Simple Summary
Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) is a notorious and polyphagous pest of several economically important agricultural crops. It is worldwide in distribution and primarily managed through typical dependence on insecticides, which resulted in health and the environmental challenges and selected for resistance development in S. litura field populations. Resistance caused chemical control failures and S. litura outbreaks around the world. This necessitated development of eco-friendly alternative approaches such as biological control. With this view, current study investigated the functional response of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) at various growth stages (i.e., 1st, 2nd, 3rd and 4th instars, and male and female stages) and temperatures (i.e., 15, 20, 25, 30 and 35 °C) against S. litura eggs to enable the recognition of efficient biocontrol stages that could be utilized to suppress S. litura populations. In our findings, egg consumption depended on the growth stage of the predator as well as temperature. All stages consumed S. litura eggs, but more promising stages with active egg consumption were the 4th instar and adults (male and female) typically at higher temperatures (25–35 °C). We conclude that these stages may be exploited to suppress S. litura populations in fields and greenhouses.
Abstract
Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) is a major pest of several economically important crops with worldwide distribution. Use of insecticides is the principal strategy for its management, which has subsequently led to insecticide resistance and control failures. Functional response of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) at larval and adult stages was evaluated in this study, using S. litura eggs as the prey at various temperatures varying between 15 and 35 °C. Based on logistic model findings, linear parameters of various predatory stages of H. axyridis at various temperatures were significantly negative, which indicate a type II functional response. The theoretical maximum number (T/Th) of eggs consumed increased with increasing temperature across all predatory stages. According to the random predator equation, the coefficients of attack rate increased and that of handling time decreased as the temperature increased. The 4th instar and adult stages were superior candidates for biocontrol of the target prey, typically at higher temperatures. The maximum attack rate (0.546 ± 0.058 h−1) and lowest handling time (0.189 ± 0.004 h) were exhibited by the females at 30 and 35 °C, respectively, whereas these parameters were inferior for early instars. These findings clearly depict that the 4th instar and adult predators are efficient egg consumers and can serve as potential suppressors of S. litura field populations. The limitations of the predictions formulated by functional response trials are also discussed.
1. Introduction
The tobacco cutworm, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) is well-known as a notorious cosmopolitan pest insect with extensive host range of economically important crops such as mallorca cabbage, Brassica balearica L. (Brassicales: Brassicaceae), maize, Zea mays L., rice, Oryza sativa L. (Poales: Poaceae), cotton, Gossypium hirsutum L., jute, Corchorus olitorius L., okra, Abelmoschus esculentus L. (Malvales: Malvaceae), tobacco, Nicotiana tabacum L., potato, Solanum tuberosum L., capsicum, Capsicum annuum L., tomato, Solanum lycopersicum L., eggplant, Solanum melongena L. (Solanales: Solanaceae), tea, Camellia sinensis L. (Ericales: Theaceae), soybean, Glycine max L. and peanut, Arachis hypogaea L. (Fabales: Fabaceae) [1]. It is a polyphagous pest of about 27 host species belonging to 25 genera of 14 families including cultivated crops, vegetables, weeds, fruits and ornamental plants [2,3]. High reproductive rate, rapid damage potential and extensive host range have contributed towards the economic status of the pest. A single female can lay over 2000 eggs during her entire life span of one week [2]. The early caterpillars are gregarious leaf eaters [4], become solitary as growth advances with potential to feed on the reproductive and fruit structures [5] of the attacked plant. Larval feeding can result in crop losses of 25.8% to 100% depending upon the susceptibility of the attacked host [6,7,8]. To protect these losses, use of insecticides is the primary strategy against S. litura in almost all agricultural areas of the world [9].
The control of agricultural pest challenges has depended mostly on the application of synthetic pesticides [10]. About 80% of the pesticides are used by the developed world and remaining is by the developing world [11]. In last years, pesticide use has considerably increased in China [12,13,14], corresponding to over 60% of the pesticide applied worldwide [11]. Resistance evolution is a major challenge associated with pesticides among other challenges for health and the environment [15,16]. Spodoptera litura has been shown to be resistant to a range of chemicals, and is ranked among the top ten most resistant insect pests of the world, with 638 reported case of resistance against 39 active ingredients [17]. Resistance to insecticides from synthetic origin including conventional and new chemistry chemicals has been reported in S. litura populations from Pakistan, India, China and United States [18,19]. Resistance development led to outbreaks by S. litura and failures of the crop around the world [3], particularly in Asia including India, Pakistan [20,21] and China [22]. Hence, the management of this pest has become increasingly difficult all over the world. The commonly used chemicals are ineffective in controlling the pest owing to resistance development and exploration of efficient and environmentally benign approaches such as biocontrol agents are desirable.
Biological control agents are considered the major forces regulating insect populations in global agroecosystems [23,24]. In particular, the coccinellids ladybeetles are an important group of insect predators, as they have large body size and prey upon multiple prey resources [25]. The assessment regarding natural enemy potential to suppress pest population is mainly carried out via evaluation of functional response, which depicts the interaction between prey density and predation capacity [26,27,28], enabling suitable choice of predator to be utilized in classical and augmentative biological control programs [29,30]. However, functional response may be regulated by a range of factors such as host plant of the prey [31,32,33,34,35,36], feeding behaviour and history [37], prey species [38,39], predator and its phenology [40,41] and temperature also [42,43,44,45]. In these, temperature is regarded as one of the most fundamental aspect, with ability to affect predator growth, development and foraging behaviour and thus the functional response [44].
The multicolored Asian ladybird beetle, Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) is a voracious predator that preys upon soft bodied hemipterans [46,47] and early-instar caterpillars [48]. Predation is also reported on the eggs of Monarch butterfly [49]. Many prior researches exploited H. axyridis to evaluate functional response against many crop pests [50,51,52,53], but information is lacking in S. litura. The current study aimed to assess the functional response of various stages of H. axyridis at five different temperatures using the eggs of S. litura as the prey. The information generated from the findings would assist to strengthen the biological control of S. litura under field conditions.
2. Materials and Methods
2.1. Insect Cultures
2.1.1. Mass Rearing of H. axyridis
In January 2019, colonies of Harmonia axyridis and Acyrthosiphon pisum (Harris) (Hemiptera: Aphididae) were established from a population of adults taken from a stock culture at Key Laboratory of Hubei, Insect Resources Utilization and Sustainable Pest Management, located at Huazhong Agricultural University (HZAU), Wuhan, China. Harmonia axyridis was fed with A. pisum and both colonies were maintained under constant laboratory conditions at 23 ± 1 °C, 70 ± 5% RH and 16:8 (L:D) h photoperiod. The aphid species, A. pisum was reared on bean plants Phaseolus vulgaris L. (Fabales: Fabaceae). A total of 20 pairs of adult (male: female) were released in mesh covered cages (60 × 44 × 34 cm), containing bean plants heavily infested with aphids. Aphid culture was refreshed daily and plants were carefully observed for H. axyridis egg batches. Eggs batches were removed and shifted to a controlled conditioned growth chamber, maintained at 23 ± 1 °C, 70 ± 5% RH and 16:8 (L:D) photoperiod, in a Petri dish (6 cm) with tissue paper lined at the bottom. Eclosed larvae were removed and secluded in another Petri dish along with aphid prey.
