Mixed-Species Gardens Increase Monarch Oviposition without Increasing Top-Down Predation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
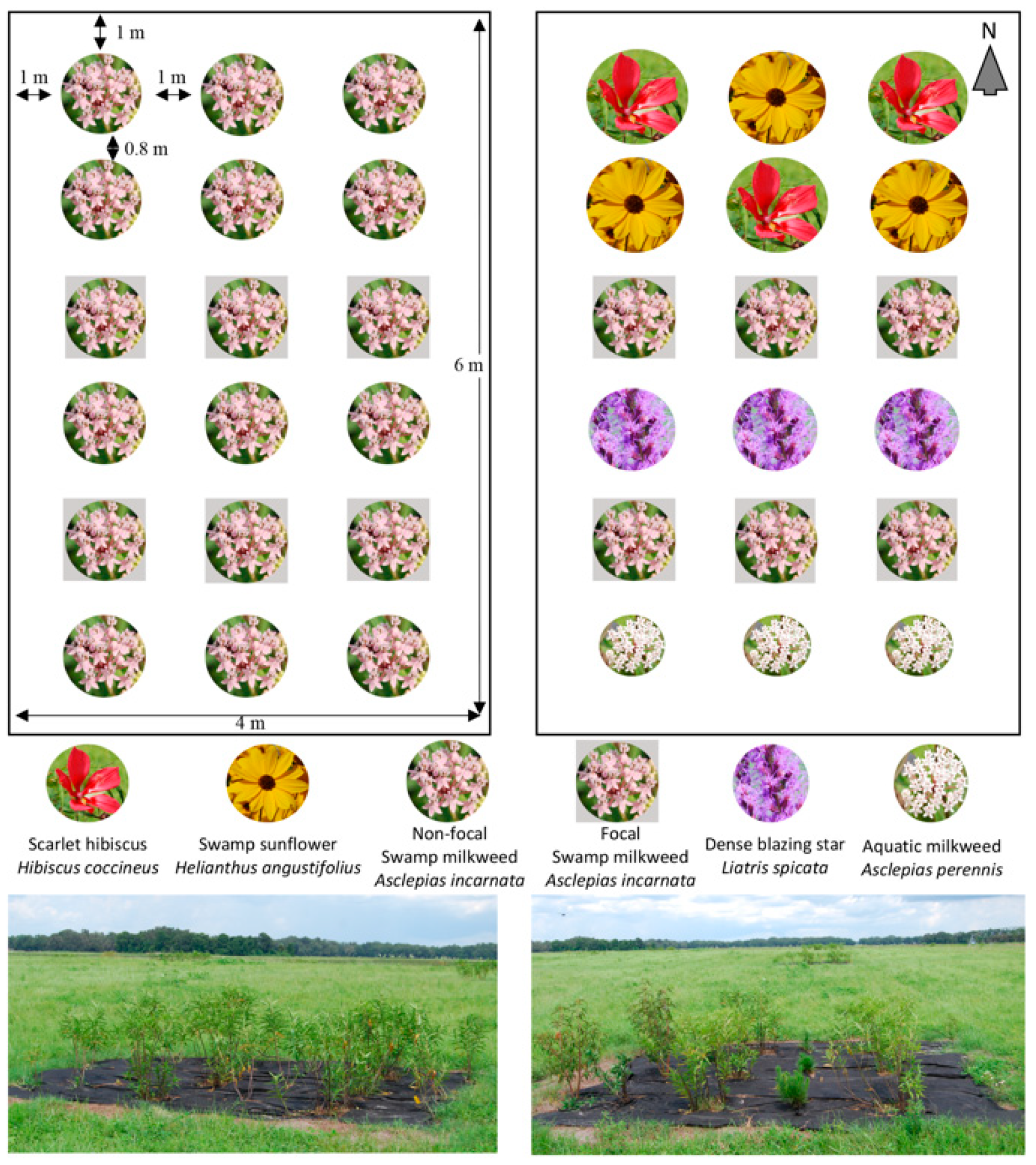
2.1. Plant Selection and Experimental Design
2.2. Floral Abundance and Richness
2.3. Effects of Plant Diversity on Monarch Colonization and Establishment
2.4. Effects of Plant Diversity on Monarch Natural Enemies
2.5. Separating Top-Down and Bottom-Up Drivers of Monarch Success
2.6. Statistical Analysis
3. Results
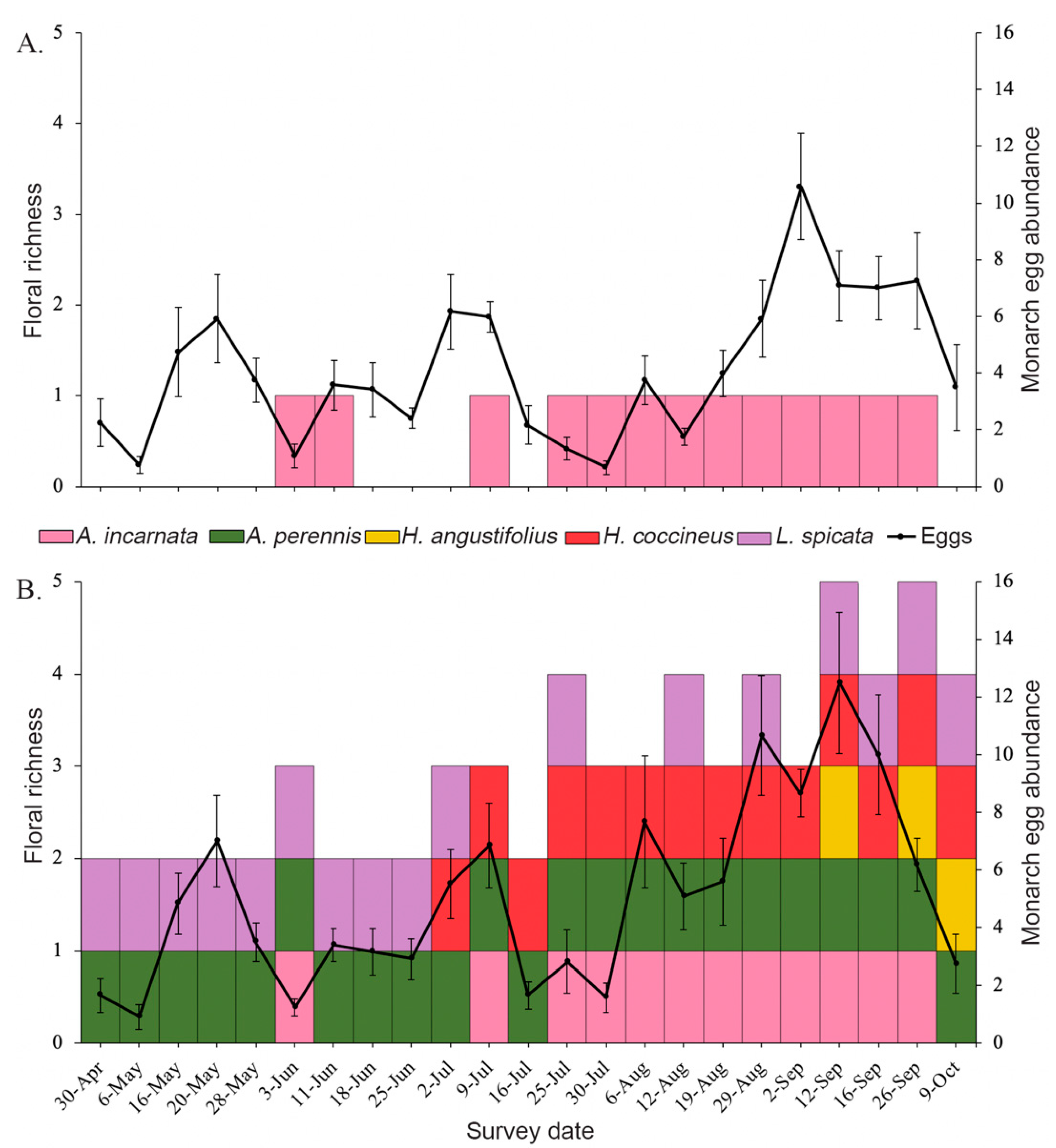
3.1. Floral Abundance and Richness
3.2. Effects of Plant Diversity on Monarch Colonization and Establishment
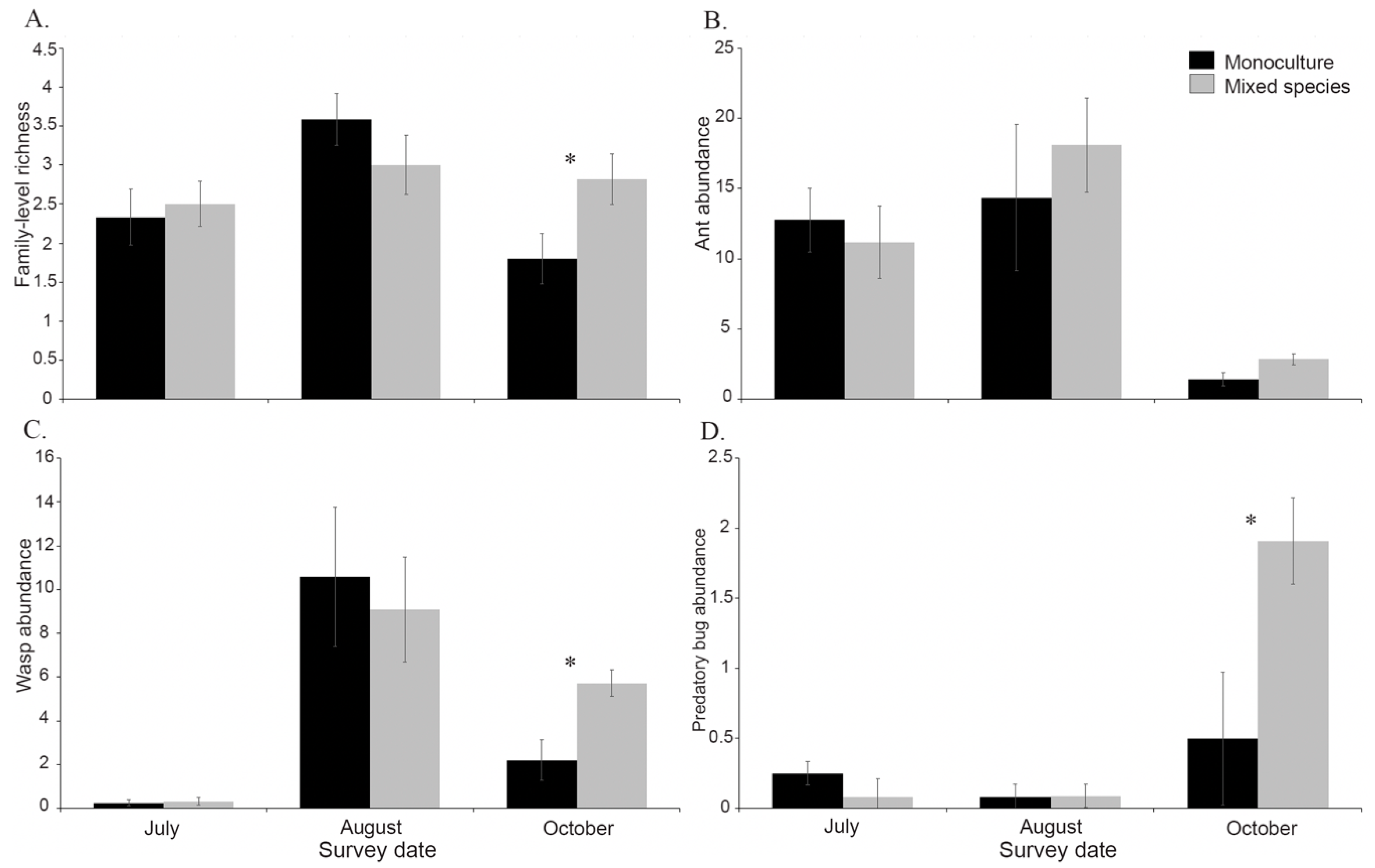
3.3. Effects of Plant Diversity on Monarch Natural Enemies
3.4. Separating Top-Down and Bottom-Up Drivers of Monarch Success
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brower, L.P.; Williams, E.H.; Dunford, K.S.; Dunford, J.C.; Knight, A.L.; Daniels, J.; Cohen, J.A.; Van Hook, T.; Saarinen, E.; Standridge, M.J.; et al. A Long-Term Survey of Spring Monarch Butterflies in North-Central Florida. J. Nat. Hist. 2018, 52, 2025–2046. [Google Scholar] [CrossRef]
- Pelton, E.M.; Schultz, C.B.; Jepsen, S.J.; Black, S.H.; Crone, E.E. Western Monarch Population Plummets: Status, Probable Causes, and Recommended Conservation Actions. Front. Ecol. Evol. 2019, 7, 258. [Google Scholar] [CrossRef]
- Pleasants, J.M.; Oberhauser, K.S. Milkweed Loss in Agricultural Fields Because of Herbicide Use: Effect on the Monarch Butterfly Population. Insect Conserv. Divers. 2013, 6, 135–144. [Google Scholar] [CrossRef]
- Pleasants, J. Milkweed Restoration in the Midwest for Monarch Butterfly Recovery: Estimates of Milkweeds Lost, Milkweeds Remaining and Milkweeds That Must Be Added to Increase the Monarch Population. Insect Conserv. Divers. 2017, 10, 42–53. [Google Scholar] [CrossRef]
- Thogmartin, W.E.; López-Hoffman, L.; Rohweder, J.; Diffendorfer, J.; Drum, R.; Semmens, D.; Black, S.; Caldwell, I.; Cotter, D.; Drobney, P.; et al. Restoring Monarch Butterfly Habitat in the Midwestern US: ‘All Hands on Deck’. Environ. Res. Lett. 2017, 12, 074005. [Google Scholar] [CrossRef]
- Malcolm, S.B. Anthropogenic Impacts on Mortality and Population Viability of the Monarch Butterfly. Annu. Rev. Entomol. 2018, 63, 277–302. [Google Scholar] [CrossRef]
- Stenoien, C.; Nail, K.R.; Zalucki, J.M.; Parry, H.; Oberhauser, K.S.; Zalucki, M.P. Monarchs in Decline: A Collateral Landscape-Level Effect of Modern Agriculture. Insect Sci. 2018, 25, 528–541. [Google Scholar] [CrossRef]
