Morphology of the Novel Basimandibular Gland in the Ant Genus Strumigenys (Hymenoptera, Formicidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
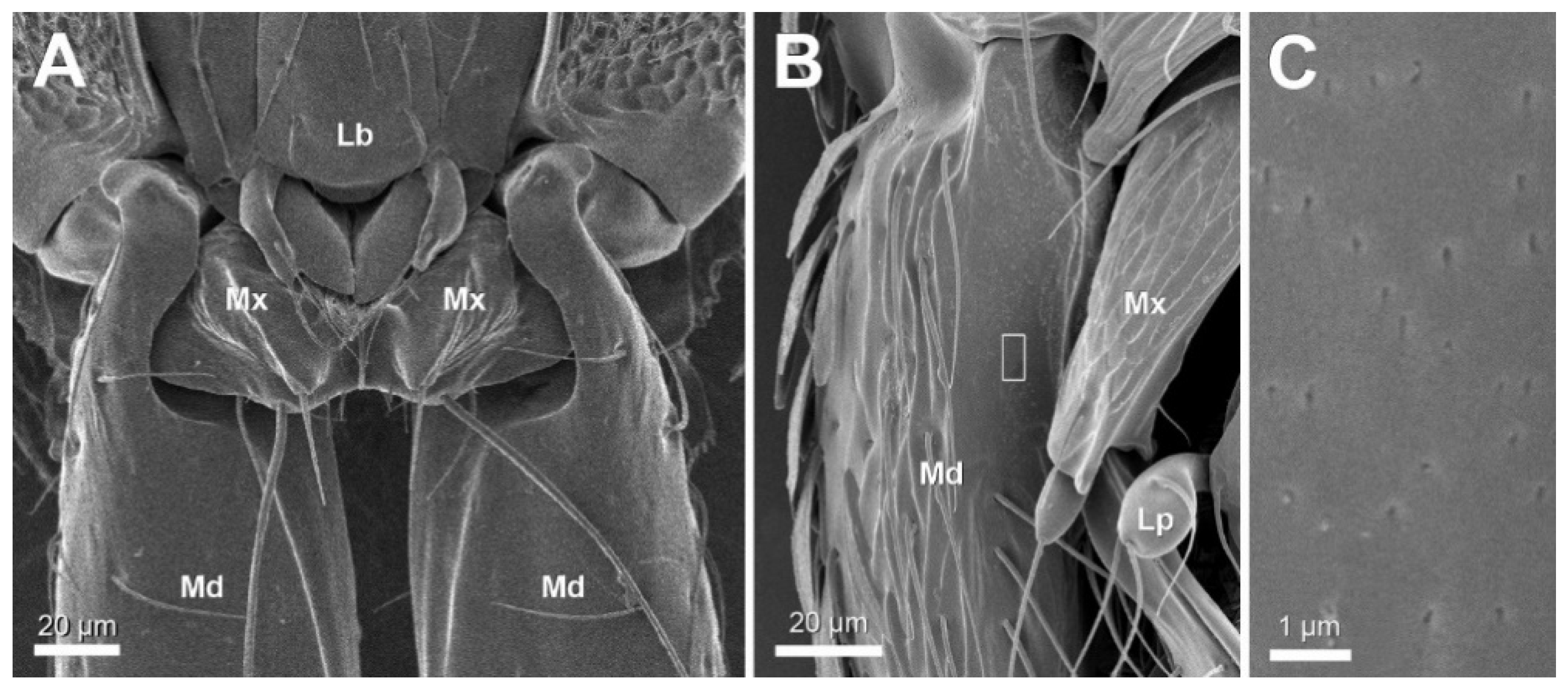
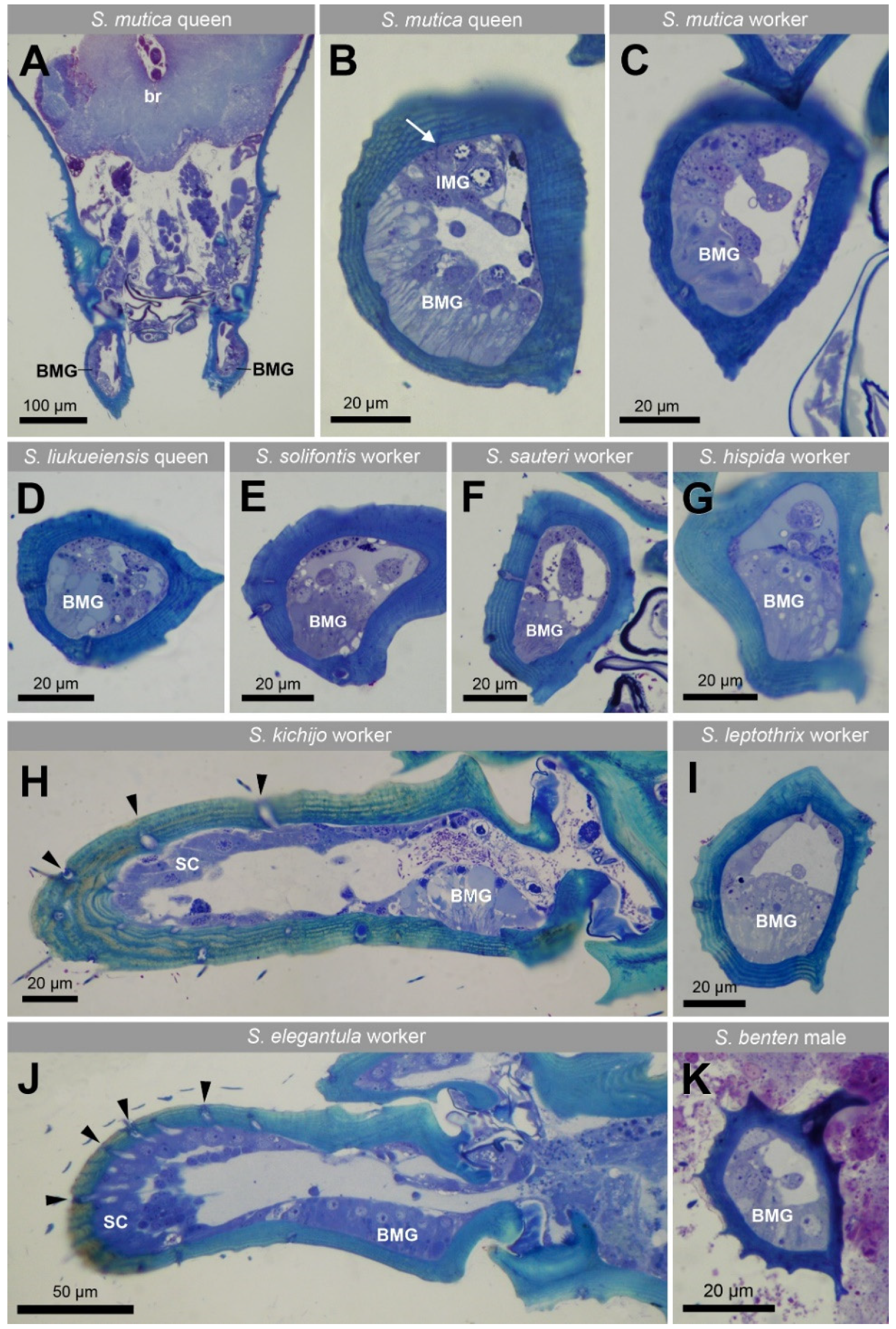
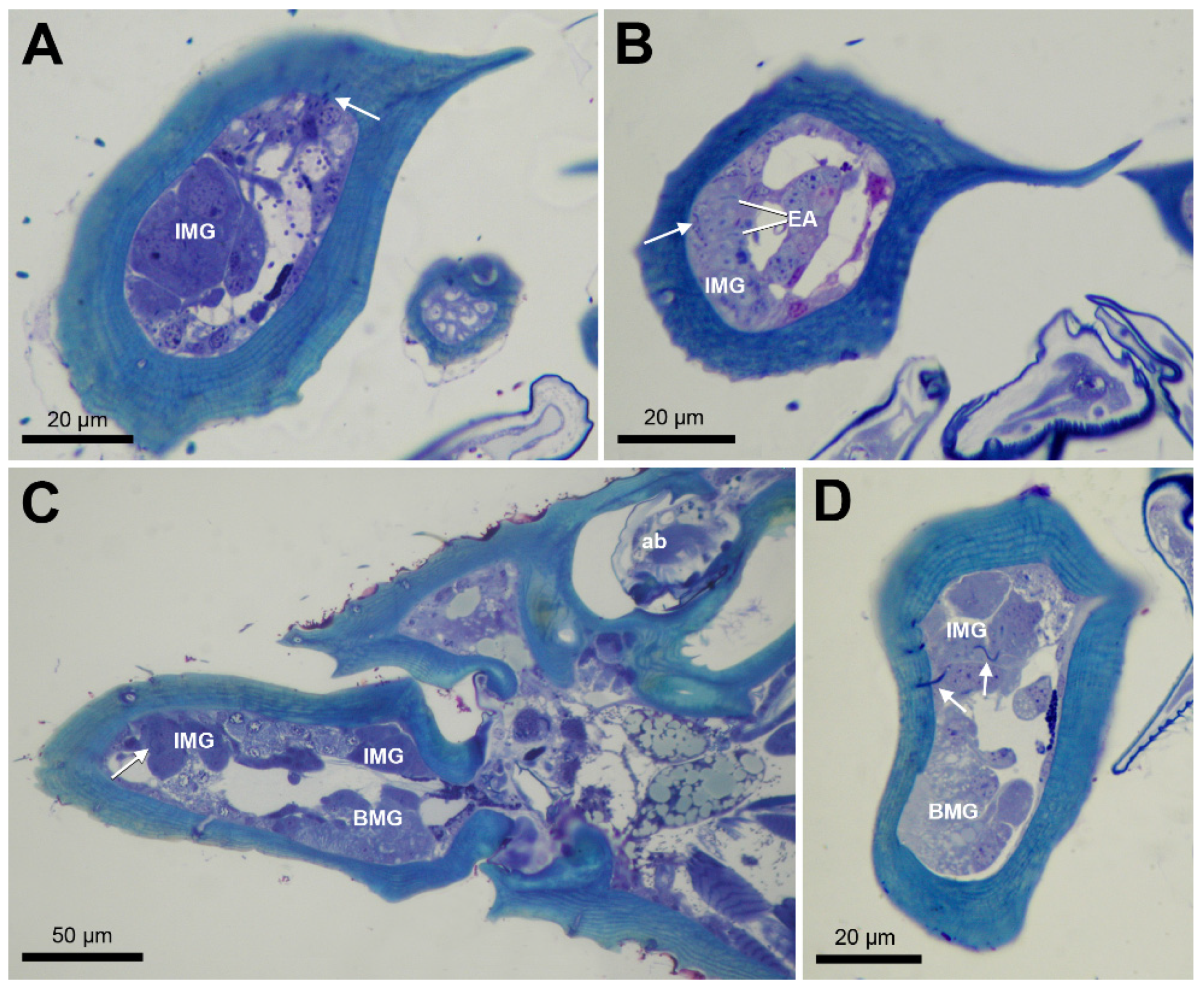
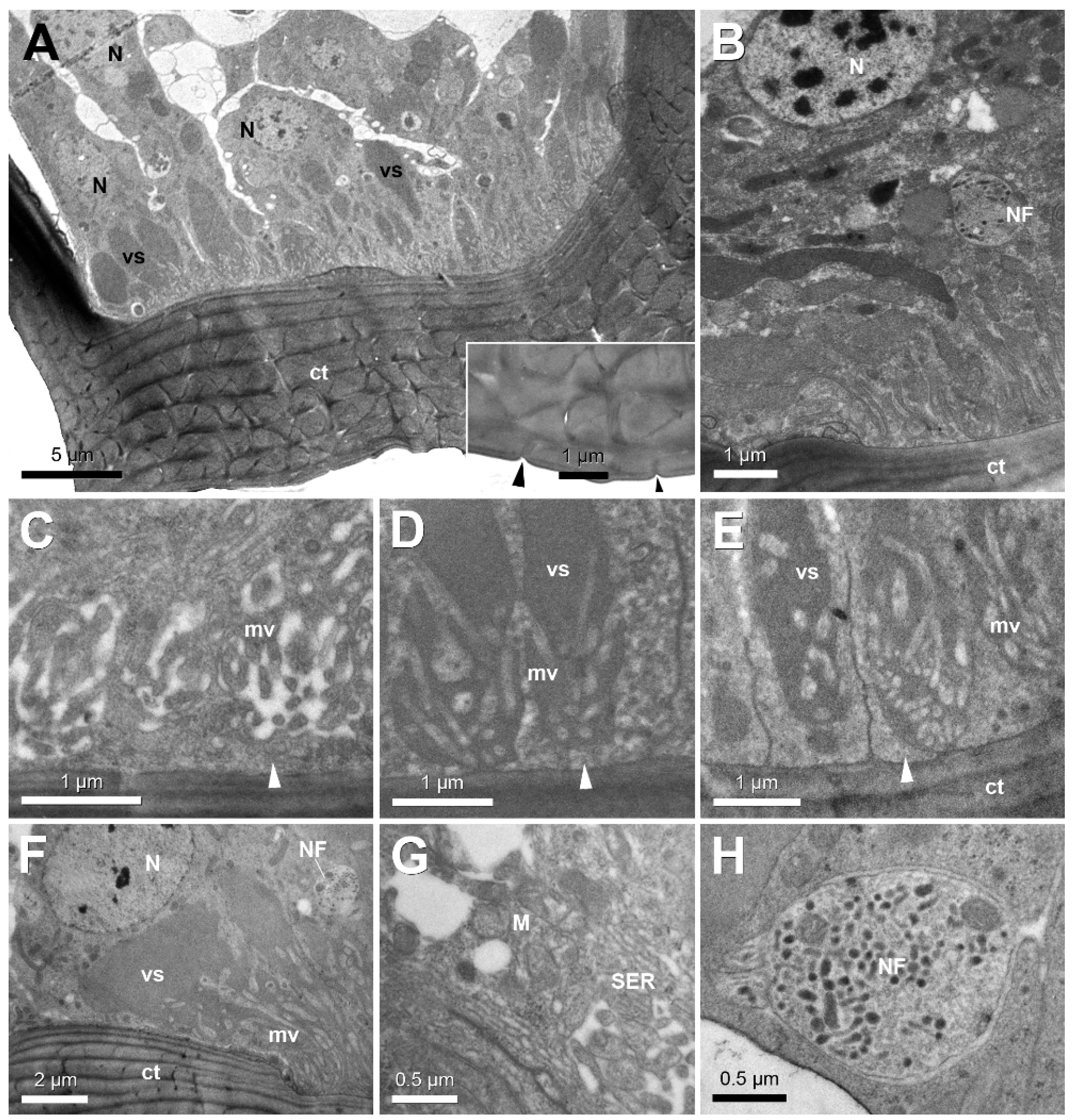
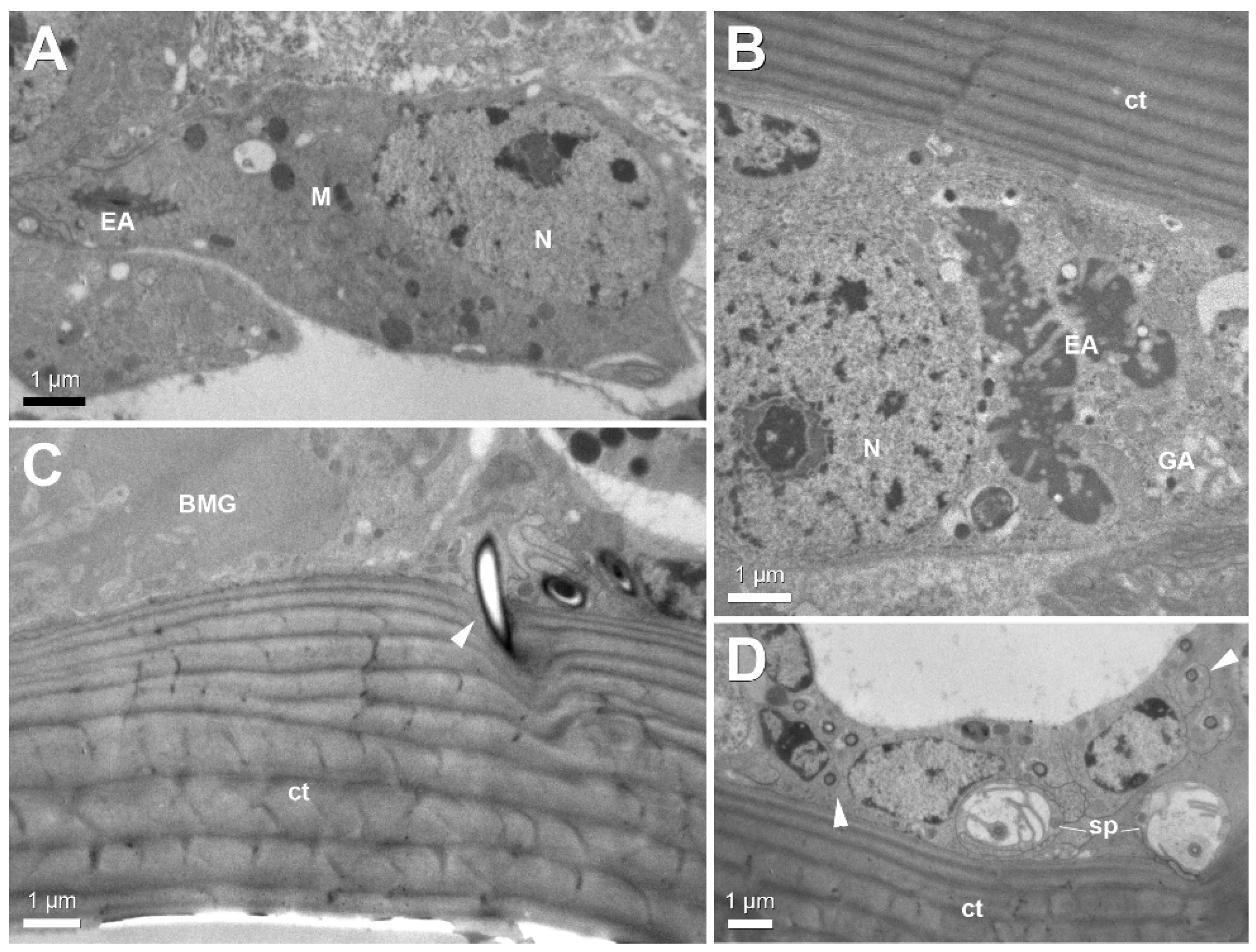
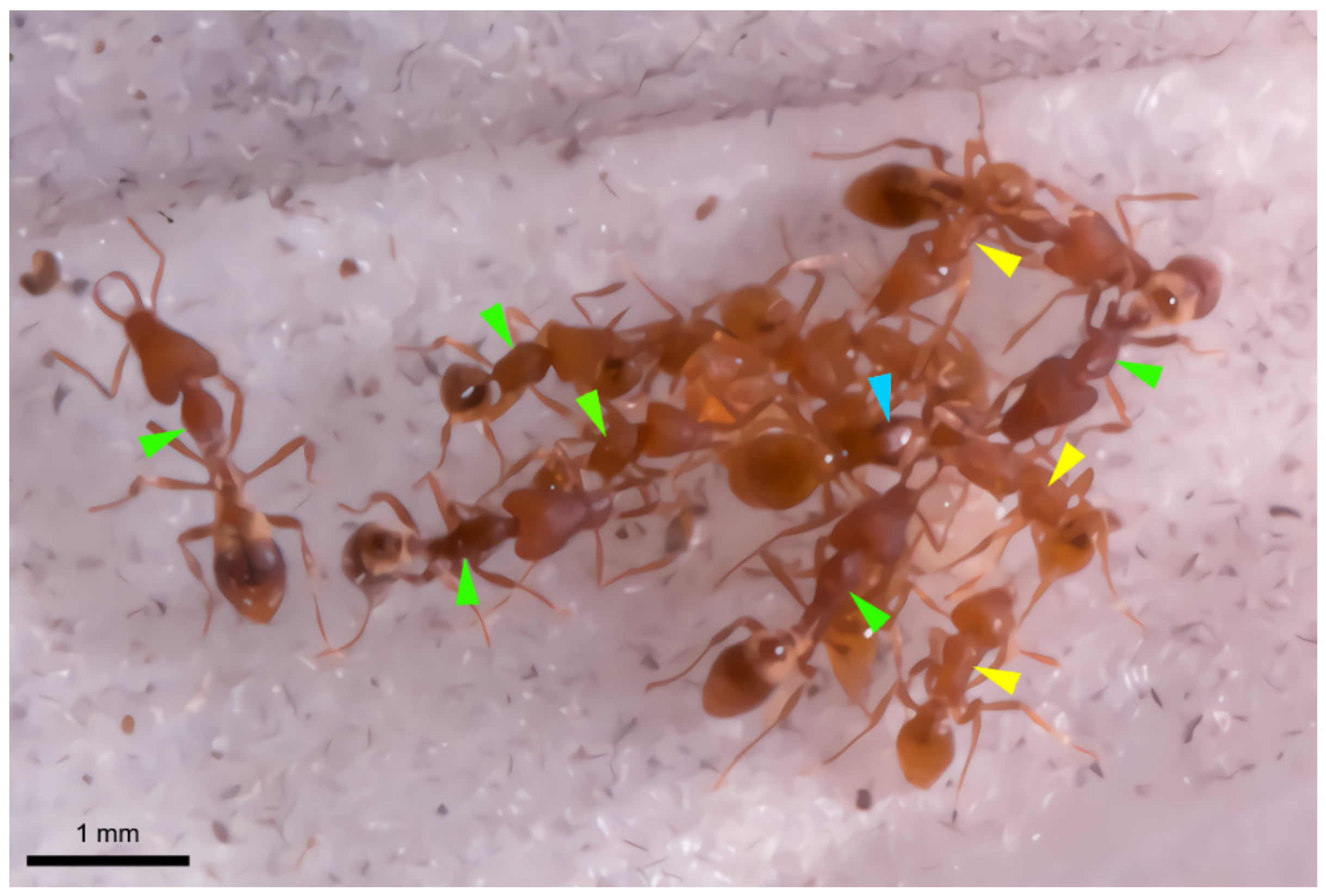
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Billen, J. Diversidad y morfología de las glándulas exocrinas en las hormigas. In Hormigas de Colombia; Fernández, F., Guerrero, R.J., Delsinne, T., Eds.; Universidad Nacional de Colombia Press: Bogota, Colombia, 2019; pp. 165–174. [Google Scholar]
- Romani, R.; Grasso, D.A.; Mori, A.; Isidoro, N.; Le Moli, F. Antennal glands of the slave-making ant Polyergus rufescens and its slave species Formica cunicularia (Hymenoptera, Formicidae). Can. J. Zool. 2006, 84, 490–494. [Google Scholar] [CrossRef]
- Billen, J. Occurrence and structural organization of the exocrine glands in the legs of ants. Arthropod Struct. Dev. 2009, 38, 2–15. [Google Scholar] [CrossRef]
