Organic Control of Pear Psylla in Pear with Trunk Injection
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Plots and Treatment Compounds
2.2. Trunk Injection
2.3. Foliar Application
2.4. Field Evaluations
2.5. Residue Sample Collection and Preparation
2.6. Residue Sample Analysis
2.7. Statistical Analysis
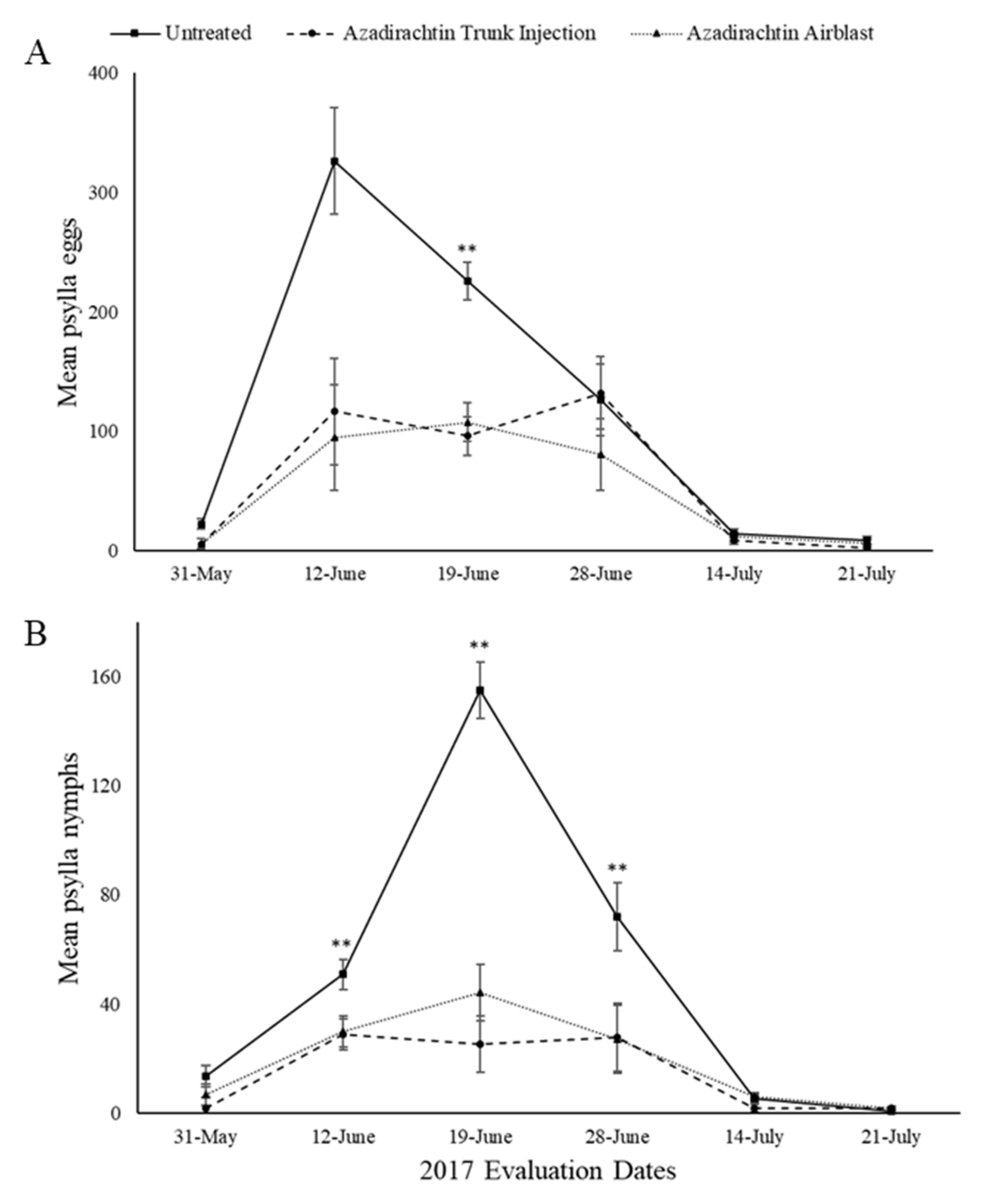
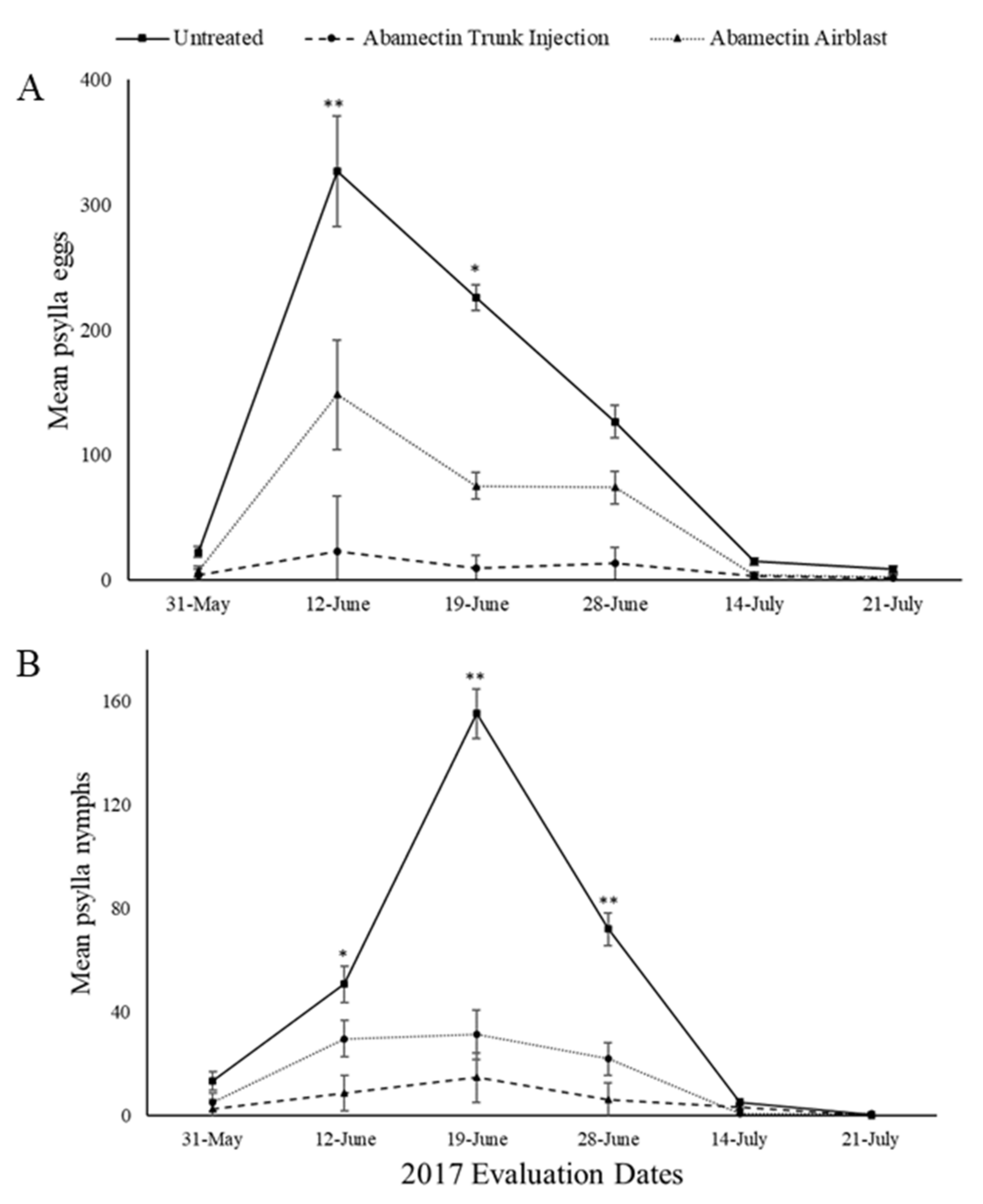
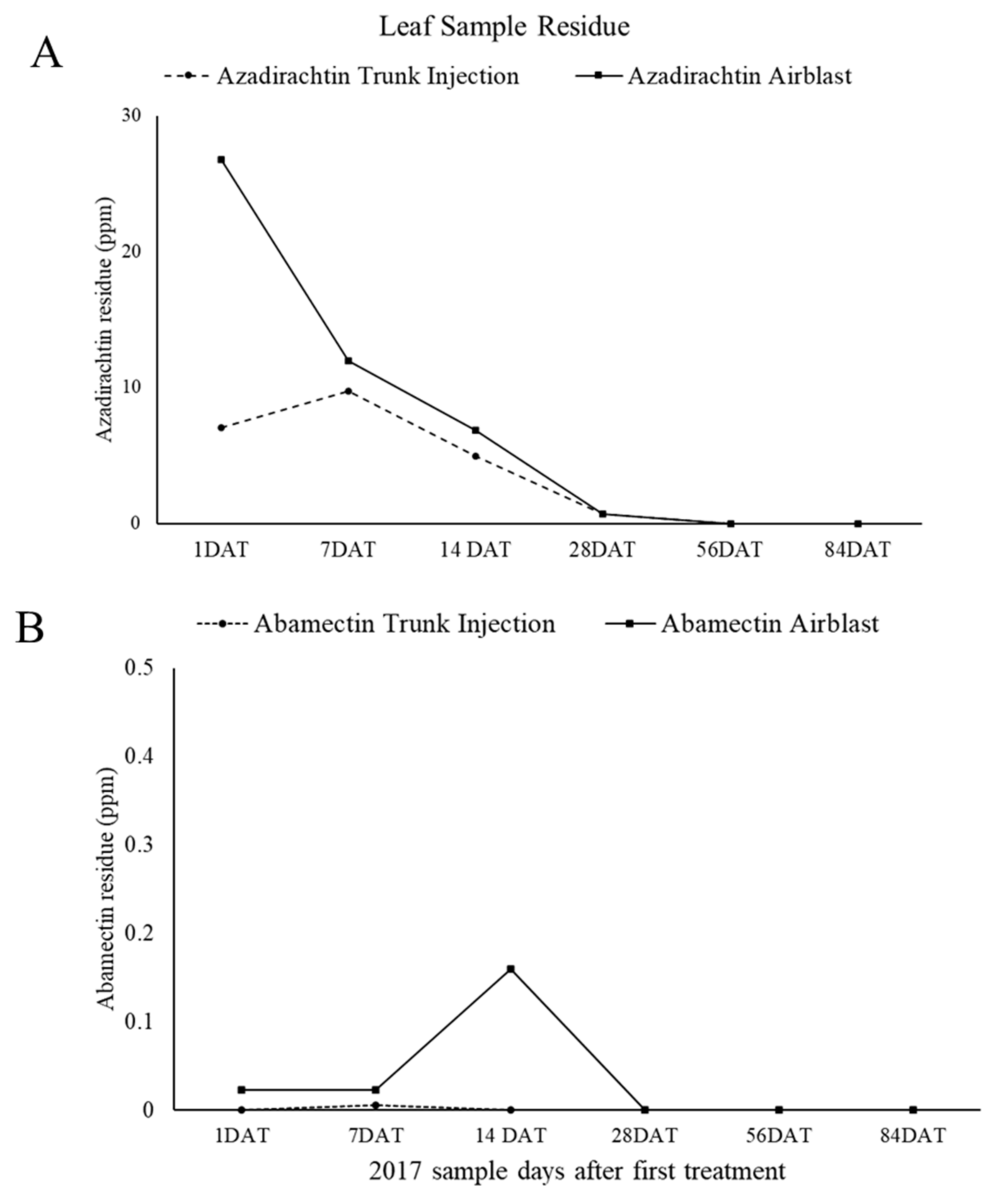
3. Results
3.1. Field Evaluations
3.1.1. 2017 Season
3.1.2. 2018 Season
3.2. Residue Profiling
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Horton, D.R. Monitoring of pear psylla for pest management decisions and research. Integr. Pest Manag. Rev. 1999, 4, 1–20. [Google Scholar] [CrossRef]
- Westigard, P.H.; Allen, R.B.; Gut, L.J. Pear psylla: Relationship of early-season nymph densities to honeydew-induced fruit damage on two pear cultivars13. J. Econ. Entomol. 1981, 74, 532–534. [Google Scholar] [CrossRef]