2.1.2. Mass Rearing of S. litura
Spodoptera litura population was established from a collection of 50 pupae obtained from, The Institute of Plant Protection and Soil Fertility Hubei Academy of Agricultural Sciences, Wuhan, China in 2019. All pupae were kept in a circular glass transparent jar (21 × 9 cm diameter; Model No. GG-17, manufactured by Sichuan Shubo Group Co., Ltd., Chongzhou, China) in an incubator (Shanghai Xinmiao Medical Device Manufacturing Co., Ltd., Shanghai, China: Model No QHX-250 BSH-III) at 27 ± 2 °C and 70 ± 5% RH with a photoperiod of 14:10 h (L:D). Emerged moths were collected and reared in another similar sized circular glass transparent jar (21 × 9 cm diameter) carrying a piece of cotton swab dipped in 20% honey solution as their diet. The jar was lined with tissue papers at the bottom as an egg laying substrate and the lid had multiple small holes for aeration. Once egg lying started, laid batches were removed daily and transferred to a small plastic box along with artificial diet [54] for eclosing larvae. The artificial diet (semi-solid) was consisted of kidney bean flour (150 g), yeast powder (24 g), methyl-4-hydroxy benzoate (1.5 g), ascorbic acid (2.35 g), distilled water 550 mL, agar (8.4 g), sorbic acid (0.75 g), streptomycin (0.75 g), and formaldehyde solution (1 mL) [55]. Feces were removed daily from the jar and the food was changed every 24 h. Larvae were reared in these boxes since they reached the final instar (6th instar), afterward shifted to another plastic box (12 × 7.5 cm diameter, purchased from Wenling Daxi Lingping Plastic Products Factory, Wenling, China) carrying diet and arrangement for pupation. This protocol was followed for developing and maintaining adult colonies.
2.2. Functional Response
For functional response studies, H. axyridis adults from the stock culture were shifted into similar sized plastic containers of 27 cm length, 14 cm width and 18 cm height. In total, twelve sets of plastic containers were prepared. Adults H. axyridis were adapted to prey on S. litura eggs. For this purpose, one pair of adult predator (male and female) was released in each container with 200 S. litura eggs refreshed every 12 h. A cotton wick was added to each container as an egg laying substrate. This whole apparatus was placed in an incubator under constant laboratory conditions at 23 ± 1 °C, 70 ± 5% RH and 16:8 (L:D) h photoperiod. The predator was reared on S. litura eggs for at least one complete generation, which ensured predator adaptation to successfully exploit eggs as prey. After this, functional response experiment was formally initiated. One fundamental aspect for the true assessment of predation capability is to maintain homogeneity of predator (i.e., instar/stage) age. To meet this crucial aspect, we kept male-female pairs together in a container carrying cotton wick as an egg lying substrate, checked, and collected every 12 h interval. Using this approach, different instars of approximately same age (0–6 h old after molting) were obtained. Upon getting each desired stage i.e., larval instars (first, second, third, and fourth) and adults (male and female), they were kept individually in a Petri dish of same size (6 cm). The first instar after eclosion was starved for about six hours, whereas subsequent instars/stages were starved for about 24 h within 24 h of their last molting. During starvation, only humidity was available in each petri dish by moist cotton roll. The experiments were performed in Petri dishes (6 cm diameter) on cotton leaves lined with fine layer of agar solution to prevent cotton (Gossypium hirsutum L. cv. Varamin) leaf discs (5 cm diameter) from drying during the experiment. A single fresh cotton leaf disc was centered upside down on the agar solution to obtain uniform leaf surface [56]. Freshly laid eggs were used for each replication. A fine camel hairbrush was used to transfer eggs into the Petri dish. First and second instar larvae were given 3, 6, 10, 15, 20, 25, 30, 35 and 5, 10, 15, 20, 30, 40, 50, 60 eggs, respectively, whereas third and fourth instar, and adult male and female were given same densities of eggs i.e., 5, 10, 25, 50, 100, 150, 250, 350 eggs. The temperature ranges considered reflect the thermal conditions experienced by predator in various protected and field crops in temperate areas. The experiment was conducted at five different constant temperatures (15 °C, 20 °C, 25 °C, 30 °C and 35 °C) with 70 ± 5% RH and photoperiod of 16:8 (L:D). The experiment was replicated 10 times simultaneously for each instar/stage, density and temperature. Eggs consumed/damaged by H. axyridis were not replaced during the entire experiment. After 24 h, the larval and adult predators were removed from the Petri dishes to count about the numbers of egg consumed.
2.3. Data Analysis
The Shapiro–Wilk test was used to assess the normality of data regarding mean and proportionate prey consumption by H. axyridis [57], prior to the analysis. The data were typically found to be non-normal (p < 0.05). The data on average prey consumption were analyzed with generalized linear model assuming negative binomial distribution due to over-dispersion and group means were separated with Tukey’s Honestly Significant Difference (HSD) test (p < 0.05). The data on proportionate prey consumption were subjected to the Kruskal–Wallis test (non-parametric ANOVA) for assessing significant effects, followed by Dunn’s multiple comparison test to differentiate between group means (p < 0.05). Egg consumption rate by predator at various predatory stages and temperatures was assessed separately.
A polynomial logistic regression equation was fitted for the proportion of prey eaten on the prey density offered, assuming a binomial distribution of data to determine the type of functional response [58] (Equation (1)).
where Ne and No represent the number of prey consumed and initial prey density, respectively, and is the proportion of prey consumed. The regression parameters P0, , , are the intercept, and the linear, quadratic and cubic coefficients, respectively. The coefficients were estimated by the maximum likelihood method. The signs of the linear and quadratic coefficients indicated the type of functional response. When P1 < 0, the functional response was type II, and if P1 > 0 and P2 < 0, the response was type III [58]. It is important to determine the nature of functional response for estimating the functional response parameters using an appropriate method. The type II response indicated that the prey consumption declined monotonically with prey density offered, and a type III response indicated that the proportionate prey consumption was positively density dependent [58]. The Roger’s random predator equation is appropriate for modelling predation or parasitism whenever predation or parasitism results in a significant reduction of prey or host densities [59] (Equation (2)).
where and N0 represent the number of consumed and prey density offered, respectively, is the instantaneous attack rate, is the handling time, T is the duration of the experiment (24 h). We used “glm” function to fit the logistic regression, and the “friar” [60] package to determine the coefficients of attack rate and handling time. All statistical analyses were done in R 4.0.0 [61].