- Meinert, F. Bidrag til de Danske Myrers Naturhistorie. In Det Kongelige Danske Videnskabernes Selskabs Skrifter; Nabu Press: Kobenhagen, Denmark, 1860; pp. 273–340. [Google Scholar]
- Noirot, C.; Quennedey, A. Fine structure of insect epidermal glands. Annu. Rev. Entomol. 1974, 19, 61–80. [Google Scholar] [CrossRef]
- Baroni Urbani, C.; Andrade, M. The ant tribe Dacetini: Limits and constituent genera, with descriptions of new species (Hymenoptera, Formicidae). Annali del Museo Civico di Storia Naturale “G. Doria” 2007, 99, 1–191. [Google Scholar]
- Lattke, J.E.; Da Silva, T.S.R.; Delsinne, T. Taxonomy and natural history of Strumigenys thaxteri Wheeler and Strumigenys reticeps (Kempf) (Hymenoptera: Formicidae). Zootaxa 2018, 4438, 137–147. [Google Scholar] [CrossRef]
- Brown, W.L. A preliminary generic revision of the higher Dacetini (Hymenoptera: Formicidae). Trans. Am. Entomol. Soc. 1948, 74, 101–129. [Google Scholar]
- Ward, P.S.; Brady, S.G.; Fisher, B.L.; Schultz, T.R. The evolution of myrmicine ants: Phylogeny and biogeography of a hyperdiverse ant clade (Hymenoptera: Formicidae). Syst. Entomol. 2015, 40, 61–81. [Google Scholar] [CrossRef]
- Bolton, B. Ant genera of the tribe Dacetonini (Hymenoptera: Formicidae). J. Nat. Hist. 1999, 33, 1639–1689. [Google Scholar] [CrossRef]
- Billen, J.; Ito, F.; Bolton, B. Femoral and tibial glands in the ant genus Strumigenys (Hymenoptera, Formicidae). Belg. J. Zool. 2000, 130, 111–115. [Google Scholar]
- Schoeters, E.; Billen, J. The intramandibular gland, a novel exocrine structure in ants (Insecta, Hymenoptera). Zoomorphology 1994, 114, 125–131. [Google Scholar] [CrossRef]
- Roux, O.; Billen, J.; Orivel, J.; Dejean, A. An overlooked mandibular-rubbing behavior used during recruitment by the African weaver ant, Oecophylla longinoda. PLoS ONE 2010, 5, e8957. [Google Scholar] [CrossRef] [PubMed]
- Billen, J.; Verbesselt, S. The intramandibular gland of Aneuretus simoni (Formicidae, Aneuretinae). Asian Myrmecol. 2016, 8, 95–99. [Google Scholar] [CrossRef]