- Howitt, A.J. Common Tree Fruit Pests; Michigan State University Extension: East Lansing, MI, USA, 1993. [Google Scholar]
- Seemüller, E.; Schneider, B. “Candidatus Phytoplasma Mali”, “Candidatus Phytoplasma Pyri” and “Candidatus Phytoplasma Prunorum”, the casual agents of apple proliferation, pear decline and european stone fruit yellows, respectively. Int. J. Syst. Evol. Microbiol. 2004, 54, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Ing, G. Pear production and utilization in North America. Acta Hortic. 2002, 8, 61–65. [Google Scholar] [CrossRef]
- (Dataset) USDA National Agricultural Statistics Service. NASS—Quick Stats. USDA National Agricultural Statistics Service, 2017. Available online: https://data.nal.usda.gov/dataset/nass-quick-stats (accessed on 1 September 2020).
- Horton, D.R. Oviposition by overwintering morph of pear psylla (Homoptera: Psyllidae) with information on conditioning. Environ. Entomol. 1990, 19, 357–361. [Google Scholar] [CrossRef]
- McMullen, R.D.; Jong, C. Dithiocarbamate fungicides for control of pear psylla. J. Econ. Entomol. 1971, 64, 1266–1270. [Google Scholar] [CrossRef]
- Whalon, M.E.; Mota-Sanchez, D.; Hollingworth, R.M. Global pesticide resistance in arthropods. Entomol. Exp. Appl. 2008, 131, 106. [Google Scholar]
- Marcic, D.; Ogurlic, I.; Prijovic, M.; Peric, P. Effectiveness of azadirachtin (NeemAzal-T/S) in controlling pear psylla (Cacopsylla Pyri) and European red mite (Panonychus Ulmi). Pestic. Fitomedicina 2009, 24, 123–131. [Google Scholar] [CrossRef]
- Bond, C.; Buhl, K.; Stone, D. Neem Oil General Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services: Corvallis, OR, USA, 2012; Available online: http://npic.orst.edu/factsheets/neemgen.html (accessed on 1 October 2019).
- Wise, J.C. Enhancing performance of biorational insecticides with novel delivery systems in tree fruit IPM. In Advances in Insect Control and Resistance Management; Rami Horowitz, A., Ishaaya, I., Eds.; Springer Publishing Ltd.: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2016; Chapter 5; pp. 77–92. [Google Scholar] [CrossRef]
- Zhu, H.; Derksen, R.C.; Guler, H.; Krause, C.R.; Ozkan, H.E. Foliar deposition and off-target loss with different spray techniques in nursery applications. Trans. ASABE 2006, 49, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Steiner, P.W. The Distribution of Spray Material between Target and Non-Target Areas of a Mature Apple Orchard by Airblast Equipment. Master’s Thesis, Cornell University, Ithaca, NY, USA, 1969. [Google Scholar]
- EU. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. Official J. Eur. Union 2009, L309, 1e50. [Google Scholar]
- US EPA. PRN 2001-X Draft: Spray and Dust Drift Label Statements for Pesticide Products. 2016. Available online: https://www.epa.gov/pesticide-registration/prn-2001-x-draft-spray-and-dust-drift-label-statements-pesticide-products (accessed on 14 February 2019).