- Cutting, B.T.; Tallamy, D.W. An Evaluation of Butterfly Gardens for Restoring Habitat for the Monarch Butterfly (Lepidoptera: Danaidae). Environ. Entomol. 2015, 44, 1328–1335. [Google Scholar] [CrossRef]
- Dale, A.G.; Perry, R.L.; Cope, G.C.; Benda, N. Floral Abundance and Richness Drive Beneficial Arthropod Conservation and Biological Control on Golf Courses. Urban Ecosyst. 2020, 23, 55–66. [Google Scholar] [CrossRef]
- Kasten, K.; Stenoien, C.; Caldwell, W.; Oberhauser, K.S. Can Roadside Habitat Lead Monarchs on a Route to Recovery? J. Insect Conserv. 2016, 1047–1057. [Google Scholar] [CrossRef]
- Baker, A.M.; Potter, D.A. Configuration and Location of Small Urban Gardens Affect Colonization by Monarch Butterflies. Front. Ecol. Evol. 2019, 7, 474. [Google Scholar] [CrossRef]
- Pocius, V.M.; Debinski, D.M.; Pleasants, J.M.; Bidne, K.G.; Hellmich, R.L. Monarch Butterflies Do Not Place All of Their Eggs in One Basket: Oviposition on Nine Midwestern Milkweed Species. Ecosphere 2018, 9, e02064. [Google Scholar] [CrossRef]
- Majewska, A.A.; Sims, S.; Wenger, S.J.; Davis, A.K.; Altizer, S. Do Characteristics of Pollinator-Friendly Gardens Predict the Diversity, Abundance, and Reproduction of Butterflies? Insect Conserv. Divers. 2018, 11, 370–382. [Google Scholar] [CrossRef]
- Geest, E.A.; Wolfenbarger, L.L.; McCarty, J.P. Recruitment, Survival, and Parasitism of Monarch Butterflies (Danaus plexippus) in Milkweed Gardens and Conservation Areas. J. Insect Conserv. 2019, 23, 211–224. [Google Scholar] [CrossRef]
- Hairston, N.G.; Smith, F.E.; Slobodkin, L.B. Community Structure, Population Control, and Competition. Am. Nat. 1960, 94, 421–425. [Google Scholar] [CrossRef]
- Root, R.B. Organization of a Plant-Arthropod Association in Simple and Diverse Habitats: The Fauna of Collards (Brassica oleracea). Ecol. Monogr. 1973, 43, 95–124. [Google Scholar] [CrossRef]
- Barbosa, P.; Hines, J.; Kaplan, I.; Martinson, H.; Szczepaniec, A.; Szendrei, Z. Associational Resistance and Associational Susceptibility: Having Right or Wrong Neighbors. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 1–20. [Google Scholar] [CrossRef]
- Stenoien, C.; Nail, K.R.; Oberhauser, K.S. Habitat Productivity and Temporal Patterns of Monarch Butterfly Egg Densities in the Eastern United States. Ann. Entomol. Soc. Am. 2015, 108, 670–679. [Google Scholar] [CrossRef]
- Pitman, G.M.; Flockhart, D.T.T.; Norris, D.R. Patterns and Causes of Oviposition in Monarch Butterflies: Implications for Milkweed Restoration. Biol. Conserv. 2018, 217, 54–65. [Google Scholar] [CrossRef]