The theoretical maximum predation rate, given by the ratio of T/Th, represents the maximal prey consumption in the given time interval. We calculated the maximum predation rate from the estimates of Th as determined above and subjected to non-parametric ANOVA [62].
3. Results
Generally, the mean prey consumption rate increased with increasing temperature. The mean prey consumption rate increased from 20.18 ± 6.25 to 43.70 ± 10.12 (mean ± SE) eggs per day per predator as the temperature increased from 15 to 35 °C. The mean prey consumption was lowest at 15 °C, intermediate at 20 and 25 °C and greatest at 30 and 35 °C (Table 1).

Table 1.
Mean prey consumption (±SE) rate on Spodoptera litura eggs by Harmonia axyridis at various predatory stages and temperatures.
The predator consumed 42% of the offered prey at 15 °C, which increased to 75% at 35 °C (Table 2). Mean prey consumption rate increased with advancing predator growth stages. The 1st and 2nd instar consumed the lowest. The highest prey consumption was noted for final instar and adult stages. Among these stages, consumption rate was highest for adult females (57.39 ± 4.36) but similar to that by 4th instar (52.45 ± 6.66) and adult males (43.12 ± 6.00) (Table 1). The 1st instar predator could consume 45% of the prey offered while the adult females and the 4th instar consumed 75% and 72% of the prey offered, respectively in the same time interval (Table 2). When effects were assessed on various temperatures, 4th instar and adult predators (male and female) were superior egg consumers over the other growth stages.

Table 2.
Proportionate prey consumption (±SE) rate on Spodoptera litura eggs by Harmonia axyridis at various predatory stages and temperatures.
Based on S. litura egg consumption rates, all stages of H. axyridis showed a type II functional response across all temperatures, as confirmed by linear estimates being significantly negative for all stages at all tested temperatures, which is an inverse density dependent response and the random predator equation is more appropriate for experimental data of type II functional response. The proportionate prey consumption rate was greater at low prey densities but lowered at higher prey densities. This type of functional response was further confirmed by negative linear estimates < 0) according to logistic regression analysis (Table 3 and Figure 1).

Table 3.
Maximum likelihood estimates from logistic regression analyses of the proportion of prey eaten by different stages of Harmonia axyridis against initial number of Spodoptera litura eggs offered.
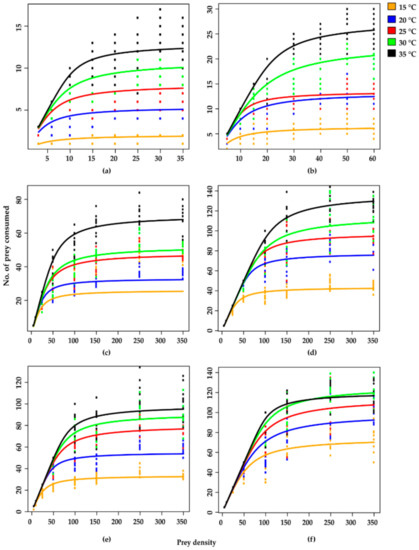
Figure 1.
Type II functional response curves fitted by Roger’s random predator equation (RRPE) of different stages of Harmonia axyridis against Spodoptera litura eggs at various temperatures (a–f). Here, (a) 1st instar, (b) 2nd instar, (c) 3rd instar, (d) 4th instar, (e) adult male and (f) female.
The curves reflect that at initially low prey densities, egg consumption rates quickly rose across all predator stages at all tested temperatures but levelled off with a decreasing trend with beyond a certain increase in prey density (Figure 1).
The attack rate increased with increasing temperature across all predatory stages; however, significant effects were obtained for the initial instars (1st and 2nd) and for adult females only. For adult females, the attack rate increased from 0.155 ± 0.01 h−1 at 15 °C to 0.546 ± 0.05 h−1 at 35 °C. Similarly, the attack rate for fourth instar grubs increased from 0.194 ± 0.018 h−1 to 0.33 ± 0.02 h−1 from 15 to 35 °C (Table 4). Attack rate estimate was significantly greater at 30 and 35 °C as compared to lower temperatures. The highest attack rate was observed for females (0.546 ± 0.058 h−1) and males (0.313 ± 0.024 h−1) at 35 °C whereas for the 4th instar, highest attack rate (0.243 ± 0.016 h−1) was noted at 30 °C. Overall, the attack rates of the fourth instar and adult female were significantly superior over the rest of stages for which the attack rates did not vary significantly. Therefore, attack rate was probably not a good indicator of the predator efficiency for the predator-prey combination under study.

Table 4.
Coefficients of attack rate (mean ± SE) of Harmonia axyridis at various predatory stages and temperatures, preying upon Spodoptera litura eggs (according to Roger’s random predator equation).
The handing time of predator decreased with increasing temperature, and with advancing in growth stages. The lowest handling times was exhibited by all predatory stages at 35 °C. For adult females, the handling time decreased from 0.322 ± 0.008 h at 15 °C to 0.199 ± 0.004 h at 35 °C. For the 4th instar, the handling time decreased from 0.55 ± 0.014 h at 15 °C to 0.174 ± 0.003 h at 35 °C (Table 5). At 15 °C, the highest handling time was noted for the 1st instar (11.71 ± 1.75 h) while the lowest handling time was noted for adult female (0.322 ± 0.008 h) followed by the 4th instar (0.551 ± 0.014 h). The female took significantly much less time to handle prey than the 4th instar at 15 and 20 °C whereas no significant difference was found at other temperature for these predatory stages. Overall, the handling time across all temperatures was similar for 1st, 2nd and 3rd instar grubs and significantly higher as compared to 4th instar and both adult stages.

Table 5.
Coefficients of handling time (mean± SE) of Harmonia axyridis at various predatory stages and temperatures, preying upon Spodoptera litura eggs (according to Rogers’ random predator equation).
The theoretical maximum predation rate (T/Th) significantly increased with advancing in growth stages, and with increasing temperature. For the 4th instar grub, the maximum predation rate increased from 43.64 ± 5.63 to 137.93 ± 19.45 as the temperature increased from 15 to 35 °C. Similarly, for adult females, the predation rate increased from 75.00 ± 11.10 to 120.60 ± 3.76 as the temperature increased from 15 to 35 °C. Among the predator growth stages, maximum predation rate was exhibited by the adult females followed by the 4th instar across all temperatures with the exception of 35 °C (Figure 2). The average maximum predation rate (averaged across all temperatures) was highest for adult females (108.52 ± 9.53), followed by 4th instars (96.31 ± 16.35) and adult males (72.94 ± 12.31); rest of growth stages performed comparatively lower.