- Aćimović, S.G.; Vanwoerkom, A.H.; Reeb, P.D.; Vandervoort, C.; Garavaglia, T.; Cregg, B.M.; Wise, J.C. Spatial and temporal distribution of trunk-injected imidacloprid in apple tree canopies. Pest Manag. Sci. 2014, 70, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Byrne, F.J.; Urena, A.A.; Robinson, L.J.; Krieger, R.I.; Doccola, J.; Morse, J.G. Evaluation of neonicotinoid, organophosphate and avermectin trunk injections for the management of avocado thrips in California Avocado Groves. Pest Manag. Sci. 2012, 68, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Coslor, C.C.; Vandervoort, C.; Wise, J.C. Insecticide dose and seasonal timing of trunk injection in apples influence efficacy and residues in nectar and plant parts. Pest Manag. Sci. 2018, 75, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, M.Z.; Alrubeai, H.F. Chemical control of date palm tree borers, oryctes species (Coloeptera: Scrabaidae: Dynastinae). Pak. Entomol. 2016, 38, 1–5. [Google Scholar]
- Civolani, S.; Cassanelli, S.; Rivi, M.; Manicardi, G.C.; Peretto, R.; Chicca, M.; Pasqualini, E.; Leis, M. Survey of susceptibility to abamectin of pear psylla (Hemiptera: Psyllidae) in Northern Italy. J. Econ. Entomol. 2010, 103, 816–822. [Google Scholar] [CrossRef]
- Bayer. Residue Analytical Method for the Determination of Residues of Imidacloprid in Plant Material by HPLC; Method no 00573; Bayer AG: Leverkusen, Germany, 1998. [Google Scholar]
- Wise, J.C.; Coombs, A.B.; Vandervoort, C.; Gut, L.J.; Hoffmann, E.J.; Whalon, M.E. Use of residue profile analysis to identify modes of insecticide activity contributing to control of plum curculio in apples. J. Econ. Entomol. 2006, 99, 2055–2064. [Google Scholar] [CrossRef]
- US EPA. Title 40 eCFR, Chapter I, Subchapter E, Part 180, Subpart C 180.449. 2020. Available online: https://www.ecfr.gov/cgi-bin/text-idx?tpl=/ecfrbrowse/Title40/40cfr180_main_02.tpl (accessed on 7 July 2020).
- Karnavar, G.K. Influence of azadirachtin on insect nutrition and reproduction. Proc. Anim. Sci. 1987, 96, 341–347. [Google Scholar] [CrossRef]
- Subrahmanyam, B. Azadirachtin—A naturally occurring insect growth regulator. Proc. Indian Acad. Sci. Anim. Sci. 1990, 99, 277–288. [Google Scholar] [CrossRef]
- US EPA. Azadirachtin: Tolerance Exemption. Federal Register. Vol. 58, No. 30. Rules and Regulations. Available online: https://www.federalregister.gov/documents/2009/10/28/E9-25455/cold-pressed-neem-oil-exemption-from-the-requirement-of-a-tolerance (accessed on 10 October 2019).
