Scent of Jasmine Attracts Alien Invaders and Records on Citizen Science Platforms: Multiple Introductions of the Invasive Lacebug Corythauma ayyari (Drake, 1933) (Heteroptera: Tingidae) in Italy and the Mediterranean Basin
Simple Summary
Abstract
1. Introduction
2. Material and Methods
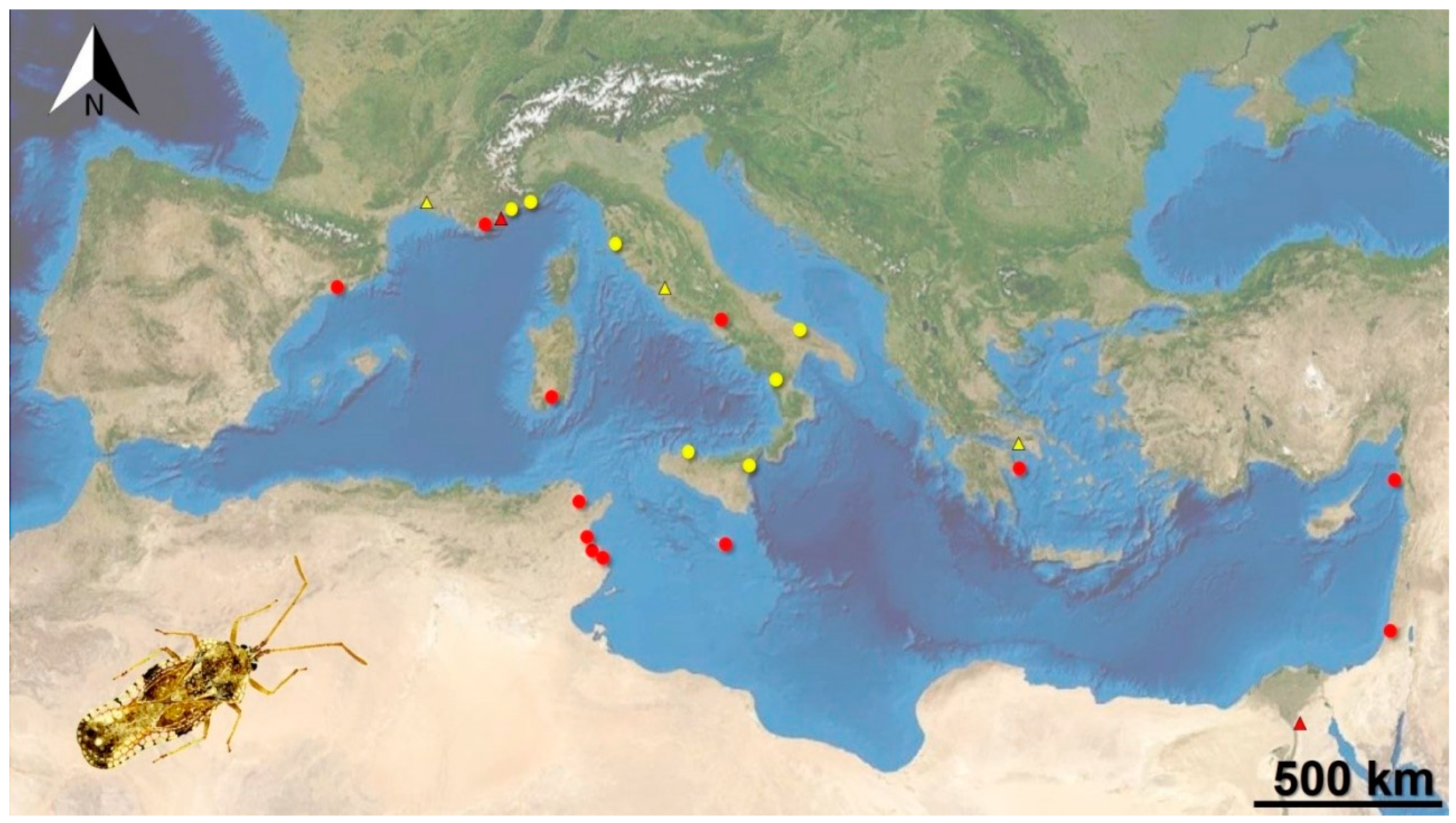
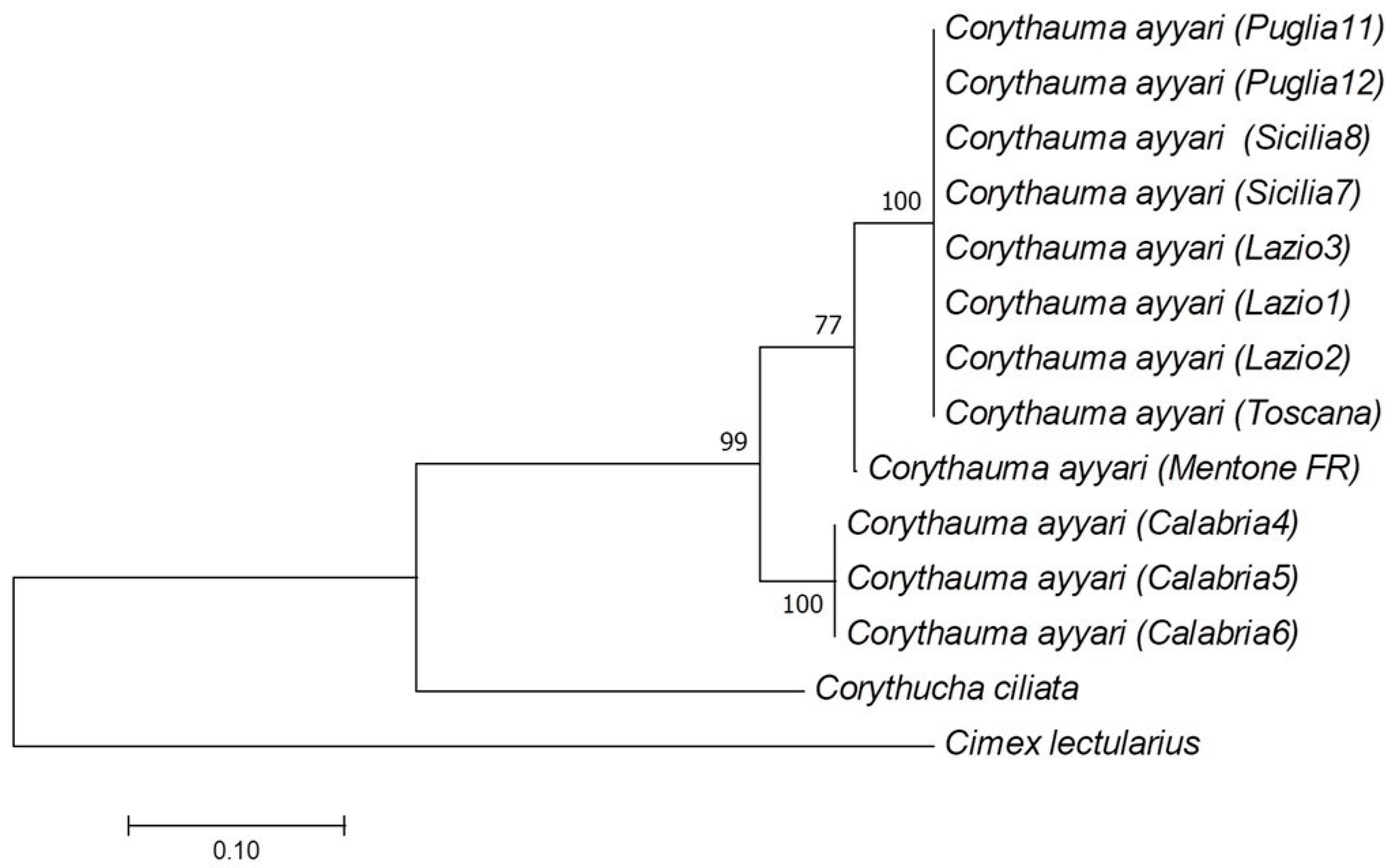
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ballantyne, R.; Packer, J.; Hughes, K. Tourists’ support for conservation messages and sustainable management practices in wildlife tourism experiences. Tour. Manag. 2009, 30, 658–664. [Google Scholar] [CrossRef]
- Dickinson, J.L.; Zuckerberg, B.; Bonter, D.N. Citizen science as an ecological research tool: Challenges and benefits. Annu. Rev. Ecol. Evol. S. 2010, 41, 149–172. [Google Scholar] [CrossRef]
- Bik, H.M.; Goldstein, M.C. An introduction to social media for scientists. PLoS Biol. 2013, 11, 1001535. [Google Scholar] [CrossRef] [PubMed]
- Kobori, H.; Dickinson, J.L.; Washitani, I.; Sakurai, R.; Amano, T.; Komatsu, N.; Kitamura, W.; Takagawa, S.; Koyama, K.; Ogawara, T.; et al. Citizen science: A new approach to advance ecology, education, and conservation. Ecol. Res. 2016, 31, 1–19. [Google Scholar] [CrossRef]
- Zapponi, L.; Cini, A.; Bardiani, M.; Hardersen, S.; Maura, M.; Maurizi, E.; de Zan, L.R.; Audisio, P.; Bologna, M.A.; Carpaneto, G.M.; et al. Citizen science data as an efficient tool for mapping protected saproxylic beetles. Biol. Conserv. 2016, 208, 139–145. [Google Scholar] [CrossRef]
- Campanaro, A.; Hardersen, S.; De Zan, L.R.; Antonini, G.; Bardiani, M.; Maura, M.; Maurizi, E.; Mosconi, F.; Zauli, A.; Bologna, M.A.; et al. Analyses of occurrence data of protected insect species collected by citizens in Italy. Nat. Conserv. 2017, 20, 265–297. [Google Scholar] [CrossRef]
- Tiago, P.; Pereira, H.M.; Capinha, C. Using citizen science data to estimate climatic niches and species distributions. Basic Appl. Ecol. 2017, 20, 75–85. [Google Scholar] [CrossRef]