- Zalucki, M.P.; Kitching, R.L. Dynamics of Oviposition in Danaus plexippus (Insecta: Lepidoptera) on Milkweed, Asclepias spp. J. Zool. Proc. Zool. Soc. Lond. 1982, 198, 103–116. [Google Scholar] [CrossRef]
- Grez, A.A.; González, R.H. Resource Concentration Hypothesis: Effect of Host Plant Patch Size on Density of Herbivorous Insects. Oecologia 1995, 103, 471–474. [Google Scholar] [CrossRef]
- Kral-O’Brien, K.C.; Hovick, T.J.; Limb, R.F.; Harmon, J.P.; Gillam, E.H. Incorporating Field Behaviors into Monarch Surveys to Promote Informed Conservation Actions. J. Nat. Conserv. 2020, 53, 125761. [Google Scholar] [CrossRef]
- Frank, S.D.; Shrewsbury, P.M. Effect of Conservation Strips on the Abundance and Distribution of Natural Enemies and Predation of Agrotis ipsilon (Lepidoptera: Noctuidae) on Golf Course Fairways. Environ. Entomol. 2004, 33, 1662–1672. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Isaacs, R. Larger Wildflower Plantings Increase Natural Enemy Density, Diversity, and Biological Control of Sentinel Prey, without Increasing Herbivore Density. Ecol. Entomol. 2012, 37, 386–394. [Google Scholar] [CrossRef]
- Rosas-Ramos, N.; Baños-Picón, L.; Tormos, J.; Asís, J.D. The Complementarity between Ecological Infrastructure Types Benefits Natural Enemies and Pollinators in a Mediterranean Vineyard Agroecosystem. Ann. Appl. Biol. 2019, 175, 193–201. [Google Scholar] [CrossRef]
- Oberhauser, K.; Elmquist, D.; Perilla-López, J.M.; Gebhard, I.; Lukens, L.; Stireman, J. Tachinid Fly (Diptera: Tachinidae) Parasitoids of Danaus plexippus (Lepidoptera: Nymphalidae). Ann. Entomol. Soc. Am. 2017, 110, 536–543. [Google Scholar] [CrossRef]
- Prysby, M.D. Natural Enemies and Survival of Monarch Eggs and Larvae. In The Monarch Butterfly: Biology and Conservation; Cornell University Press: Ithaca, NY, USA, 2004; pp. 27–37. [Google Scholar]
- Nail, K.R.; Stenoien, C.; Oberhauser, K.S. Immature Monarch Survival: Effects of Site Characteristics, Density, and Time. Ann. Entomol. Soc. Am. 2015, 108, 680–690. [Google Scholar] [CrossRef]
- Hermann, S.L.; Blackledge, C.; Haan, N.L.; Myers, A.T.; Landis, D.A. Predators of Monarch Butterfly Eggs and Neonate Larvae Are More Diverse than Previously Recognised. Sci. Rep. 2019, 9, 14304. [Google Scholar] [CrossRef]
- Vander Zanden, H.B.; Chaffee, C.L.; González-Rodríguez, A.; Flockhart, D.T.T.; Norris, D.R.; Wayne, M.L. Alternate Migration Strategies of Eastern Monarch Butterflies Revealed by Stable Isotopes. Anim. Migr. 2018, 5, 74–83. [Google Scholar] [CrossRef]