- Billen, J.; Delsinne, T. A novel intramandibular gland in the ant Tatuidris tatusia (Hymenoptera: Formicidae). Myrmecol. News 2014, 19, 61–64. [Google Scholar]
- do Amaral, J.B.; Caetano, F.H. The intramandibular gland of leaf-cutting ants (Atta sexdens rubropilosa Forel 1908). Micron 2006, 37, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Billen, J.; Al-Khalifa, M. A novel intramandibular gland in the ant Brachyponera sennaarensis. Insect. Soc. 2016, 63, 321–326. [Google Scholar] [CrossRef]
- Billen, J.; Espadaler, X. A novel epithelial intramandibular gland in the ant Pyramica membranifera (Hymenoptera, Formicidae). Belg. J. Zool. 2002, 132, 175–176. [Google Scholar]
- Billen, J.; Bauweleers, E.; Hashim, R.; Ito, F. Survey of the exocrine system in Protanilla wallacei (Hymenoptera, Formicidae). Arthropod Struct. Dev. 2013, 42, 173–183. [Google Scholar] [CrossRef]
- Costa Leonardo, A.M. Glândulas intramandibulares em abelhas sociais. Ciencia e cultura 1978, 30, 835–838. [Google Scholar]
- Da Cruz-Landim, C.; Gracioli-Vitti, L.F.; Abdalla, F.C. Ultrastructure of the intramandibular gland of workers and queens of the stingless bee, Melipona quadrifasciata. J. Insect Sci. 2011, 11, 1–9. [Google Scholar] [CrossRef]
- Penagos-Arevalo, A.C.; Billen, J.; Sarmiento, C.E. Uncovering head gland diversity in neotropical Polistinae wasps (Hymenoptera, Vespidae): Comparative analysis and description of new glands. Arthropod Struct. Dev. 2015, 44, 415–425. [Google Scholar] [CrossRef]
- Vancova, M.; Bily, T.; Nebesarova, J.; Grubhoffer, L.; Bonnet, S.; Park, Y.; Simo, L. Ultrastructural mapping of salivary gland innervation in the tick Ixodes ricinus. Sci. Rep. 2019, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.C.B.; Do Nascimento, F.S.; Campos, M.C.G.; Lima, E.R.; Zanuncio, J.C.; Serrão, J.E. Chemical composition of the intramandibular glands of the ant Neoponera villosa (Fabricius, 1804) (Hymenoptera: Ponerinae). Chemoecology 2015, 25, 25–31. [Google Scholar] [CrossRef]