- Pavela, R.; ŽABKA, M.; Kalinkin, V.; Kotenev, E.; Gerus, A.; Shchenikova, A.; Chermenskaya, T. Systemic applications of azadirachtin in the control of corythucha ciliata (Say, 1832)(Hemiptera, Tingidae), a pest of platanus sp. Plant Prot. Sci. 2013, 49, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Cevenini, L.; Minelli, A. Translocation of active ingredient using three trunk injection methods. Acta Hortic. 2010, 881, 409–412. [Google Scholar] [CrossRef]
- Barnby, M.A.; Yamasaki, R.B.; Klocke, A.A. Biological activity of azadirachtin., three derivatives., and their ultraviolet radiation degradation products against tobacco budworm (Lepidoptera: Noctuidae) larvae. J. Econ. Entomol. 1989, 82, 58–63. [Google Scholar] [CrossRef]
- Dureja, P.; Johnson, S. Photodegradation of azadirachtin-A: A neem-based pesticide. Curr. Sci. 2000, 79, 1700–1703. [Google Scholar]
- Stokes, J.B.; Redfern, R.E. Effect of sunlight on azadirachtin: Antifeeding potency. J. Environ. Sci. Health Part A Environ. Sci. Eng. 1982, 17, 57–65. [Google Scholar] [CrossRef]
- Lasota, J. Avermectins, a novel class of compounds: Implications for use in arthropod pest control. Annu. Rev. Entomol. 2002, 36, 91–117. [Google Scholar] [CrossRef]
- Bai, S.H.; Ogbourne, S.M. Eco-toxicological effects of the avermectin family with a focus on abamectin and ivermectin. Chemosphere 2016, 154, 204–214. [Google Scholar] [CrossRef]
- VanWoerkom, A.H.; Aćimović, S.G.; Sundin, G.W.; Cregg, B.M.; Mota-Sanchez, D.; Vandervoort, C.; Wise, J.C. Trunk injection: An alternative technique for pesticide delivery in apples. Crop Prot. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Wise, J.C.; VanWoerkom, A.H.; Acimovic, S.G.; Sundin, G.W.; Cregg, B.M.; Vandervoort, C. Trunk injection: A discriminating delivering system for horticulture crop IPM. Entomol. Ornithol. Herpetol. Curr. Res. 2014, 3, 3–9. [Google Scholar]
- Coslor, C.C.; Sundin, G.W.; Wise, J.C. The efficacy of trunk injections of emamectin benzoate and phosphorous acid for control of obliquebanded leafroller and apple scab on semi-dwarf apple. Crop Prot. 2019, 118, 44–49. [Google Scholar] [CrossRef]


| Treatment/Application Method | Trade Name | Active Ingredient | Application Rate | Active Ingredient per Tree |
|---|---|---|---|---|
| Untreated Control | - | - | - | - |
| Abamectin/Trunk Injection | Agri-Mek 0.15 EC | abamectin | 2.40 mL/tree | 0.04 g |
| Abamectin/Airblast | Agri-Mek 0.7 SC | abamectin | 0.29 L/ha | 0.04 g |
| Azadirachtin/Trunk Injection | Azasol 6% | azadirachtin | 4.0 g/tree | 0.24 g |
| Azadirachtin/Airblast | Azasol 6% | azadirachtin | 2.45 kg/ha | 0.24 g |
| Treatment/Application Method | Mean Leaves with Black Sooty Mold |
|---|---|
| Untreated | 71.5 a |
| Abamectin/Trunk Injection | 15.8 b |
| Abamectin/Airblast | 10.3 b |
| Azadirachtin/Trunk Injection | 8.5 b |
| Azadirachtin/Airblast | 14 b |
| Treatment/Application Method | 7 DAT (Days after First Treatment) | 84 DAT |
|---|---|---|
| Untreated | nd | nd |
| Azadirachtin/Trunk Injection | 1.507 | nd |
| Azadirachtin/Airblast | nd | nd |
| Treatment/Application Method | 7 DAT | 84 DAT |
|---|---|---|
| Untreated | nd | nd |
| Abamectin/Trunk Injection | nd | nd |
| Abamectin/Airblast | nd | nd |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wheeler, C.E.; Vandervoort, C.; Wise, J.C. Organic Control of Pear Psylla in Pear with Trunk Injection. Insects 2020, 11, 650. https://doi.org/10.3390/insects11090650
Wheeler CE, Vandervoort C, Wise JC. Organic Control of Pear Psylla in Pear with Trunk Injection. Insects. 2020; 11(9):650. https://doi.org/10.3390/insects11090650
Chicago/Turabian StyleWheeler, Celeste E., Christine Vandervoort, and John C. Wise. 2020. "Organic Control of Pear Psylla in Pear with Trunk Injection" Insects 11, no. 9: 650. https://doi.org/10.3390/insects11090650
APA StyleWheeler, C. E., Vandervoort, C., & Wise, J. C. (2020). Organic Control of Pear Psylla in Pear with Trunk Injection. Insects, 11(9), 650. https://doi.org/10.3390/insects11090650