- Baker, A.M.; Potter, D.A. Colonization and Usage of Eight Milkweed (Asclepias) Species by Monarch Butterflies and Bees in Urban Garden Settings. J. Insect Conserv. 2018, 22, 405–418. [Google Scholar] [CrossRef]
- Tofangsazi, N.; Cherry, R.H.; Beeson, R.C.; Arthurs, S.P. Concentration-Response and Residual Activity of Insecticides to Control Herpetogramma phaeopteralis (Lepidoptera: Crambidae) in St. Augustinegrass. J. Econ. Entomol. 2015, 108, 730–735. [Google Scholar] [CrossRef]
- Redmond, C.T.; Potter, D.A. Chlorantraniliprole: Reduced-Risk Insecticide for Controlling Insect Pests of Woody Ornamentals with Low Hazard to Bees. Arboric. Urban For. 2017, 43, 242–256. [Google Scholar]
- Larson, J.L.; Redmond, C.T.; Potter, D.A. Comparative Impact of an Anthranilic Diamide and Other Insecticidal Chemistries on Beneficial Invertebrates and Ecosystem Services in Turfgrass. Pest Manag. Sci. 2012, 68, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Larson, J.L.; Redmond, C.T.; Potter, D.A. Assessing Insecticide Hazard to Bumble Bees Foraging on Flowering Weeds in Treated Lawns. PLoS ONE 2013, 8, e66375. [Google Scholar] [CrossRef]
- de Roode, J.C.; Rarick, R.M.; Mongue, A.J.; Gerardo, N.M.; Hunter, M.D. Aphids Indirectly Increase Virulence and Transmission Potential of a Monarch Butterfly Parasite by Reducing Defensive Chemistry of a Shared Food Plant. Ecol. Lett. 2011, 14, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Kroes, A.; van Loon, J.J.A.; Dicke, M. Density-Dependent Interference of Aphids with Caterpillar-Induced Defenses in Arabidopsis: Involvement of Phytohormones and Transcription Factors. Plant Cell Physiol. 2015, 56, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Zalucki, M.P. Temperature and Rate of Development in Danaus plexippus L. and D. chrysippus L. (Lepidoptera: Nymphalidae). Aust. J. Entomol. 1982, 21, 241–246. [Google Scholar] [CrossRef]
- Howard, E.; Aschen, H.; Davis, A.K. Citizen Science Observations of Monarch Butterfly Overwintering in the Southern United States. Psyche A J. Entomol. 2010, 2010, 1–6. [Google Scholar] [CrossRef]
- Knight, A.; Brower, L.P. The Influence of Eastern North American Autumnal Migrant Monarch Butterflies (Danaus plexippus L.) on Continuously Breeding Resident Monarch Populations in Southern Florida. J. Chem. Ecol. 2009, 35, 816–823. [Google Scholar] [CrossRef]