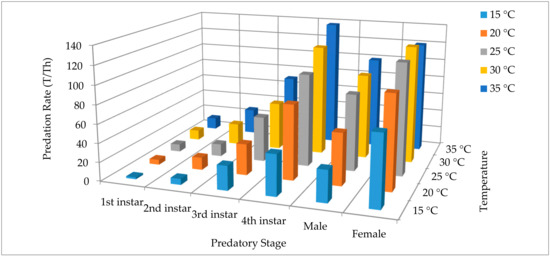
Figure 2.
Comparison of the theoretical maximum predation rates for all the predatory stages of Harmonia axyridis on Spodoptera litura eggs at five different temperatures.
4. Discussion
Functional response is an imperative criterion for assessing the efficiency of a predator on a given prey. Predation by H. axyridis using functional response experiments has been investigated against many crop pests [63,64]. However, no prior study reports predatory potential on S. litura eggs. As of today, current study assessed the predatory efficiency of H. axyridis, at various predatory stages, against S. litura eggs at a range of temperature that are likely to be experienced by the predator under field conditions.
Our findings showed that all developmental stages of H. axyridis across all tested temperatures exhibited a type II functional response. This type II functional response has been commonly reported for coccinellids [40,65,66] including H. axyridis when fed on various prey species such as Rhopalosiphum prunifoliae L. [50], Cinara sp. [51], Aphis craccivora K. [67], Schizaphis graminum R. [68], Lipaphis erysimi K. [52], Myzus persicae S. [69], Myzus nicotianae S. [70], Rhopalosiphum nymphaeae L. [50], Melanaphis sacchari Z. [63] (Hemiptera: Aphididae) and eggs of Danaus plexippus L. (Lepidoptera: Nymphlidae) [49]. In our findings, temperature affected the magnitude of the egg predation by various stages of predator. Increased temperature increased the numbers of eggs eaten. Higher consumption at higher temperature could be attributed to the faster growth and development. Temperature-related changes affect predator biology and physiology, such as higher reproduction, metabolism, and activity of predator and are likely to increase with increasing temperature [71,72]. High temperature increased the functional response of H. axyridis by decreasing handling time and increasing attack rate, in accord with many previous researches [73,74,75,76,77]. These findings suggest regulatory role of temperature towards searching efficiency and handling time across all predatory stages [64].
Handling time and attack rate (also called search rate or searching time or rate of discovery or space clearance rate) are important parameters of the functional response. The attack rate determines the ability of a predator to catch prey in a given time, and handling time indicates the time a predator spends to identify, subjugate, attack and consume a particular prey [1]. These parameters determine the foraging behavior of a predator and explain how much efficient a predator is against a given density of prey. As the foraging behavior increases, the attack rate will also increase which leads to a decrease in handling time. It means the predator will consume more prey and we can use it to control pest. The parameters of functional response help to explain relative changes in the efficacy of the predator with changing density of the prey, along the growth of the predator. For example, early in the season when the pest density is low, the predator efficiency is also expected to be low, based on the parameters of functional response. Similarly, as the predator grows through different instars, the functional response parameters also improve, which means that the capacity of the predator to regulate the pest population will improve. Therefore, the functional response parameters give an indication of the temporal variation in the efficacy of the predator to regulate the pest population, which could be exploited to improve the fate of the control program involving agents of biological control.
Our findings showed that both the handling time and attack rate depend on temperature as well as size of the predator. The attack rate for first instars of H. axyridis was very low compared to other stages at all tested temperatures, in line with findings of another study where this predator was fed with aphid species [64]. We found very low attack rate for first instar among all stages i.e., 0.03 ± 0.016 h−1, and these findings are supported by another study where attack rate for first instar i.e., 0.003 ± 0.006 h−1 was also very low when H. axyridis was fed with A. gossypii [64]. The low attack rate might be due to smaller sizes, slower movements and low nutrition requirement of the early instar. The larvae in second and third instars had relatively higher S. litura eggs consumption compared to the first instar larvae. Moreover, fourth instars larvae and female adults of H. axyridis were more voracious than other developmental stages, likely due to nutritional requirements for development and for reproduction [49]. The highest attack rate was exhibited by females i.e., 0.546 ± 0.058 h−1 at 35 °C. Similarly, high attack rate was observed in female i.e., 0.94 ± 0.374 h−1 and 0.78 h−1 when H. axyridis allowed to prey on D. plexippus eggs and Aphis glycines Matsumura (Hemiptera: Aphididae) respectively [49,78]. Likewise, fourth instar and female of Hippodamia variegata G. (Coleoptera: Coccinellidae) showed highest attack rate against A. craccivora i.e., 0.144 ± 0.013 h−1 and 0.106 ± 0.011 h−1 respectively [36]. Literature suggests that the attack rate varies from predator species and stage, arena size, temperature and prey stage also [79]. Fourth instar larvae and females generally had higher search rates than third instar larvae, possibly reflecting increased mobility skills with developmental stage. The same results were observed when H. axyridis was fed on the aphid M. persicae [80]. Fourth instar is a more voracious feeder compared to other immature stages because of higher food and energy demands in order to grow and achieve the desired weight for pupation [81].
Handling time is an accurate measure for consumption rate and to determine the productivity of a predator as it represents the combined effect of time used to catch, kill, and digesting the prey [82]. In case of our study, handling time decreased with increasing temperature and by advancing in predator size also, indicating that these stages would more efficiently contribute in handling prey and typically at higher temperature. Handling time for fourth instar (0.174 ± 0.003 h) was shorter than adult female (0.189 ± 0.004 h) and male (0.243 ± 0.05 h). Better efficacy by fourth instar and adult predators over earlier instars, as found in our study, has been supported by findings of many earlier studies where a coccinellid, Nephus arcuatus K. (Coleoptera: Coccinellidae) was fed on spherical mealybug, Nipaecoccus viridis N. (Hemiptera: Pseudococcidae) [83], and also when H. axyridis was fed on aphid, M. persicae [80] and when H. variegata was fed on Aphis fabae S. [40]. Seko and Miura [80] reported lower handling time for female (0.127 h) followed by the male (0.146 h) and fourth instar (0.156 h) H. axyridis, whereas Farhadi, Allahyari and Juliano [40] reported lower handling time for female (0.409 ± 0.048 h) followed by fourth instar (0.454 ± 0.028 h) and male (1.194 ± 0.069 h) H. variegate. This also suggests that handling time is likely to be different according to prey and predator species, their phenology as well as physical nature of the prey, either static or mobile [84].