- Poursanidis, D.; Zenetos, A. The role played by citizen scientists in monitoring marine alien species in Greece. Cah. Biol. Mar. 2013, 54, 419–426. [Google Scholar]
- Gardiner, M.M.; Allee, L.L.; Brown, P.M.; Losey, J.E.; Roy, H.E.; Smyth, R.R. Lessons from lady beetles: Accuracy of monitoring data from US and UK citizen-science programs. Front. Ecol. Environ. 2012, 10, 471–476. [Google Scholar] [CrossRef]
- Maistrello, L.; Dioli, P.; Bariselli, M.; Mazzoli, G.L.; Giacalone-Forini, I. Citizen science and early detection of invasive species: Phenology of first occurrences of Halyomorpha halys in Southern Europe. Biol. Invasions 2016, 18, 3109–3116. [Google Scholar] [CrossRef]
- Pocock, M.J.; Roy, H.E.; Fox, R.; Ellis, W.N.; Botham, M. Citizen science and invasive alien species: Predicting the detection of the oak processionary moth Thaumetopoea processionea by moth recorders. Biol. Conserv. 2017, 208, 146–154. [Google Scholar] [CrossRef]
- Carapezza, A. Corythauma ayyari (Drake, 1933) new pest of jasmine in Italy (Heteroptera Tingidae). Nat. Sicil. 2014, 38, 381–384. [Google Scholar]
- Novoselsky, T.; Freidberg, A. Note: Corythauma ayyari (Drake) (Hemiptera: Heteroptera: Tingidae)—A new pest of ornamentals in Israel. Phytoparasitica 2013, 41, 149–150. [Google Scholar] [CrossRef]
- Carapezza, A.; Linnavuori, R.E.; Kment, P. Order Hemiptera, suborder Heteroptera Infraorder Cimicomorpha, families Tingidae, Nabidae and Anthocoridae. Arthropod Fauna UAE 2014, 5, 148–186. [Google Scholar]
- Streito, J.C.; Matocq, A.; Guilbert, E. Découverte d’un foyer de Corythauma ayyari (Drake, 1933) et point sur la présence de plusieurs espèces de Stephanitis envahissants en France (Hemiptera Tingidae). L’Entomologiste 2010, 66, 7–12. [Google Scholar]