- McKinney, M.L. Effects of Urbanization on Species Richness: A Review of Plants and Animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Goddard, M.A.; Dougill, A.J.; Benton, T.G. Scaling up from Gardens: Biodiversity Conservation in Urban Environments. Trends Ecol. Evol. 2010, 25, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Mata, L.; Threlfall, C.G.; Williams, N.S.G.; Hahs, A.K.; Malipatil, M.; Stork, N.E.; Livesley, S.J. Conserving Herbivorous and Predatory Insects in Urban Green Spaces. Sci. Rep. 2017, 7, 40970. [Google Scholar] [CrossRef] [PubMed]
- Derby Lewis, A.; Bouman, M.J.; Winter, A.M.; Hasle, E.A.; Stotz, D.F.; Johnston, M.K.; Klinger, K.R.; Rosenthal, A.; Czarnecki, C.A. Does Nature Need Cities? Pollinators Reveal a Role for Cities in Wildlife Conservation. Front. Ecol. Evol. 2019, 7, 220. [Google Scholar] [CrossRef]
- Pywell, R.F.; Warman, E.A.; Sparks, T.H.; Greatorex-Davies, J.N.; Walker, K.J.; Meek, W.R.; Carvell, C.; Petit, S.; Firbank, L.G. Assessing Habitat Quality for Butterflies on Intensively Managed Arable Farmland. Biol. Conserv. 2004, 118, 313–325. [Google Scholar] [CrossRef]
- Tahvanainen, J.O.; Root, R.B. The Influence of Vegetational Diversity on the Population Ecology of a Specialized Herbivore, Phyllotreta cruciferae (Coleoptera: Chrysomelidae). Oecologia 1972, 10, 321–346. [Google Scholar] [CrossRef]
- Castagneyrol, B.; Giffard, B.; Péré, C.; Jactel, H. Plant Apparency, an Overlooked Driver of Associational Resistance to Insect Herbivory. J. Ecol. 2013, 101, 418–429. [Google Scholar] [CrossRef]
- Feeny, P. Plant Apparency and Chemical Defense. In Biochemical Interaction between Plants and Insects; Springer: Boston, MA, USA, 1976; pp. 1–40. [Google Scholar]
- Brower, L.P.; Fink, L.S.; Walford, P. Fueling the Fall Migration of the Monarch Butterfly. Integr. Comp. Biol. 2006, 46, 1123–1142. [Google Scholar] [CrossRef]
- Moscardini, V.F.; Gontijo, P.C.; Michaud, J.P.; Carvalho, G.A. Sublethal Effects of Chlorantraniliprole and Thiamethoxam Seed Treatments When Lysiphlebus testaceipes Feed on Sunflower Extrafloral Nectar. BioControl B 2014, 59, 503–511. [Google Scholar] [CrossRef]
- Oliveira, R.L.; Gontijo, P.C.; Sâmia, R.R.; Carvalho, G.A. Long-Term Effects of Chlorantraniliprole Reduced Risk Insecticide Applied as Seed Treatment on Lady Beetle Harmonia axyridis (Coleoptera: Coccinellidae). Chemosphere 2019, 219, 678–683. [Google Scholar] [CrossRef]
- Majewska, A.A.; Altizer, S. Planting Gardens to Support Insect Pollinators. Conserv. Biol. 2020, 34, 15–25. [Google Scholar] [CrossRef]