- Grasso, D.A.; Romani, R.; Castracani, C.; Visicchio, R.; Mori, A.; Isidoro, N.; Le Moli, F. Mandible associated glands in queens of the slave-making ant Polyergus rufescens (Hymenoptera, Formicidae). Insect. Soc. 2004, 51, 74–80. [Google Scholar] [CrossRef]
- Visicchio, R.; Mori, A.; Grasso, D.A.; Castracani, C.; Le Moli, F. Glandular sources of recruitment, trail, and propaganda semiochemicals in the slave-making ant Polyergus rufescens. Ethol. Ecol. Evol. 2001, 13, 361–372. [Google Scholar] [CrossRef]
| Species | Collection Locality | W | AQ | M | |
|---|---|---|---|---|---|
| short-mandibulate | S. bentenT | Yuchi Township, Nantou County, Taiwan | 3 | 0 | 1 |
| S. canina | Kobe, Japan | 2 | 0 | 0 | |
| S. elegantulaT | Yuchi Township, Nantou County, Taiwan | 4 | 0 | 0 | |
| S. emmaeS | Baihe District, Tainan City, Taiwan | 2 | 0 | 0 | |
| S. hexamera | Takamatsu, Japan | 5 | 0 | 0 | |
| S. kichijoT | Lugu Township, Nantou County, Taiwan | 4 | 0 | 0 | |
| S. leptothrixS,T | Ren’ai Township, Nantou County, Taiwan | 4 | 0 | 0 | |