- Pedata, P.A.; Guilbert, É.; Nugnes, F.; Manicini, D. Rinvenimento di una popolazione di Corythauma ayyari (Heteroptera Tingidae) su Jasminum officinale (Oleaceae): Un nuovo fitofago per l’Italia. Prot. Delle Colt. 2013, 3, 36–39. [Google Scholar]
- Haouas, D.; Guilbert, E.; Halima-Kamel, M.B. First report of Corythauma ayyari (Drake) (Hemiptera: Tingidae) on Arabian and Spanish jasmine in Tunisia. EPPO Bull. 2015, 45, 144–147. [Google Scholar] [CrossRef]
- Carapezza, A.; Mifsud, D. New records of true bugs (Hemiptera, Heteroptera) from the Maltese Islands. Bull. Entomol. Soc. Malta 2015, 7, 27–50. [Google Scholar]
- Roca-Cusachs, M.; Goula, M. First record of the invasive tingid species Corythauma ayyari (Drake, 1933) in the Iberian Peninsula (Insecta: Hemiptera: Heteroptera: Tingidae). Butll. Inst. Catalana Hist. Nat. 2014, 78, 119–123. [Google Scholar]
- Rietschel, S. Corythauma ayyari (Drake, 1933)—Erstnachweis der invasiven Tingide in Griechenland (Heteroptera, Tingidae). Heteropteron 2015, 44, 5–8. [Google Scholar]
- Zeity, M.; Ali, A.Y. First report of the lacebug Corythauma ayyari (Drake) (Hemiptera: Tingidae) on Jasminum grandiflorum L. and Jasminum sambac (L.) from Syria. EPPO Bull. 2019, 49, 398–400. [Google Scholar] [CrossRef]
- Van der Heyden, T. First record of Corythauma ayyari (Drake) (Heteroptera: Tingidae) in Monaco. Rev. Chil. Entomol. 2019, 45, 579–581. [Google Scholar]
- Van der Heyden, T. First record of Corythauma ayyari (Drake, 1933) (Hemiptera: Heteroptera: Tingidae) in Egypt. J. Heteroptera Turk 2020, 2, 1–2. [Google Scholar]
- Fancello, L.; Cillo, D.; Rattu, A. Prima intercettazione in Sardegna dell’emittero “alieno” Corythauma ayyari (Drake, 1933), specie potenzialmente invasiva dannosa a piante ornamentali (Insecta, Hemiptera, Heteroptera, Tingidae). Mediterr. Nat. 2019, 2, 56–59. [Google Scholar]