In general, predators spent more time handling preys at lower temperatures than at higher temperatures [44]. Ladybugs feeding on lepidopterans pests showed higher space clearance rates compared to the other pests. This is due to the high success of the attack rate, because all lepidopteran preys were relatively easy to subdue at egg or first instar stage [85,86,87]. Although our findings demonstrate that predators have increased space clearance rates at higher temperatures indicating that low-density prey populations in warmer environments can be reduced most successfully, many individuals have high feeding rate at intermediate temperature [88]. This strong temperature influence on functional response parameters can lead to important changes in predator-prey relationship, population dynamics and food web interactions [89,90,91].
From the above findings, it is clear that 4th instar and adults of H. axyridis are the important biocontrol agents of S. litura eggs, with more consumption typically at higher temperature. Due to its high attack rate over a wide range of temperatures, particularly at high temperatures (25–35 °C), H. axyridis is a good natural enemy for use against S. litura eggs in warm locations such as greenhouses. Although, the type of functional response is considered to be a significant aspect, it is not the only standard measuring the failure or success of a natural enemy. Functional response experiments under small scale laboratories are very unlike to natural environment and therefore the outcomes should be explained correctly with due caution [92,93]. Moreover, biocontrol efficiency of this ladybug can be appreciated only when considering all relevant attributes of their biology, including their population growth parameters. The studies under lab may depict the predation capacity of H. axyridis when eggs are abundant, but they do not count cannibalism or emigration of the natural enemy that may occur when S. litura populations drop significantly, leaving few natural enemies in the field, which would favor resurgence of the pest. Furthermore, it is paramount to consider prey development with respect to temperature when investigating functional response. As increasing temperature can also accelerate egg hatching, it is likely that predation may decrease with plenty of prey availability at higher temperatures. It thus worth to account for predator as well as prey biology with respect to temperature to synthesize careful conclusions. Therefore, studies performed under greenhouse or field conditions are required to comprehend the feeding behavior of H. axyridis in various cropping systems, in order to design practical release approaches for this promising coccinellids predator for natural suppression of S. litura field populations.
5. Conclusions
This study concluded that H. axyridis is an efficient S. litura egg consumer. The biocontrol efficacy of the predator is growth stage and temperature dependent. All stages of the predator feed on eggs but 4th instar and adults are the most efficient consumers than other stages, and predation is typically more active at higher temperatures (25–35 °C). We can use 4th instar and adult predators in greenhouses and fields to control this notorious pest.
Author Contributions
Conceptualization, X.Z.; methodology, Y.I. and X.Z.; software, Y.I., F.M.S. and M.A.S.; validation, Y.I., F.M.S., M.A.S., M.M.K., M.A.R., S.U.R., S.A. and X.Z.; formal analysis, Y.I. and M.A.S.; investigation, X.Z. and Y.I.; resources, Y.I. and M.M.K.; data curation, Y.I., F.M.S., M.A.S., M.M.K., M.A.R., S.U.R., S.A. and X.Z.; writing—original draft preparation, Y.I.; writing—review and editing, X.Z., F.M.S., M.A.S., M.M.K.; visualization, Y.I., F.M.S., M.A.S., M.M.K., M.A.R., S.U.R., S.A. and X.Z.; supervision, X.Z.; project administration, Y.I., F.M.S., M.A.S. and X.Z.; funding acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R and D Program of China (2017YFD0201000), and The National Natural Science Foundation of China, Grant No. 31872023.
Acknowledgments
We are thankful to Muhammad Razaq (Department of Entomology, Faculty of Agricultural Sciences and Technology, Bahauddin Zakariya University, Multan, 60000, Pakistan) for commenting on earlier version of manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Zhou, Z.; Chen, Z.; Xu, Z. Potential of trap crops for integrated management of the tropical armyworm. Spodoptera litura in tobacco. J. Insect Sci. 2010, 10, 1–11. [Google Scholar] [CrossRef]
- Ahmad, M.; Ghaffar, A.; Rafiq, M. Host plants of leaf worm, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) in Pakistan. Asian J. Agric. Biol. 2013, 1, 23–28. [Google Scholar]
- Ahmad, M.; Arif, M.I.; Ahmad, M. Occurrence of insecticide resistance in field populations of Spodoptera litura (Lepidoptera: Noctuidae) in Pakistan. Crop Prot. 2007, 26, 809–817. [Google Scholar] [CrossRef]
- Lee, K.P.; Raubenheimer, D.; Behmer, S.T.; Simpson, S.J. A correlation between macronutrient balancing and insect host-plant range: Evidence from the specialist caterpillar Spodoptera exempta (Walker). J. Insect Physiol. 2003, 49, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.I.; Arshad, M.; Afzal, M.; Khalid, S.; Saleem, M.; Mustafa, I.; Iftikhar, Y.; Molina-Ochoa, J.; Foster, J.E. Incidence of Spodoptera litura (Lepidoptera: Noctuidae) and its feeding potential on various citrus (Sapindales: Rutaceae) cultivars in the Sargodha Region of Pakistan. Fla. Entomol. 2016, 99, 192–195. [Google Scholar] [CrossRef]
- Shah, F.M.; Razaq, M.; Ali, Q.; Ali, A.; Shad, S.A.; Aslam, M.; Hardy, I.C.W. Action threshold development in cabbage pest management using synthetic and botanical insecticides. Entomol. Gen. 2020, 40, 157–172. [Google Scholar] [CrossRef]
- Shah, F.M.; Razaq, M.; Ali, Q.; Shad, S.A.; Aslam, M.; Hardy, I.C.W. Field evaluation of synthetic and neem-derived alternative insecticides in developing action thresholds against cauliflower pests. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Tuan, S.J.; Li, N.J.; Yeh, C.C.; Tang, L.-C.; Chi, H. Effects of green manure cover crops on Spodoptera litura (Lepidoptera: Noctuidae) populations. J. Econ. Entomol. 2014, 107, 897–905. [Google Scholar] [CrossRef]
- Hole, U.; Jadhav, S.; Teli, V. Bio-efficacy of Insecticides against Spodoptera litura (Fab.) infesting Soybean. Ann. Plant Protect. Sci. 2009, 17, 322–324. [Google Scholar]
- Razaq, M.; Shah, F.M.; Ahmad, S.; Afzal, M. Pest Management for Agronomic Crops. In Agronomic Crops; Hasanuzzaman, M., Ed.; Springer: Singapore, 2019; pp. 365–384. [Google Scholar]
- Caldas, E.D. Toxicological Aspects of Pesticides. In Sustainable Agrochemistry; Vaz, S., Jr., Ed.; Springer: Cham, Switzerland, 2019; pp. 275–305. [Google Scholar]
- Wiklund, K.; Dich, J.; Holm, L. Risk of malignant lymphoma in Swedish pesticide appliers. Br. J. Cancer 1987, 56, 505–508. [Google Scholar] [CrossRef]
- Huang, J.; Hu, R.; Pray, C.; Qiao, F.; Rozelle, S. Biotechnology as an alternative to chemical pesticides: A case study of Bt cotton in China. J. Agric. Econ. 2003, 29, 55–67. [Google Scholar] [CrossRef]
- Jin, S.; Zhou, J.; Ye, J. Adoption of HACCP system in the Chinese food industry: A comparative analysis. Food Control 2008, 19, 823–828. [Google Scholar] [CrossRef]
- Pu, J.; Wang, Z.; Chung, H. Climate change and the genetics of insecticide resistance. J. Pest Manag. Sci. 2020, 76, 846–852. [Google Scholar] [CrossRef]
- Kalauni, D.; Joshi, A. Pesticides Import, Use, Consumption and Residue Status among Food Crops in Nepal: A Review. Big Data Agric. 2019, 1, 21–25. [Google Scholar] [CrossRef]
- Arthropod Pesticide Resistance Database Home Page. Available online: http://www.pesticideresistance.org (accessed on 6 December 2018).
