Simple Summary
Plants often increase their odor emissions after herbivore feeding damage, which in turn attract natural enemies of the herbivores such as insect predators. Synthetic versions of these so-called herbivore-induced plant volatiles (HIPVs) can be used to monitor populations of beneficial insects in agriculture. In addition, HIPVs can potentially attract the herbivores themselves. However, whether synthetic HIPVs interact with color to affect insect communities in farms is unknown. In this study, we tested a lure containing the HIPV methyl salicylate (named ‘PredaLure’) in combination with five different colored sticky traps to monitor insect populations in cranberry fields (also known as bogs). We found that hoverflies (also called flower flies or syrphid flies), whose larvae are predators of several insect pests including aphids and thrips, were attracted to PredaLure but this attraction was affected by the color of the trap. In fact, the numbers of hoverflies were 2–4 higher on yellow and white traps baited with PredaLure than on unbaited traps. Irrespective of trap color, plant-feeding thrips were also more attracted to PredaLure-baited than unbaited traps. Our study provides guidelines for the use of odor-baited colored sticky traps to monitor natural enemies such as hoverflies in an agricultural system like cranberries.
Abstract
Synthetic herbivore-induced plant volatiles (HIPVs) could be used to monitor insect populations in agroecosystems, including beneficial insects such as natural enemies of herbivores. However, it is unknown whether insect responses to HIPVs are influenced by visual cues, e.g., color. We hypothesized that the HIPV methyl salicylate (MeSA) interacts with color to affect insect captures on sticky traps. To test this, we conducted a 5 × 2 factorial field experiment in a commercial cranberry farm to monitor numbers of insect predators, parasitoids, and herbivores by using five colored sticky traps that were either baited with a MeSA lure (named ‘PredaLure’) or unbaited. At the community level, PredaLure increased captures of predators. At the individual-taxon level, captures of the hoverfly Toxomerus marginatus (Diptera: Syrphidae) and thrips (Thysanoptera: Thripidae) were higher on PredaLure-baited traps. However, only captures of T. marginatus on PredaLure-baited traps interacted significantly with color such that the numbers of this hoverfly on yellow and white traps were 2–4 times higher when baited with PredaLure. This study is the first to document the interactive effects of synthetic HIPVs and color on an insect community. Our findings have implications for optimal selection of HIPV-baited colored traps to monitor natural enemy populations in agroecosystems.
1. Introduction
Volatile organic compounds (VOCs) emitted from plants play multiple roles in nature. They can protect plants from biotic stresses by priming plant defenses [1,2] or from abiotic stresses such as ozone, temperature, and light [3,4,5,6]. These VOCs are also important in plant–plant interactions [7,8,9] and mutualistic plant–insect interactions by attracting beneficial insects, including pollinators [10], natural enemies of herbivores [11], and seed dispersers [12,13]. However, they can also play a role in antagonistic interactions by attracting insect herbivores [11,14]. VOC emission generally increases when plants are attacked by insect herbivores (so-called herbivore-induced plant volatiles (HIPVs)) [15,16]. For natural enemies, HIPVs are more detectable and reliable cues during herbivore prey/host location than constitutive VOCs [17]. For instance, several insect predators and parasitoids are attracted to HIPVs [16], and this attraction can increase their predation and parasitism rates [18,19,20]. HIPV emissions can also affect plant-pollinator interactions [21] as well as plant-herbivore interactions [19]. Therefore, the emission of HIPVs can have profound effects on insect communities.
Due to the importance of HIPVs in tri-trophic (plant–herbivore–natural enemy) interactions, synthetic versions of them have been used to attract natural enemies (predators and parasitoids) of herbivores in agroecosystems [22,23]. An HIPV commonly used for this purpose is methyl salicylate (MeSA), which is a volatile compound emitted from leaves and flowers of many plant species [10], and that is induced by herbivory from insects belonging to different feeding guilds, such as cell-content feeders [24], sap-sucking feeders [25,26], and chewers [27]. A meta-analysis showed natural enemies have a general attraction to MeSA in agricultural fields, and this attraction was not different among predator and parasitoid taxa [28]. For example, insect predators, including the big-eyed bug Geocoris pallens Stal. (Hemiptera: Geocoridae), the lady beetle Stethorus punctum picipes (Casey) (Coleoptera: Coccinellidae), the green lacewing Chrysopa nigricornis Burmeister (Neuroptera: Chrysopidae), and hoverflies (Diptera: Syrphidae), are attracted to sticky traps baited with MeSA in hop yards [29,30,31]. In grape vineyards, sticky traps in blocks baited with MeSA captured higher numbers of the predators C. nigricornis, S. punctum picipes, Deraeocoris brevis (Uhler) (Hemiptera: Miridae), and Orius tristicolor (White) (Hemiptera: Anthocoridae) [32] and the parasitic wasp Anagrus spp. (Hymenoptera: Mymaridae) [33] than traps in unbaited blocks. In similar studies, MeSA-baited traps caught higher numbers of the spider Erigonidium graminicolum Sundevall (Araneae: Linyphiidae) and the anthocorid Orius similis Zheng in cotton [34], and the seven-spotted lady beetle Coccinella septempunctata L. in soybean [25], than unbaited traps. A MeSA-containing lure (PredaLure; AgBio Inc., Westminster, CO, USA) is commercially available to growers for the attraction of natural enemies of agricultural pests. PredaLure-baited sticky traps were more attractive to lacewings and O. tristicolor than unbaited traps in strawberry fields [35]. In soybean fields, PredaLure increased the attraction of hoverflies and green lacewings to sticky traps and reduced the abundance of soybean aphids [36].