- Guilbert, E. Tingidae (Hemiptera: Heteroptera) from Laos: New species and new records. Zootaxa 2007, 1442, 1–18. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System (http://www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Binazzi, F.; Strangi, A.; Paoli, F.; Sabbatini Peverieri, G.S.; Roversi, P.F.; Binazzi, A. A new aphid subspecies on the endemic Cyprus cedar Cedrus brevifolia: Cinara cedri brevifoliae ssp. n. (Aphididae Lachninae). Bull. Insect 2017, 70, 75–82. [Google Scholar]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G + C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar]
- Mori, E.; Milanesi, P.; Menchetti, M.; Zozzoli, R.; Monaco, A.; Capizzi, D.; Nerva, L. Genetics reveals that free-ranging chipmunks introduced to Italy have multiple origins. Hystrix 2018, 29, 239–242. [Google Scholar]
- Battisti, A.; Larsson, S. Climate change and insect pest distribution range. In Climate Change and Insect Pests; CABI: Wallingford, UK, 2015; pp. 1–15. [Google Scholar]
- Thomson, L.J.; Macfadyen, S.; Hoffmann, A.A. Predicting the effects of climate change on natural enemies of agricultural pests. Biol. Control. 2010, 52, 296–306. [Google Scholar] [CrossRef]
- Cagnacci, F.; Cardini, A.; Ciucci, P.; Ferrari, N.; Mortelliti, A.; Preatoni, D.G.; Russo, D.; Scandura, M.; Wauters, L.A.; Amori, G. Less is more: Researcher survival guide in times of economic crisis. Hystrix 2012, 23, 1–7. [Google Scholar]
- Nair, C.P.R.; Nair, M.R.G.K. Studies on the biology of the lace-wing Corythauma ayyari Drake a pest of jasmine. Agric. Res. J. Kerala 1974, 12, 172–173. [Google Scholar]
- Cariou, M.; Duret, L.; Charlat, S. The global impact of Wolbachia on mitochondrial diversity and evolution. J. Evol. Biol. 2017, 30, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Avise, J.C.; Arnold, J.; Ball, R.M.; Bermingham, E.; Lamb, T.; Neigel, J.E.; Saunders, N.C. Intraspecific phylogeography: The mitochondrial DNA bridge between population genetics and systematics. Annu. Rev. Ecol. Syst. 1987, 18, 489–522. [Google Scholar] [CrossRef]