| S. membranifera | Ren’ai Township, Nantou County, Taiwan | 1 | 0 | 0 | |
| S. muticaS,T | Yuchi Township, Nantou County, Taiwan | 8 | 5 | 0 | |
| S. sauteri | Lugu Township, Nantou County, Taiwan | 2 | 2 | 0 | |
| long-mandibulate | S. chuchihensis | Jianshi Township, Xinchu County, Taiwan | 0 | 1 | 0 |
| S. formosensisS,T | Ren’ai Township, Nantou County, Taiwan | 6 | 0 | 0 | |
| S. hispida | Yuchi Township, Nantou County, Taiwan | 3 | 0 | 0 | |
| S. koningsbergeri | Bogor Botanical Garden, Indonesia | 1 | 0 | 0 | |
| S. lacunosaT | Lugu Township, Nantou County, Taiwan | 2 | 1 | 0 | |
| S. liukueiensis | Jiji Township, Nantou County, Taiwan | 1 | 2 | 0 | |
| S. minutulaT | Hengchun Township, Pingtung County, Taiwan | 2 | 0 | 0 | |
| S. nanzanensis | Lanyu Township, Taitung County, Taiwan | 1 | 0 | 0 | |
| S. orchidensis | Lanyu Township, Taitung County, Taiwan | 1 | 0 | 0 | |
| S. perplexa | Clyde Mountain, NSW, Australia | 2 | 0 | 0 | |
| S. rogeriS | Jiji Township, Nantou County, Taiwan | 0 | 2 | 0 | |