In cranberries (Vaccinium macrocarpon Aiton), hoverflies (mainly Toxomerus marginatus (Say)), lady beetles, and green lacewings were captured in higher numbers in sticky traps baited with PredaLure than in unbaited traps [28]. In subsequent studies, MeSA, alone or in combination with other HIPVs, increased T. marginatus attraction to sticky traps but repelled megaspilid parasitoids [37]. In a 2-year study, PredaLure reliably increased T. marginatus captures on sticky traps; however, it also attracted more phytophagous thrips and, in one year, more plant bugs (Miridae) to these traps [38]. Using video recordings in a field setting, we observed lady beetles, T. marginatus, and predatory mites near the PredaLures, which resulted in increased predation of sentinel eggs [38].
Previous studies on the behavioral response of natural enemies to HIPVs have used a single trap color, either yellow e.g., [28,29,31,38,39] or white e.g., [35,39]. However, the interactive effects of HIPVs and visual cues, e.g., color, on natural enemy attraction have not been investigated. This knowledge gap is critical considering that visual cues play an essential role in the host-finding process of insects, including predators, parasitoids, and herbivores [17,40]. Thus, we tested the hypothesis that the response of insects to the HIPV MeSA is influenced by color. To achieve this, we conducted a field experiment in cranberries to address the following question: Are captures of insects from three feeding guilds (predators, parasitoids, and herbivores) on MeSA (PredaLure)-baited traps affected by the color of traps? If so, which taxa?
2. Materials and Methods
2.1. Site and Study Design
A field experiment was performed in 2019 to examine the interactive effects MeSA (PredaLure) and color on insect trap captures in cranberries in southern New Jersey (USA). In New Jersey, cranberries are grown in wet, marshy fields characterized by acidic, sandy soils called bogs (other names include beds or marshes). The experiment was conducted in five (3–4 acre) cranberry, V. macrocarpon (cv. ‘Early Black’), bogs in a commercial farm located in the Pinelands National Reserve, Chatsworth, New Jersey (Latitude: 39°44′12.17″ N; Longitude: 74°32′20.71″ W). All bogs were adjacent to a wooded habitat dominated by Pitch pine, Pinus rigida Mill., where wild Vaccinium plants are typically found in the understory [41] (Figure 1), and each bog was considered a replicate.
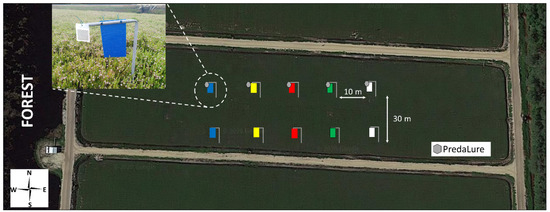
Figure 1.
Schematic layout of methyl salicylate (MeSa) (PredaLure)-baited and unbaited color sticky traps in cranberry bogs. Insert photo shows a closeup of a blue PredaLure-baited trap.
In each bog, two sets of five color traps were randomly placed in a straight line and were at least 10 m from the bog’s edge. To avoid positional bias in relation to proximity to the forest habitat (a potential source of natural enemies), traps of the same color were paired so that traps within bogs had the same sequence of colors (Figure 1). The distance between traps within each set was 10 m, and the two sets were at least 30 m apart. One set of 5 color traps was baited with MeSA lures (5 g load/lure; 90 d lure; average release rate of 35 mg/day over a 4-week period at 30 °C constant in the lab; PredaLure, AgBio Inc., Westminster, CO, USA) (insert photo, Figure 1), and the other set of 5 traps had no lures (unbaited controls). Although the position of the colored traps was randomly assigned and changed weekly so that no trap of a particular color was placed in the same position twice during the experiment, color traps always remained paired with each other. The position of the lure and non-lure traps in each pair was, however, not alternated between weeks but their position was randomly assigned among replicates. Traps and PredaLures were placed for 4 weeks, from 11 June until 09 July, which corresponds to the entire bloom period and coindices with peak hoverfly (T. marginatus) adult activity [28]. We focused our trapping on the period of T. marginatus activity because this insect was consistently the most attracted to MeSA in previous studies [28,37,38]. According to the manufacturing company, PredaLures last for at least 4 weeks in the field, so they were not replaced. None of the bogs had insecticides applied prior to or during the study.
2.2. Color Traps
Five color traps were tested: yellow (used as our standard [28,38]), white, green, blue, and red. All traps were 22.8 × 14.0 cm made of (0.24-mm-thick) colored acrylic sheets (Laird Plastics, Bristol, PA, USA; catalog no. 103617, 103416, 207486, 102249, and 103220 for yellow, white, green, blue, and red, respectively). Reflectance data for these colored traps are reported in Silva et al. [42]. Traps were coated on both sides with Tangle-Trap Insect Trap coating (The Tanglefoot Co., Grand Rapids, MI, USA) and were hung vertically with twist ties to 40-cm-high metal poles (see insert photo; Figure 1). Poles were buried in fields such that trap bottoms were <10 cm above the canopy.
