; Napoli, E.; Flamini, G.; Ascrizzi, R.; Verzera, A.; Conti, B.; Zappalà, L. Bioactivity of Different Chemotypes of Oregano Essential Oil against the Blowfly Calliphora vomitoria Vector of Foodborne Pathogens. Insects 2021, 12, 52. https://doi.org/10.3390/insects12010052
Bedini S, Farina P, Napoli E, Flamini G, Ascrizzi R, Verzera A, Conti B, Zappalà L. Bioactivity of Different Chemotypes of Oregano Essential Oil against the Blowfly Calliphora vomitoria Vector of Foodborne Pathogens. Insects. 2021; 12(1):52. https://doi.org/10.3390/insects12010052
Chicago/Turabian StyleBedini, Stefano, Priscilla Farina, Edoardo Napoli, Guido Flamini, Roberta Ascrizzi, Antonella Verzera, Barbara Conti, and Lucia Zappalà. 2021. "Bioactivity of Different Chemotypes of Oregano Essential Oil against the Blowfly Calliphora vomitoria Vector of Foodborne Pathogens" Insects 12, no. 1: 52. https://doi.org/10.3390/insects12010052
APA StyleBedini, S., Farina, P., Napoli, E., Flamini, G., Ascrizzi, R., Verzera, A., Conti, B., & Zappalà, L. (2021). Bioactivity of Different Chemotypes of Oregano Essential Oil against the Blowfly Calliphora vomitoria Vector of Foodborne Pathogens. Insects, 12(1), 52. https://doi.org/10.3390/insects12010052