- Rabitsch, W. Alien true bugs of Europe (Insecta: Hemiptera: Heteroptera). Zootaxa 2008, 1827, 1–44. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazza, G.; Nerva, L.; Strangi, A.; Mori, E.; Chitarra, W.; Carapezza, A.; Mei, M.; Marianelli, L.; Roversi, P.F.; Campanaro, A.; et al. Scent of Jasmine Attracts Alien Invaders and Records on Citizen Science Platforms: Multiple Introductions of the Invasive Lacebug Corythauma ayyari (Drake, 1933) (Heteroptera: Tingidae) in Italy and the Mediterranean Basin. Insects 2020, 11, 620. https://doi.org/10.3390/insects11090620
Mazza G, Nerva L, Strangi A, Mori E, Chitarra W, Carapezza A, Mei M, Marianelli L, Roversi PF, Campanaro A, et al. Scent of Jasmine Attracts Alien Invaders and Records on Citizen Science Platforms: Multiple Introductions of the Invasive Lacebug Corythauma ayyari (Drake, 1933) (Heteroptera: Tingidae) in Italy and the Mediterranean Basin. Insects. 2020; 11(9):620. https://doi.org/10.3390/insects11090620
Chicago/Turabian StyleMazza, Giuseppe, Luca Nerva, Agostino Strangi, Emiliano Mori, Walter Chitarra, Attilio Carapezza, Maurizio Mei, Leonardo Marianelli, Pio F. Roversi, Alessandro Campanaro, and et al. 2020. "Scent of Jasmine Attracts Alien Invaders and Records on Citizen Science Platforms: Multiple Introductions of the Invasive Lacebug Corythauma ayyari (Drake, 1933) (Heteroptera: Tingidae) in Italy and the Mediterranean Basin" Insects 11, no. 9: 620. https://doi.org/10.3390/insects11090620
APA StyleMazza, G., Nerva, L., Strangi, A., Mori, E., Chitarra, W., Carapezza, A., Mei, M., Marianelli, L., Roversi, P. F., Campanaro, A., & Cianferoni, F. (2020). Scent of Jasmine Attracts Alien Invaders and Records on Citizen Science Platforms: Multiple Introductions of the Invasive Lacebug Corythauma ayyari (Drake, 1933) (Heteroptera: Tingidae) in Italy and the Mediterranean Basin. Insects, 11(9), 620. https://doi.org/10.3390/insects11090620