2.3. Data Collection
During each week of sampling, traps were collected and processed in the laboratory where the numbers of insect predators, parasitoids, and herbivores on traps were counted under a stereomicroscope. The most abundant insect predators, parasitoids, and herbivores were identified to family and, when possible, to species levels. The “predator guild” included the following insect predators (common name (Order: Family)): hoverflies (Diptera: Syrphidae), lady beetles (Coleoptera: Coccinellidae), pirate bugs (Hemiptera: Anthocoridae), brown lacewings (Neuroptera: Hemerobiidae), and predatory thrips (Thysanoptera: Aeolothripidae). Spiders (Araneae) were also counted and included in the predator guild in the data analyses (see below). The “parasitoid guild” included the following nine parasitoid families (Order Hymenoptera): Megaspilidae, Aphelinidae, Encyrtidae, Mymaridae, Trichogrammatidae, Ceraphronidae, Figitidae, Braconidae, and Ichneumonidae. The two most commonly found insect herbivores (“herbivore guild”) were thrips (Thysanoptera: Thripidae) and click beetles (Coleoptera: Elateridae).
2.4. Data Analyses
We recorded weekly captures of each insect taxon per trap and performed data analyses as a 5 × 2 factorial, with 5 colors and 2 odor treatments (with or without MeSA). Prior to analyses, the data were checked for normality using the Shapiro–Wilk test [43] and for homoscedasticity using the Levene’s test (‘car’ package in R). At the community level, we performed multivariate analysis of variance (MANOVA) to test for the effects of MeSA (PredaLure) and color on the three insect feeding guilds (predators, parasitoids, and herbivores). The full model included treatment (PredaLure-baited versus unbaited traps), color, the interaction between treatment and color, and block (bog). At the individual-taxon level, we performed analysis of variance (ANOVA) mixed effect models (‘nlme’ package) to analyze the effects of PredaLure, color, and the interaction between them (fixed effects) on each insect taxon, with block and date of sampling as random effects. A significant ANOVA was followed by Tukey’s HSD test (α = 0.05; ‘agricolae’ package in R). If needed, data were transformed before ANOVA using ln(x + 0.5) to meet assumptions of normality. Untransformed data are presented in figures. All data analyses were performed in R version 3.4.4 [44].
3. Results
3.1. Effects of MeSA and Color on Insect Community
Captures of predators on sticky traps were influenced by MeSA (PredaLure) and color, but not by the interaction between them (Table 1). In contrast, captures of insect parasitoids and herbivores on sticky traps were influenced by color but not by MeSA or the interaction between them (Table 1).

Table 1.
Results of multivariate analysis of variance (MANOVA) for the effects of MeSA (PredaLure), color (red, blue, yellow, green, white), and their interaction on predators, parasitoids, and herbivores in commercial cranberry bogs.
3.2. Effects of MeSA and Color on Insect Predators
The color of traps had a significant effect on captures of hoverflies (Syrphidae) and lady beetles (Coccinellidae) (Table 2). The main hoverfly species captured was T. marginatus. Blue, yellow, and white traps captured the most T. marginatus; whereas green and red traps captured the fewest numbers (Figure 2). Lady beetles were most attracted to yellow and least attracted to red and white (Figure 3A).

Table 2.
Results of analysis of variance (ANOVA) mixed effect models for the effects of MeSA (PredaLure), color (red, blue, yellow, green, white), and their interaction on predators in commercial cranberry bogs.
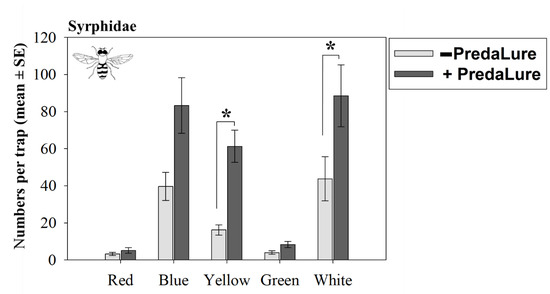
Figure 2.
Interactive effects of MeSA (PredaLure) and color on season total numbers (±1 SE) of hoverflies (Syrphidae). An asterisk (*) indicates significant differences between PredaLure-baited and unbaited traps (Tukey’s HSD test, p ≤ 0.05).

Figure 3.
Effects of color on season total numbers (±1 SE) of insect predators (A), parasitoids (B–F), and herbivores (G–H). Different letters within families indicate significant differences among colors (Tukey’s HSD test, p ≤ 0.05).
Color interacted with MeSA to affect hoverfly captures (significant PredaLure-by-color interaction; Table 2). The numbers of adult T. marginatus captured on yellow and white were significantly (2–4 times) higher on PredaLure-baited than on unbaited traps (Figure 2). Although blue traps baited with PredaLure captured 2 times more T. marginatus than unbaited blue traps, this difference was only marginal and statistically insignificant (p = 0.08; Figure 2). In contrast, the numbers of T. marginatus adults captured on red and green traps were similar between PredaLure-baited and unbaited traps.