- Cheema, H.K.; Kang, B.; Jindal, V.; Kaur, S.; Gupta, V. Biochemical mechanisms and molecular analysis of fenvalerate resistant population of Spodoptera litura (Fabricius). J. Crop Prot. 2020, 127, 104951. [Google Scholar] [CrossRef]
- Gore, J.; Adamczyk, J.J., Jr. Laboratory selection for beet armyworm (Lepidoptera: Noctuidae) resistance to methoxyfenozide. Fla. Entomol. 2004, 87, 450–453. [Google Scholar] [CrossRef]
- Dhaliwal, G.; Jindal, V.; Dhawan, A. Insect pest problems and crop losses: Changing trends. Indian J. Ecol. 2010, 37, 1–7. [Google Scholar]
- Ahmad, M.; Mehmood, R. Monitoring of resistance to new chemistry insecticides in Spodoptera litura (Lepidoptera: Noctuidae) in Pakistan. J. Econ. Entomol. 2015, 108, 1279–1288. [Google Scholar] [CrossRef]
- Zhou, Z. A review on control of tobacco caterpillar, Spodoptera litura. Chin. Bull. Entomol. 2009, 46, 354–361. [Google Scholar]
- Juen, A.; Hogendoorn, K.; Ma, G.; Schmidt, O.; Keller, M.A. Analysing the diets of invertebrate predators using terminal restriction fragments. J. Pest Sci. 2012, 85, 89–100. [Google Scholar] [CrossRef]
- Ali, S.; Li, S.; Jaleel, W.; Khan, M.M.; Wang, J.; Zhou, X. Using a Two-Sex Life Table Tool to Calculate the Fitness of Orius strigicollis as a Predator of Pectinophora gossypiella. Insects 2020, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Obrycki, J.J.; Harwood, J.D.; Kring, T.J.; O’Neil, R.J. Aphidophagy by Coccinellidae: Application of biological control in agroecosystems. Biol. Control 2009, 51, 244–254. [Google Scholar] [CrossRef]
- Holling, C. Principles of insect predation. Annu. Rev. Entomol. 1961, 6, 163–182. [Google Scholar] [CrossRef]
- Murdoch, W.W.; Oaten, A. Predation and population stability. Adv. Ecol. Res. 1975, 9, 1–131. [Google Scholar]
- Solomon, M. The natural control of animal populations. J. Anim. Ecol. 1949, 18, 1–35. [Google Scholar] [CrossRef]
- Fernández-Arhex, V.; Corley, J.C. The functional response of Lbalia leucospoides (Hymenoptera: Ibaliidae), a parasitoid of Sirex noctilio (Hymenoptera: Siricidae). Biocontrol Sci. Technol. 2005, 15, 207–212. [Google Scholar] [CrossRef]
- Van Lenteren, J.; Hemerik, L.; Lins, J.C.; Bueno, V.H. Functional responses of three neotropical mirid predators to eggs of Tuta absoluta on tomato. Insects 2016, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Cédola, C.V.; Sánchez, N.E.; Liljesthröm, G.G. Effect of tomato leaf hairiness on functional and numerical response of Neoseiulus californicus (Acari: Phytoseiidae). Exp. Appl. Acarol. 2001, 25, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Koveos, D.S.; Broufas, G.D. Functional response of Euseius finlandicus and Amblyseius andersoni to Panonychus ulmi on apple and peach leaves in the laboratory. Exp. Appl. Acarol. 2000, 24, 247–256. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, N.; Siddiqui, A.; Tripathi, C. Prey-predator relationship between Lipaphis erysimi Kalt. (Hom., Aphididae) and Coccinella septempunctata L. (Col., Coccinellidae). II. Effect of host plants on the functional response of the predator. J. Appl. Entomol. 1999, 123, 591–601. [Google Scholar] [CrossRef]
- Madadi, H.; Enkegaard, A.; Brodsgaard, H.; Kharrazi-Pakdel, A.; Mohaghegh, J.; Ashouri, A. Host plant effects on the functional response of Neoseiulus cucumeris to onion thrips larvae. J. Appl. Entomol. 2007, 131, 728–733. [Google Scholar] [CrossRef]
- Skirvin, D.; Fenlon, J. Plant species modifies the functional response of Phytoseiulus persimilis (Acari: Phytoseiidae) to Tetranychus urticae (Acari: Tetranychidae): Implications for biological control. Bull. Entomol. Res. 2001, 91, 61–67. [Google Scholar] [PubMed]
- Shah, M.A.; Khan, A. Functional response-a function of predator and prey species. Bioscan 2013, 8, 751–758. [Google Scholar]
- Castagnoli, M.; Simoni, S. Effect of long-term feeding history on functional and numerical response of Neoseiulus californicus (Acari: Phytoseiidae). Exp. Appl. Acarol. 1999, 23, 217–234. [Google Scholar] [CrossRef]
- Donnelly, B.E.; Phillips, T.W. Functional response of Xylocoris flavipes (Hemiptera: Anthocoridae)-effects of prey species and habitat. Environ. Entomol. 2001, 30, 617–624. [Google Scholar] [CrossRef]
- Hoddle, M.S. The effect of prey species and environmental complexity on the functional response of Franklinothrips orizabensis: A test of the fractal foraging model. Ecol. Entomol. 2003, 28, 309–318. [Google Scholar] [CrossRef]