- Brower, L.P.; Fink, L.S. A Natural Toxic Defense System: Cardenolides in Butterflies versus Birds. Ann. N. Y. Acad. Sci. 1985, 443, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Rayor, L. Effects of Monarch Larval Host Plant Chemistry and Body Size on Polistes Wasp Predatoin. In The Monarch Butterfly: Biology and Conservation; Cornell University Press: Ithaca, NY, USA, 2004; pp. 39–46. [Google Scholar]
- Baker, A.M.; Potter, D.A. Invasive Paper Wasp Turns Urban Pollinator Gardens into Ecological Traps for Monarch Butterfly Larvae. Sci. Rep. 2020, 10, 9553. [Google Scholar] [CrossRef] [PubMed]
- Lattin, J.D. Bionomics of the Anthocoridae. Annu. Rev. Entomol. 1999, 44, 207–231. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.A.; Souza, E.N.; Bleakley, L.A.; Burley, C.; Mott, J.L.; Rue-Glutting, G.; Fellowes, M.D. Influence of Urbanisation and Plants on the Diversity and Abundance of Aphids and Their Ladybird and Hoverfly Predators in Domestic Gardens. Eur. J. Entomol. 2018, 115, 140–149. [Google Scholar] [CrossRef]
- Gardiner, M.M.; Landis, D.A.; Gratton, C.; Difonzo, C.D.; O’Neal, M.; Chacon, J.M.; Wayo, M.T.; Schmidt, N.P.; Mueller, E.E.; Heimpel, G.E. Landscape Diversity Enhances Biological Control of an Introduced Crop Pest. Ecol. Appl. 2015, 19, 143–154. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.; Booij, C.J.; Tscharntke, T. Sustainable Pest Regulation in Agricultural Landscapes: A Review on Landscape Composition, Biodiversity and Natural Pest Control. Proc. R. Soc. B Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef]
- Borders, B.; Lee-Mäder, E. Milkweeds: A Conservation Practitioner’s Guide; The Xerces Society for Invertebrate Conservation: Portland, OR, USA, 2014; p. 143. [Google Scholar]
- Brower, L.P. Evidence for Interspecific Competition in Natural Populations of the Monarch and Queen Butterflies, Danaus plexippus and D. gilippus Berenice in South Central Florida. Ecology 1962, 43, 549–552. [Google Scholar] [CrossRef]
- Knight, A.; Brower, L.P.; Williams, E.H. Spring Remigration of the Monarch Butterfly, Danaus plexippus (Lepidoptera: Nymphalidae) in North-Central Florida: Estimating Population Parameters Using Mark-Recapture. Biol. J. Linn. Soc. 1999, 68, 531–556. [Google Scholar] [CrossRef]