3.3. Effects of MeSA and Color on Insect Parasitoids
Color, but not MeSA or the interaction between MeSA and color, affected the numbers of megaspilid, aphelinid, encyrtid, mymarid, and trichogrammatid wasps (Table 3; Figure 3). In general, these parasitoid families were most attracted to yellow and least attracted to blue (Figure 3B–F).

Table 3.
Results of analysis of variance (ANOVA) mixed effects models for the effects of MeSA (PredaLure), color (red, blue, yellow, green, white), and their interaction on parasitoids in commercial cranberry bogs.
3.4. Effects of MeSA and Color Effects on Insect Herbivores
Color significantly affected captures of phytophagous thrips and click beetles on traps (Table 4; Figure 3). Thrips were most attracted to yellow and white and least attracted to red (Figure 3G), while click beetles were most attracted to white and least attracted to yellow (Figure 3H). Thrips were 1.5 times more attracted to PredaLure-baited than unbaited traps (mean number of thrips per trap [±1 SE]: PredaLure-baited traps = 209.9 ± 24.6; unbaited traps = 144.0 ± 21.3) (Table 4). However, there was no significant interaction between MeSA (PredaLure) and color on thrips captures (Table 4), indicating that these insects responded to PredaLure in a similar manner regardless of color.

Table 4.
Results of analysis of variance (ANOVA) mixed effects models for the effects of MeSA (PredaLure), color (red, blue, yellow, green, white), and their interaction on herbivores in commercial cranberry bogs.
4. Discussion
For almost four decades, it has been well documented that the natural enemies of herbivores (e.g., predators and parasitoids) use HIPVs during prey/host location [15,16,17,18], which opens the possibility of using synthetic versions of these HIPVs to monitor their populations in agroecosystems e.g., [45,46]. In cranberries (V. macrocarpon), this study showed that (1) the HIPV MeSA (PredaLure) increases captures of predators on sticky traps, which was largely driven by an increased attraction of hoverflies (T. marginatus) to PredaLure-baited than unbaited traps; (2) T. marginatus attraction to PredaLure was influenced by the color of traps (i.e., significant PredaLure-by-color interaction); (3) captures of insect parasitoids on traps were affected by their color but not by the presence of PredaLure; and (4) PredaLure increased the attraction of phytophagous thrips to traps but, unlike T. marginatus, this effect was not influenced by trap color (i.e., no significant PredaLure-by-color interaction).
Many insect predators are known to be attracted to MeSA [28]. As in previous studies [28,37,38], we showed a consistent attraction of hoverflies to MeSA (PredaLure) throughout the cranberry flowering period; the most abundant hoverfly species in all these studies was T. marginatus. MeSA is known to not only elicit strong antennal responses [47,48], but also increase the attraction of hoverflies in other crops, including hops [29,31], soybean [36], and pear orchards [49], suggesting that physiological (i.e., antennal detection) and behavioral (i.e., attraction) responses of this predatory family to MeSA are common in agroecosystems. MeSA is generally induced by piercing-sucking herbivores, such as aphids [25,26], which are a preferred prey for hoverfly larvae [50,51]. Therefore, it is likely that female hoverflies use MeSA as a cue to locate oviposition sites that maximize immature fitness. To obtain energy while seeking for mates and oviposition sites, hoverflies frequently visit flowers to feed on nectar and pollen [52,53]. Consequently, in addition to biological control services, hoverflies can provide pollination services when visiting cranberry flowers [54]. To locate flowers, hoverflies are thought to primarily use vision, and among visual floral cues, color plays a critical role in this process e.g., [55,56]. In particular, the colors white, yellow, and blue are highly attractive to hoverflies [55,57,58,59]. In agreement with a previous study in cranberries [60], our results showed that T. marginatus is most attracted to blue and white, followed by yellow, and is least attracted to red and green. Using similar traps as those used in our study, Silva et al. [42] found that blue and yellow traps have distinct reflectance peaks (blue at 400–500 nm and yellow at 530–700 nm); however, the reflectance peaks for blue and white (at >420 nm) overlapped. Importantly, we found that the response of T. marginatus to visual cues (e.g., color) is enhanced by the presence of chemical cues (e.g., MeSA); although, this effect was dependent on the type of color. For instance, T. marginatus was 2× more attracted to blue and white and 4× more attracted to yellow in the presence of MeSA. In contrast, the response of this hoverfly to the two least preferred colors, green and red, was not enhanced by MeSA. These results suggest that both visual and chemical cues are likely important in the location and selection process of food and oviposition sites by T. marginatus. To our knowledge, this is the first study to document the interactive effects of an HIPV and color on insect predator attraction. Previous studies have shown that combining MeSA with other HIPVs could enhance hoverfly attraction [45,61]; but see [37]. However, whether more complex HIPV blends enhance the response of T. marginatus to color needs investigation.