- Farhadi, R.; Allahyari, H.; Juliano, S.A. Functional response of larval and adult stages of Hippodamia variegata (Coleoptera: Coccinellidae) to different densities of Aphis fabae (Hemiptera: Aphididae). Environ. Entomol. 2010, 39, 1586–1592. [Google Scholar] [CrossRef]
- Sharma, P.; Verma, S.; Chandel, R.; Shah, M.; Gavkare, O. Functional response of Harmonia dimidiata (fab.) to melon aphid, Aphis gossypii Glover under laboratory conditions. Phytoparasitica 2017, 45, 373–379. [Google Scholar] [CrossRef]
- Jalali, M.A.; Tirry, L.; De Clercq, P. Effect of temperature on the functional response of Adalia bipunctata to Myzus persicae. BioControl 2010, 55, 261–269. [Google Scholar] [CrossRef]
- McCaffrey, J.; Horsburgh, R. Functional response of Orius insidiosus (Hemiptera: Anthocoridae) to the European red mite, Panonychus ulmi (Acari: Tetranychidae), at different constant temperatures. Environ. Entomol. 1986, 15, 532–535. [Google Scholar] [CrossRef]
- Clercq, D. Functional response of the predators Podisus maculiventris (Say) and Podisus nigrispinus (Dallas) (Het., Pentatomidae) to the beet armyworm, Spodoptera exigua (Hübner) (Lep., Noctuidae): Effect of temperature. J. Appl. Entomol. 2001, 125, 131–134. [Google Scholar]
- Skirvin, D.J.; Fenlon, J.S. The effect of temperature on the functional response of Phytoseiulus persimilis (Acari: Phytoseiidae). Exp. Appl. Acarol. 2003, 31, 37. [Google Scholar] [CrossRef]
- Koch, R. The multicolored Asian lady beetle, Harmonia axyridis: A review of its biology, uses in biological control, and non-target impacts. Insect Sci. 2003, 3. [Google Scholar] [CrossRef]
- Roy, H.E.; Brown, P.M.; Adriaens, T.; Berkvens, N.; Borges, I.; Clusella-Trullas, S.; Comont, R.F.; De Clercq, P.; Eschen, R.; Estoup, A. The harlequin ladybird, Harmonia axyridis: Global perspectives on invasion history and ecology. Biol. Invasions 2016, 18, 997–1044. [Google Scholar] [CrossRef]
- Tedders, W.; Schaefer, P. Release and establishment of Harmonia axyridis (Coleoptera: Coccinellidae) in the southeastern United States. Entomol. News 1994, 105, 228–243. [Google Scholar]
- Koch, R.L.; Hutchison, W.D.; Venette, R.; Heimpel, G.E. Susceptibility of immature monarch butterfly, Danaus plexippus (Lepidoptera: Nymphalidae: Danainae), to predation by Harmonia axyridis (Coleoptera: Coccinellidae). Biol. Control 2003, 28, 265–270. [Google Scholar] [CrossRef]
- Luo, H. Functional response of Harmonia axyridis to the density of Rhopalosiphum prunifoliae. Nat. Enem. Insects 1987, 9, 84–87. [Google Scholar]
- Hu, Y.; Wang, Z.; Ning, C.; Pi, Z.; Gao, G. The functional response of Harmonia (Leis) axyridis to their prey of Cinara sp. Nat. Enemies Insects 1989, 11, 164–168. [Google Scholar]
- He, J.; Ma, E.; Shen, Y.; Chen, W.; Sun, X. Observations of the biological characteristics of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). J. Shanghai Agric. Coll. 1994, 12, 119–124. [Google Scholar]
- Seo, M.; Youn, Y. The Asian ladybird, Harmonia axyridis, as biological control agents: I. Predacious behavior and feeding ability. Kor. J. App. Entomol. 2000, 39, 59–71. [Google Scholar]
- Ul Haq, R.; Khan, J.; Ali, G. Rearing of Spodoptera litura (Fabricius) on different artificial diets and its parasitization with Trichogramma chilonis (Ishii). Pak. J. Zool. 2015, 47, 169–175. [Google Scholar]
- Ayyub, M.B.; Nawaz, A.; Arif, M.J.; Amrao, L. Individual and combined impact of nuclear polyhedrosis virus and spinosad to control the tropical armyworm, Spodoptera litura (Fabricius)(Lepidoptera: Noctuidae), in cotton in Pakistan. Egypt. J. Biol. Pest Control 2019, 29, 67. [Google Scholar] [CrossRef]
- Hassanpour, M.; Mohaghegh, J.; Iranipour, S.; Nouri-Ganbalani, G.; Enkegaard, A. Functional response of Chrysoperla carnea (Neuroptera: Chrysopidae) to Helicoverpa armigera (Lepidoptera: Noctuidae): Effect of prey and predator stages. Insect Sci. 2011, 18, 217–224. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Juliano, S.A. Nonlinear curve fitting: Predation and functional response curves. In Design and Analysis of Ecological Experiments; Scheiner, S.M., Gurevitch, J., Eds.; Chapman and Hall: New York, NY, USA, 2001; Volume 2, pp. 178–196. [Google Scholar]
- Rogers, D. Random search and insect population models. J. Anim. Ecol. 1972, 1, 369–383. [Google Scholar] [CrossRef]
- Pritchard, D.; Paterson, R.; Bovy, H.; Barrios-O’Neill, D. Frair: An R package for fitting and comparing consumer functional responses. Methods Ecol. Evol. 2017, 8, 1528–1534. [Google Scholar] [CrossRef]
- R core Team. R: A Language and Environment for Statistical Computing; Version 4.00; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 24 May 2020).