- Satterfield, D.A.; Maerz, J.C.; Hunter, M.D.; Flockhart, D.T.T.; Hobson, K.A.; Norris, D.R.; Streit, H.; de Roode, J.C.; Altizer, S. Migratory Monarchs That Encounter Resident Monarchs Show Life-History Differences and Higher Rates of Parasite Infection. Ecol. Lett. 2018, 21, 1670–1680. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 Percent Decline over 27 Years in Total Flying Insect Biomass in Protected Areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global Pollinator Declines: Trends, Impacts and Drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- van Klink, R.; Bowler, D.E.; Gongalsky, K.B.; Swengel, A.B.; Gentile, A.; Chase, J.M. Meta-Analysis Reveals Declines in Terrestrial but Increases in Freshwater Insect Abundances. Science 2020, 368, 417–420. [Google Scholar]
- Wagner, D.L. Insect Declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef] [PubMed]
- Forister, M.L.; Pelton, E.M.; Black, S.H. Declines in Insect Abundance and Diversity: We Know Enough to Act Now. Conserv. Sci. Pract. 2019, 1, 1–8. [Google Scholar] [CrossRef]
- Dobbs, E.K.; Potter, D.A. Naturalized Habitat on Golf Courses: Source or Sink for Natural Enemies and Conservation Biological Control? Urban Ecosyst. 2016, 19, 899–914. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Isaacs, R. Wildflower Plantings Enhance the Abundance of Natural Enemies and Their Services in Adjacent Blueberry Fields. Biol. Control 2015, 91, 94–103. [Google Scholar] [CrossRef]
- Tao, L.; Berns, A.R.; Hunter, M.D. Why Does a Good Thing Become Too Much? Interactions between Foliar Nutrients and Toxins Determine Performance of an Insect Herbivore. Funct. Ecol. 2014, 28, 190–196. [Google Scholar] [CrossRef]
- Andow, D. Vegetational Diversity and Arthropod Population Response. Annu. Rev. Entomol. 1991, 36, 561–586. [Google Scholar] [CrossRef]
| Entire Survey Period | Mean | SE | Test Statistic | p-Value | ||
|---|---|---|---|---|---|---|
| Floral richness | X223 = 683 | <0.001* | ||||
| Diversity trt | Monoculture | 0.35 | 0.03 | 499 | <0.001 * | |
| Mixed-spp | 1.72 | 0.07 | ||||
| Date | 417 | <0.001 * | ||||
| Weekly egg count per focal milkweed plantings | F23,528 = 15 | <0.001 * | ||||
| Diversity trt | Monoculture | 4.13 | 0.26 | 6.57 | 0.01 * | |
| Mixed-spp | 5.05 | 0.32 | ||||
| Date | 15 | <0.001 * | ||||
| Total egg count per focal milkweed plantings | Diversity trt | Monoculture | 95.0 | 8.31 | F1,19 = 3.71 | 0.07 |
| Mixed-spp | 116.2 | 8.78 | ||||
| Focal milkweed umbel abundance | F7,145 = 9.30 | <0.001 * | ||||
| Diversity trt | Monoculture | 39.6 | 7.43 | 1.85 | 0.18 | |
| Mixed-spp | 44.2 | 7.34 | ||||
| Date | 10.66 | <0.001 * | ||||
| Swamp Milkweed Bloom Period | Mean | SE | Test Statistic | p-Value | ||
| Floral richness | X210 = 294 | <0.001 * | ||||
| Diversity trt | Monoculture | 0.75 | 0.04 | 283 | <0.001 * | |
| Mixed-spp | 2.56 | 0.07 | ||||