Previous studies in other crops showed attraction of insect parasitoids to MeSA e.g., [28,33]. In our study, however, none of the common parasitoid families (Hymenoptera) found in cranberries were attracted to MeSA. Unlike predators, it is possible that parasitoids need more complex HIPV blends to find their host [62]. De Lange et al. [37] tested the field attraction of natural enemies to MeSA alone and in combination with other HIPVs emitted by cranberries after herbivore feeding damage but found that adding these HIPVs does not change the lack of attraction of parasitoids to MeSA. In contrast, treating cranberries exogenously with methyl jasmonate, a phytohormone involved in HIPV emissions, did increase parasitoid attraction in the field [63]. Altogether, these findings indicate that, in cranberries, the use of HIPVs to manipulate insect parasitoid behavior is a challenging task. In cranberries, all recorded parasitoid families were highly attracted to yellow. Greater numbers of Hymenoptera parasitoids were also caught on yellow sticky traps than on any other colored trap in a previous study in cranberries [60], supporting the importance of visual cues for this beneficial insect group. In recent studies, combining HIPVs with companion plants in a so-called ‘attract-and-reward’ scenario increased the attraction of parasitic wasps [64]. Therefore, finding the ‘right’ attractive HIPV blend that could be used in combination with rewarding (yellow) flowering plants might be needed to enhance and conserve parasitoid populations in habitats near cranberries.
Although HIPV lures like PredaLure are meant to attract natural enemies, insect herbivores can also respond to these lures. In fact, we found that phytophagous thrips were attracted to MeSA-baited traps in cranberries. According to the literature, the behavioral response of phytophagous thrips to MeSA is mixed, possibly due to species identity and crop type [65]. For example, there are reports that MeSA repels and deters oviposition by western flower thrips, Frankliniella occidentalis (Pergande) [66,67]. In grapes, however, MeSA had no effects on the abundance of F. occidentalis [32]. In another study, grapevines treated with MeSA increased the abundance of phytophagous thrips [68]. In cranberries, higher phytophagous thrips numbers were caught on yellow sticky traps baited with PredaLure than unbaited traps [38]. Phytophagous thrips are also influenced by color [69]. For instance, in cranberries, higher numbers of phytophagous thrips were caught on yellow and white sticky traps than in blue, green, and red traps [60]. Two thrips species, Frankliniella fusca (Hinds) and Scirtothrips sp., are common in cranberry bogs in New Jersey (C.R-S., pers. observation), and F. fusca are most attracted to yellow and white [69], which agrees with results from our current study. At present, thrips are not considered pests of cranberries but are thought to potentially transmit pathogens, such as the tobacco streak virus [70]. However, unlike the predator T. marginatus, the attraction of phytophagous thrips to MeSA was not influenced by color.
5. Conclusions
In this study, we showed that white and yellow sticky traps baited with the HIPV MeSA (e.g., PredaLure or other similar formulated lures) could be used to monitor populations of the hoverfly T. marginatus and thrips in cranberry farms. Yellow and white sticky traps are commercially available and already widely used to monitor insect pest populations in many agroecosystems. Hoverflies provide two important ecosystem services to crops: biological control and pollination. Therefore, by monitoring seasonal fluctuations in hoverfly population size, cranberry growers could increase these ecosystem services through augmentative and conservation biological control. Pest management practices, such as the use of low-risk insecticides and timing and rate of insecticide applications, could be then tailored according to natural enemy trap counts to enhance biological control. To implement MeSA-baited colored traps to monitor hoverfly and thrips populations in cranberries, further studies are needed to estimate their range of attraction.
Author Contributions
C.R.-S., P.U.-B., and J.S. conceived research. C.R.-S., P.U.-B., J.S., and V.G.-T. conducted the experiment. V.G.-T. collected data. J.S. analyzed data and conducted statistical analyses. C.R.-S. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Partial financial support for this project was provided by the New Jersey Cranberry Research Council and the Hatch project Nos. NJ08252 and NJ08140 to C.R.-S. V.G.-T. was funded by a project from the Universidad Nacional Abierta y a Distancia (UNAD) (No. PS-30-2018 announcement 007).