| S. solifontis | Lugu Township, Nantou County, Taiwan | 6 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Steenhuyse-Vandevelde, M.; Lin, C.-C.; Billen, J. Morphology of the Novel Basimandibular Gland in the Ant Genus Strumigenys (Hymenoptera, Formicidae). Insects 2021, 12, 50. https://doi.org/10.3390/insects12010050
Wang C, Steenhuyse-Vandevelde M, Lin C-C, Billen J. Morphology of the Novel Basimandibular Gland in the Ant Genus Strumigenys (Hymenoptera, Formicidae). Insects. 2021; 12(1):50. https://doi.org/10.3390/insects12010050
Chicago/Turabian StyleWang, Chu, Michael Steenhuyse-Vandevelde, Chung-Chi Lin, and Johan Billen. 2021. "Morphology of the Novel Basimandibular Gland in the Ant Genus Strumigenys (Hymenoptera, Formicidae)" Insects 12, no. 1: 50. https://doi.org/10.3390/insects12010050
APA StyleWang, C., Steenhuyse-Vandevelde, M., Lin, C.-C., & Billen, J. (2021). Morphology of the Novel Basimandibular Gland in the Ant Genus Strumigenys (Hymenoptera, Formicidae). Insects, 12(1), 50. https://doi.org/10.3390/insects12010050