| Date | 42 | <0.001 * | ||||
| Weekly egg count per focal milkweed plantings | F10,229 = 17 | <0.001 * | ||||
| Diversity trt | Monoculture | 4.93 | 0.44 | 14 | 0.002 * | |
| Mixed-spp | 7.08 | 0.58 | ||||
| Date | 17 | <0.001 * | ||||
| Total egg count per focal milkweed plantings | Diversity trt | Monoculture | 49.3 | 4.65 | F1,19 = 6.88 | 0.02 * |
| Mixed-spp | 70.6 | 6.72 | ||||
| Monarch Natural Enemies | Mean | SE | F | p-Value | ||
|---|---|---|---|---|---|---|
| Natural Enemy Richness | F5,62 = 3.79 | <0.005 * | ||||
| Diversity trt | Monoculture | 2.62 | 0.23 | 1.53 | 0.22 | |
| Mixed-spp | 2.76 | 0.18 | ||||
| Date | 5.48 | 0.01 * | ||||
| Diversity × Date | 3.42 | 0.04 * | ||||
| Formicidae Abundance | F3,56 = 10.9 | <0.001 * | ||||
| Diversity trt | Monoculture | 9.76 | 1.77 | 0.39 | 0.54 | |
| Mixed-spp | 10.1 | 2.09 | ||||
| Date | 16 | <0.001 * | ||||
| Flying Hymenoptera Abundance | F5,66 = 17 | <0.001 * | ||||
| Diversity trt | Monoculture | 4.47 | 1.16 | 1.12 | 0.29 | |
| Mixed-spp | 4.91 | 1.22 | ||||
| Date | 36 | <0.001 * | ||||
| Diversity × Date | 4.34 | 0.02 * | ||||
| Hemiptera Abundance | F5,62 = 9.22 | <0.001 * | ||||
| Diversity trt | Monoculture | 0.26 | 0.11 | 4.74 | 0.03 * | |
| Mixed-spp | 0.68 | 0.21 | ||||
| Date | 13 | <0.001 * | ||||
| Diversity × Date | 6.57 | 0.002 * | ||||
| Monarch Larval Disappearance Rates | Mean % | SE | F | p | ||
|---|---|---|---|---|---|---|
| Overall model | F2,45 = 7.08 | 0.002 * | ||||
| Diversity trt | Monoculture | 59 | 8 | 1.13 | 0.29 | |
| Mixed-spp | 49 | 8 | ||||
| Caged trt | Predator Inclusion | 73 | 7 | 13 | <0.001 * | |
| Predator Exclusion | 36 | 7 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nestle, R.; Daniels, J.C.; Dale, A.G. Mixed-Species Gardens Increase Monarch Oviposition without Increasing Top-Down Predation. Insects 2020, 11, 648. https://doi.org/10.3390/insects11090648
Nestle R, Daniels JC, Dale AG. Mixed-Species Gardens Increase Monarch Oviposition without Increasing Top-Down Predation. Insects. 2020; 11(9):648. https://doi.org/10.3390/insects11090648
Chicago/Turabian StyleNestle, Rebecca, Jaret C. Daniels, and Adam G. Dale. 2020. "Mixed-Species Gardens Increase Monarch Oviposition without Increasing Top-Down Predation" Insects 11, no. 9: 648. https://doi.org/10.3390/insects11090648
APA StyleNestle, R., Daniels, J. C., & Dale, A. G. (2020). Mixed-Species Gardens Increase Monarch Oviposition without Increasing Top-Down Predation. Insects, 11(9), 648. https://doi.org/10.3390/insects11090648