Acknowledgments
The authors would like to thank Pine Island Cranberry Co. for providing field sites for this study. We also thank Giovanna Jimenez, Fernando Sanchez-Pedraza, and Stephanie Aponte for field assistance, Robert Holdcraft for assistance making the color traps, and two anonymous reviewers for insightful comments on an earlier draft of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Heil, M.; Kost, C. Priming of indirect defences. Ecol. Lett. 2006, 9, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.J.; Mescher, M.C.; Carlson, J.E.; De Moraes, C.M. Plant defense priming against herbivores: Getting ready for a different battle. Plant Physiol. 2008, 146, 818–824. [Google Scholar] [CrossRef]
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.T.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Possell, M.; Loreto, F. The Role of Volatile Organic Compounds in Plant Resistance to Abiotic Stresses: Responses and Mechanisms. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Niinemets, Ü., Monson, R., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 209–235. [Google Scholar]
- Loreto, F.; Schnitzler, J.P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.K. Multiple functions of inducible plant volatiles. Trends Plant Sci. 2004, 9, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, C.R.; Mescher, M.C.; De Moraes, C.M. The Role of Volatiles in Plant–Plant Interactions. In Long-Distance Systemic Signaling and Communication in Plants; Baluška, F., Ed.; Springer: Berlin, Germany, 2013; pp. 393–412. [Google Scholar]
- Glinwood, R.; Ahmed, E.; Qvarfordt, E.; Ninkovic, V.; Pettersson, J. Airborne interactions between undamaged plants of different cultivars affect insect herbivores and natural enemies. Arthropod Plant Interact. 2009, 3, 215–224. [Google Scholar] [CrossRef]
- Vucetic, A.; Dahlin, I.; Petrovic-Obradovic, O.; Glinwood, R.; Webster, B.; Ninkovic, V. Volatile interaction between undamaged plants affects tritrophic interactions through changed plant volatile emission. Plant Signal Behav. 2014, 9, 37–41. [Google Scholar] [CrossRef]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Peñaflor, M.F.G.V.; Bento, J.M.S. Role of Plant Odors to Arthropod Natural Enemies and Herbivores. In The Biology of Odors; Weiss, L.E., Atwood, J.M., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2011; pp. 361–380. [Google Scholar]
- Baldwin, I.T. Plant volatiles. Curr. Biol. 2010, 20, 392–397. [Google Scholar] [CrossRef]
- Borges, R.M.; Bessière, J.M.; Hossaert-McKey, M. The chemical ecology of seed dispersal in monoecious and dioecious figs. Funct. Ecol. 2008, 22, 484–493. [Google Scholar] [CrossRef]
- Unsicker, S.B.; Kunert, G.; Gershenzon, J. Protective perfumes: The role of vegetative volatiles in plant defense against herbivores. Curr. Opin. Plant Biol. 2009, 12, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M.; Van Loon, J.J.A. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Exp. Appl. 2000, 97, 237–249. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Vet, L.E.M.; Dicke, M. Ecology of infochemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol. 1992, 37, 141–172. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef]
- Halitschke, R.; Stenberg, J.A.; Kessler, D.; Kessler, A.; Baldwin, I.T. Shared signals-“Alarm calls” from plants increase apparency to herbivores and their enemies in nature. Ecol. Lett. 2008, 11, 24–34. [Google Scholar] [CrossRef]
- Poelman, E.H.; Oduor, A.M.O.; Broekgaarden, C.; Hordijk, C.A.; Jansen, J.J.; Van Loon, J.J.A.; Van Dam, N.M.; Vet, L.E.M.; Dicke, M. Field parasitism rates of caterpillars on Brassica oleracea plants are reliably predicted by differential attraction of Cotesia parasitoids. Funct. Ecol. 2009, 23, 951–962. [Google Scholar] [CrossRef]
- Lucas-Barbosa, D.; Van Loon, J.J.A.; Dicke, M. The effects of herbivore-induced plant volatiles on interactions between plants and flower-visiting insects. Phytochemistry 2011, 72, 1647–1654. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Blaauw, B.R.; Isaacs, R. Manipulation of Natural Enemies in Agroecosystems: Habitat and Semiochemicals for Sustainable Insect Pest Control. In Integrated Pest Management and Pest Control-Current and Future Tactics; Soloneski, S., Larramendy, M.L., Eds.; InTech Open: London, UK, 2012; pp. 89–126. [Google Scholar]
- Peñaflor, M.F.G.V. Use of Semiochemical-Based Strategies to Enhance Biological Control. In Natural Enemies of Insect Pests in Neotropical Agroecosystems; Souza, B., Vázquez, L.L., Marucci, R.C., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 509–522. [Google Scholar]
- Ament, K.; Kant, M.R.; Sabelis, M.W.; Haring, M.A.; Schuurink, R.C. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 2004, 135, 2025–2037. [Google Scholar] [CrossRef]