- Hassell, M. The Spatial and Temporal Dynamics of Host-Parasitoid Interactions; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Wu, P.; Zhang, J.; Haseeb, M.; Yan, S.; Kanga, L. Functional responses and intraspecific competition in the ladybird Harmonia axyridis (Coleoptera: Coccinellidae) provided with Melanaphis sacchari (Homoptera: Aphididae) as prey. Eur. J. Entomol. 2018, 115, 232–241. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, T.J. Functional response of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) to Aphis gossypii Glover (Homoptera: Aphididae) in the laboratory. Biol. Control 2004, 31, 306–310. [Google Scholar] [CrossRef]
- Moura, R.; Cabral, S.; Soares, A.O. Does pirimicarb affect the voracity of the euriphagous predator, Coccinella undecimpunctata L. (Coleoptera: Coccinellidae)? Biol. Control 2006, 38, 363–368. [Google Scholar] [CrossRef]
- Timms, J.; Oliver, T.; Straw, N.; Leather, S. The effects of host plant on the coccinellid functional response: Is the conifer specialist Aphidecta obliterata (L.) (Coleoptera: Coccinellidae) better adapted to spruce than the generalist Adalia bipunctata (L.) (Coleoptera: Coccinellidae)? Biol. Control 2008, 47, 273–281. [Google Scholar] [CrossRef]
- Mogi, M. Predation response of the larvae of Harmonia axyridis Pallas (Coccinellidae) to different prey density. Jpn. J. Appl. Entomol. Zool. 1969, 13, 9–16. [Google Scholar] [CrossRef]
- Hu, G. Functional Response of larvae of three ladybirds, Adonia variegata, Coccinella septempunctata and C. transversogutata to the aphid Schizaphis graminum (Homoptera; Aphididae). Nat. Enemies Insects 1992, 14, 180–185. [Google Scholar]
- Wu, H.; Cheng, X.; Zou, Y.; Wei, C.; Lu, F.; Ma, F. Predatism of Harmonia axyridis adults on different ranges of starvation to Myzus persicae. J. Anhui Agric. Coll. 2000, 27, 348–351. [Google Scholar]
- Deng, J.; Tan, Z.; Shan, Q.; Wu, X.; Liu, J. Functional Responses and density Interference Effect in Harmonis axyridis Pallas A Predator to Myzus nicotianae (Blackman). J. Southwest Agric Univ. 2002, 24, 433–435. [Google Scholar]
- McCoull, C.; Swain, R.; Barnes, R. Effect of temperature on the functional response and components of attack rate in Naucoris congrex Stål (Hemiptera: Naucoridae). Aust. J. Entomol. 1998, 37, 323–327. [Google Scholar] [CrossRef]
- De Clercq, P.; Degheele, D. Quality of predatory bugs of the genus Podisus (Heteroptera: Pentatomidae) reared on natural and artificial diets. In Proceedings of the 7th Workshop of the IOBC Global Working Group ‘Quality Control of Mass Reared Arthropods’, Rimini, Italy, 13–16 September 1993; pp. 129–142. [Google Scholar]
- Thompson, D.J. Towards a realistic predator-prey model: The effect of temperature on the functional response and life history of larvae of the damselfly, Ischnura elegans. J. Anim. Ecol. 1978, 47, 757–767. [Google Scholar] [CrossRef]
- Gresens, S.E.; Cothran, M.L.; Thorp, J.H. The influence of temperature on the functional response of the dragonfly Celithemis fasciata (Odonata: Libellulidae). Oecologia 1982, 53, 281–284. [Google Scholar] [CrossRef]
- Bailey, P. The effect of water temperature on the functional response of the water stick insect Ranatra dispar (Heteroptera: Nepidae). Aust. J. Entomol. 1989, 14, 381–386. [Google Scholar] [CrossRef]
- Anderson, M.T.; Kiesecker, J.M.; Chivers, D.P.; Blaustein, A.R. The direct and indirect effects of temperature on a predator prey relationship. Can. J. Zool. 2001, 79, 1834–1841. [Google Scholar]
- Gotoh, T.; Nozawa, M.; Yamaguchi, K. Prey consumption and functional response of three acarophagous species to eggs of the two-spotted spider mite in the laboratory. Appl. Entomol. Zool. 2004, 39, 97–105. [Google Scholar] [CrossRef]
- Xue, Y.; Bahlai, C.A.; Frewin, A.; Sears, M.; Schaafsma, A.; Hallett, R.H. Predation by Coccinella septempunctata and Harmonia axyridis (Coleoptera: Coccinellidae) on Aphis glycines (Homoptera: Aphididae). Environ. Entomol. 2009, 38, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Holling, C.S. Some characteristics of simple types of predation and parasitism. J. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Seko, T.; Miura, K. Functional response of the lady beetle Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) on the aphid Myzus persicae (Sulzer) (Homoptera: Aphididae). Appl. Entomol. Zool. 2008, 43, 341–345. [Google Scholar] [CrossRef]
- Hodek, I. Diapause/dormancy. In Ecology and Behaviour of the Ladybird Beetles (Coccinellidae); Hodek, I., van Emden, H.F., Honk, A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2012; pp. 275–342. [Google Scholar]
- Athan, R.; Guldal, H. Prey density-dependent feeding activity and life history of Scymnus subvillosus Goeze. (Coleoptera: Coccinellidae). Phytoparasitica 2009, 37, 35–41. [Google Scholar] [CrossRef]
- Zarghami, S.; Mossadegh, M.S.; Kocheili, F.; Allahyari, H.; Rasekh, A. Functional responses of Nephus arcuatus Kapur (Coleoptera: Coccinellidae), the most important predator of spherical mealybug Nipaecoccus viridis (Newstead). Psyche A J. Entomol. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Ganjisaffar, F.; Perring, T.M. Prey stage preference and functional response of the predatory mite Galendromus flumenis to Oligonychus pratensis. Biol. Control 2015, 82, 40–45. [Google Scholar] [CrossRef]
- Aljetlawi, A.A.; Sparrevik, E.; Leonardsson, K. Prey–predator size-dependent functional response: Derivation and rescaling to the real world. J. Anim Ecol. 2004, 73, 239–252. [Google Scholar] [CrossRef]
- Price, P.W. Resource-driven terrestrial interaction webs. Ecol. Res. 2002, 17, 241–247. [Google Scholar] [CrossRef]
- Uiterwaal, S.F.; DeLong, J.P. Multiple factors, including arena size, shape the functional responses of ladybird beetles. J. Appl. Ecol. 2018, 55, 2429–2438. [Google Scholar] [CrossRef]
- Englund, G.; Öhlund, G.; Hein, C.L.; Diehl, S. Temperature dependence of the functional response. Ecol. Lett. 2011, 14, 914–921. [Google Scholar] [CrossRef]
- Vasseur, D.A.; McCann, K.S. A mechanistic approach for modeling temperature-dependent consumer-resource dynamics. Am. Nat. 2005, 166, 184–198. [Google Scholar] [CrossRef]
- Vucic-Pestic, O.; Ehnes, R.B.; Rall, B.C.; Brose, U. Warming up the system: Higher predator feeding rates but lower energetic efficiencies. Glob. Chang. Biol. 2011, 17, 1301–1310. [Google Scholar] [CrossRef]
- Sentis, A.; Hemptinne, J.-L.; Brodeur, J. Using functional response modeling to investigate the effect of temperature on predator feeding rate and energetic efficiency. Oecologia 2012, 169, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Kareiva, P. The spatial dimension in pest-enemy interactions. In Critical Issues in Biological Control; Mackauer, M., Ehler, L.E., Roland, J., Eds.; Intercept: Andover, MA, USA, 1990; pp. 213–227. [Google Scholar]
- Zamani, A.; Talebi, A.; Fathipour, Y.; Baniameri, V. Temperature-dependent functional response of two aphid parasitoids, Aphidius colemani and Aphidius matricariae (Hymenoptera: Aphidiidae), on the cotton aphid. J. Pest Sci. 2006, 79, 183–188. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).