- Zhu, J.; Park, K.C. Methyl salicylate, a soybean aphid-induced plant volatile attractive to the predator Coccinella septempunctata. J. Chem. Ecol. 2005, 31, 1733–1746. [Google Scholar] [CrossRef]
- Salamanca, J.; Pareja, M.; Rodriguez-Saona, C.; Resende, A.L.S.; Souza, B. Behavioral responses of adult lacewings, Chrysoperla externa, to a rose-aphid-coriander complex. Biol. Control 2015, 80, 103–112. [Google Scholar] [CrossRef]
- Bolter, C.J.; Dicke, M.; Van Loon, J.A.; Visser, J.H.; Posthumus, M.A. Attraction of Colorado potato beetles to herbivore-damaged plants during herbivory and after its termination. J. Chem. Ecol. 1997, 23, 1003–1023. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Kaplan, I.; Braasch, J.; Chinnasamy, D.; Williams, L. Field responses of predaceous arthropods to methyl salicylate: A meta-analysis and case study in cranberries. Biol. Control 2011, 59, 294–303. [Google Scholar] [CrossRef]
- James, D.G. Synthetic herbivore-induced plant volatiles as field attractants for benecial insects. Environ. Entomol. 2003, 32, 977–982. [Google Scholar] [CrossRef]
- James, D.G. Field evaluation of herbivore-induced plant volatiles as attractants for beneficial insects: Methyl salicylate and the green lacewing, Chrysopa nigricornis. J. Chem. Ecol. 2003, 29, 1601–1609. [Google Scholar] [CrossRef]
- James, D.G. Further field evaluation of synthetic herbivore-induced plan volatiles as attractants for beneficial insects. J. Chem. Ecol. 2005, 31, 481–495. [Google Scholar] [CrossRef]
- James, D.G.; Price, T.S. Field-testing of methyl salicylate for recruitment and retention of beneficial insects in grapes and hops. J. Chem. Ecol. 2004, 30, 1613–1628. [Google Scholar] [CrossRef]
- James, D.G.; Grasswitz, T.R. Synthetic herbivore-induced plant volatiles increase field captures of parasitic wasps. BioControl 2005, 50, 871–880. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Wu, K.; Gao, X.W.; Guo, Y.Y. Field-testing of synthetic herbivore-induced plant volatiles as attractants for beneficial insects. Environ. Entomol. 2008, 37, 1410–1415. [Google Scholar] [CrossRef]
- Lee, J.C. Effect of Methyl salicylate-based lures on beneficial and pest arthropods in strawberry. Environ. Entomol. 2010, 39, 653–660. [Google Scholar] [CrossRef]
- Mallinger, R.E.; Hogg, D.B.; Gratton, C. Methyl salicylate attracts natural enemies and reduces populations of soybean aphids (Hemiptera: Aphididae) in soybean agroecosystems. J. Econ. Entomol. 2011, 104, 115–124. [Google Scholar] [CrossRef] [PubMed]
- De Lange, E.S.; Salamanca, J.; Polashock, J.; Rodriguez-Saona, C. Genotypic variation and phenotypic plasticity in gene expression and emissions of herbivore-induced volatiles, and their potential tritrophic implications, in Cranberries. J. Chem. Ecol. 2019, 45, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, J.; Souza, B.; Kyryczenko-Roth, V.; Rodriguez-Saona, C. Methyl salicylate increases attraction and function of beneficial arthropods in cranberries. Insects 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Lucchi, A.; Loni, A.; Gandini, L.M.; Scaramozzino, P.; Ioriatti, C.; Ricciardi, R.; Shearer, P.W. Using herbivore-induced plant volatiles to attract lacewings, hoverflies and parasitoid wasps in vineyards: Achievements and constraints. Bull. Insectol. 2017, 70, 273–282. [Google Scholar]
- Prokopy, R.J.; Owens, E.D. Visual detection of plants by herbivorous insects. Annu. Rev. Entomol. 1983, 28, 337–364. [Google Scholar] [CrossRef]
- McCormick, J. The vegetation of the New Jersey Pine Barrens. In Pine Barrens: Ecosystem and Landscape; Forman, R., Ed.; Academic Press, Inc.: New York, NY, USA, 1979; pp. 229–243. [Google Scholar]
- Silva, D.; Salamanca, J.; Kyryczenko-Roth, V.; Alborn, H.T.; Rodriguez-Saona, C. Comparison of trap types, placement, and colors for monitoring Anthonomus musculus (Coleoptera: Curculionidae) adults in highbush blueberries. J. Insect Sci. 2018, 18, 19. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- R Development Core Team. R, A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Jones, V.P.; Steffan, S.A.; Wiman, N.G.; Horton, D.R.; Miliczky, E.; Zhang, Q.H.; Baker, C.C. Evaluation of herbivore-induced plant volatiles for monitoring green lacewings in Washington apple orchards. Biol. Control 2011, 56, 98–105. [Google Scholar] [CrossRef]
- Jones, V.P.; Horton, D.R.; Mills, N.J.; Unruh, T.R.; Baker, C.C.; Melton, T.D.; Milickzy, E.; Steffan, S.A.; Shearer, P.W.; Amarasekare, K.G. Evaluating plant volatiles for monitoring natural enemies in apple, pear and walnut orchards. Biol. Control 2016, 102, 53–65. [Google Scholar] [CrossRef]
- Shonouda, M.L. New approach to evaluate the antennal response of an adult predator insect to different volatile chemical compounds by using electroantennogram technique. J. Appl. Sci. 2008, 8, 1692–1698. [Google Scholar]
- Primante, C.; Dötterl, S. A syrphid fly uses olfactory cues to find a non-yellow flower. J. Chem. Ecol. 2010, 36, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Molleman, F.; Drukker, B.; Blommers, L. A trap for monitoring pear psylla predators using dispensers with the synomone methylsalicylate. Proc. Exp. Appl. Entomol. 1997, 8, 177–182. [Google Scholar]
- Dixon, T.J. Studies on oviposition behaviour of syrphidae (Diptera). Trans. R. Entomol. Soc. Lond. 1959, 111, 57–80. [Google Scholar] [CrossRef]
- Almohamad, R.; Verheggen, F.J.; Haubruge, É. Searching and oviposition behavior of aphidophagous hoverflies (Diptera: Syrphidae): A review. Biotechnol. Agron. Soc. Environ. 2009, 13, 467–481. [Google Scholar]
- Weems, H.V. Notes on collecting syrphid flies (Diptera: Syrphidae). Fla. Entomol. Soc. 1953, 36, 91–98. [Google Scholar] [CrossRef]
- Branquart, E.; Hemptinne, J.-L. Selectivity in the exploitation of floral resources by hoverflies (Diptera: Syrphinae). Ecography 2000, 23, 732–742. [Google Scholar] [CrossRef]
- Gervais, A.; Chagnon, M.; Fournier, V. Diversity and pollen loads of flower flies (Diptera: Syrphidae) in cranberry crops. Ann. Entomol. Soc. Am. 2018, 111, 326–334. [Google Scholar] [CrossRef]
- Campbell, D.R.; Bischoff, M.; Lord, J.M.; Robertson, A.W. Flower color influences insect visitation in alpine New Zealand. Ecology 2010, 91, 2638–2649. [Google Scholar] [CrossRef]
- Day, R.L.; Hickman, J.M.; Sprague, R.I.; Wratten, S.D. Predatory hoverflies increase oviposition in response to colour stimuli offering no reward: Implications for biological control. Basic Appl. Ecol. 2015, 16, 544–552. [Google Scholar] [CrossRef]
- Campbell, J.W.; Hanula, J.L. Efficiency of Malaise traps and colored pan traps for collecting flower visiting insects from three forested ecosystems. J. Insect Conserv. 2007, 11, 399–408. [Google Scholar] [CrossRef]
- Broughton, S.; Harrison, J. Evaluation of monitoring methods for thrips and the effect of trap colour and semiochemicals on sticky trap capture of thrips (Thysanoptera) and beneficial insects (Syrphidae, Hemerobiidae) in deciduous fruit trees in Western Australia. Crop. Prot. 2012, 42, 156–163. [Google Scholar] [CrossRef]
- Klecka, J.; Hadrava, J.; Biella, P.; Akter, A. Flower visitation by hoverflies (Diptera: Syrphidae) in a temperate plant-pollinator network. PeerJ 2018, 6, e26516v2. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, C.R.; Byers, J.A.; Schiffhauer, D. Effect of trap color and height on captures of blunt-nosed and sharp-nosed leafhoppers (Hemiptera: Cicadellidae) and non-target arthropods in cranberry bogs. Crop. Prot. 2012, 40, 132–144. [Google Scholar] [CrossRef]
- Gencer, N.S.; Kumral, N.A.; Altin, I.; Pehlevan, B. Response of aphid predators to synthetic herbivore induced plant volatiles in an apple orchard. Rev. Colomb. Entomol. 2019, 45, 1–7. [Google Scholar]
- Braasch, J.; Wimp, G.M.; Kaplan, I. Testing for phytochemical synergism: Arthropod community responses to induced plant volatile blends across crops. J. Chem. Ecol. 2012, 38, 1264–1275. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.R.; Polashock, J.; Malo, E.A. Jasmonate-mediated induced volatiles in the American cranberry, Vaccinium macrocarpon: From gene expression to organismal interactions. Front. Plant. Sci. 2013, 4, 1–18. [Google Scholar] [CrossRef]
- Simpson, M.; Gurr, G.M.; Simmons, A.T.; Wratten, S.D.; James, D.G.; Leeson, G.; Nicol, H.I.; Orre-Gordon, G.U.S. Attract and reward: Combining chemical ecology and habitat manipulation to enhance biological control in field crops. J. Appl. Ecol. 2011, 48, 580–590. [Google Scholar] [CrossRef]
- Koschier, E.H. Essential oil compounds for thrips control-A review. Nat. Prod. Commun. 2008, 3, 1171–1182. [Google Scholar] [CrossRef]
- Chermenskaya, T.D.; Burov, V.N.; Maniar, S.P.; Pow, E.M.; Roditakis, N.; Selytskaya, O.G.; Shamshev, I.V.; Wadhams, L.J.; Woodcock, C.M. Behavioural responses of western flower thrips, Frankliniella occidentals (pergande), to volatiles from three aromatic plants. Int. J. Trop. Insect Sci. 2001, 21, 67–72. [Google Scholar] [CrossRef]
- Allsopp, E.; Prinsloo, G.J.; Smart, L.E.; Dewhirst, S.Y. Methyl salicylate, thymol and carvacrol as oviposition deterrents for Frankliniella occidentalis (Pergande) on plum blossoms. Arthropod Plant. Interact. 2014, 8, 421–427. [Google Scholar] [CrossRef]
- Simpson, M.; Gurr, G.M.; Simmons, A.T.; Wratten, S.D.; James, D.G.; Leeson, G.; Nicol, H.I.; Orre, G.U.S. Field evaluation of the “attract and reward” biological control approach in vineyards. Ann. Appl. Biol. 2011, 159, 69–78. [Google Scholar] [CrossRef]
- Walker, W.F. Responses of selected Thysanoptera to colored surfaces. Environ. Entomol. 1974, 3, 295–304. [Google Scholar] [CrossRef]
- Wells-Hansen, L.D.; McManaus, P.S. Tobacco Streak Virus in Cranberry; University of Wisconsin-Extension: Madison, WI, USA, 2016; p. 4. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).