Seasonal Dynamics of the Alien Invasive Insect Pest Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) in Manica Province, Central Mozambique
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
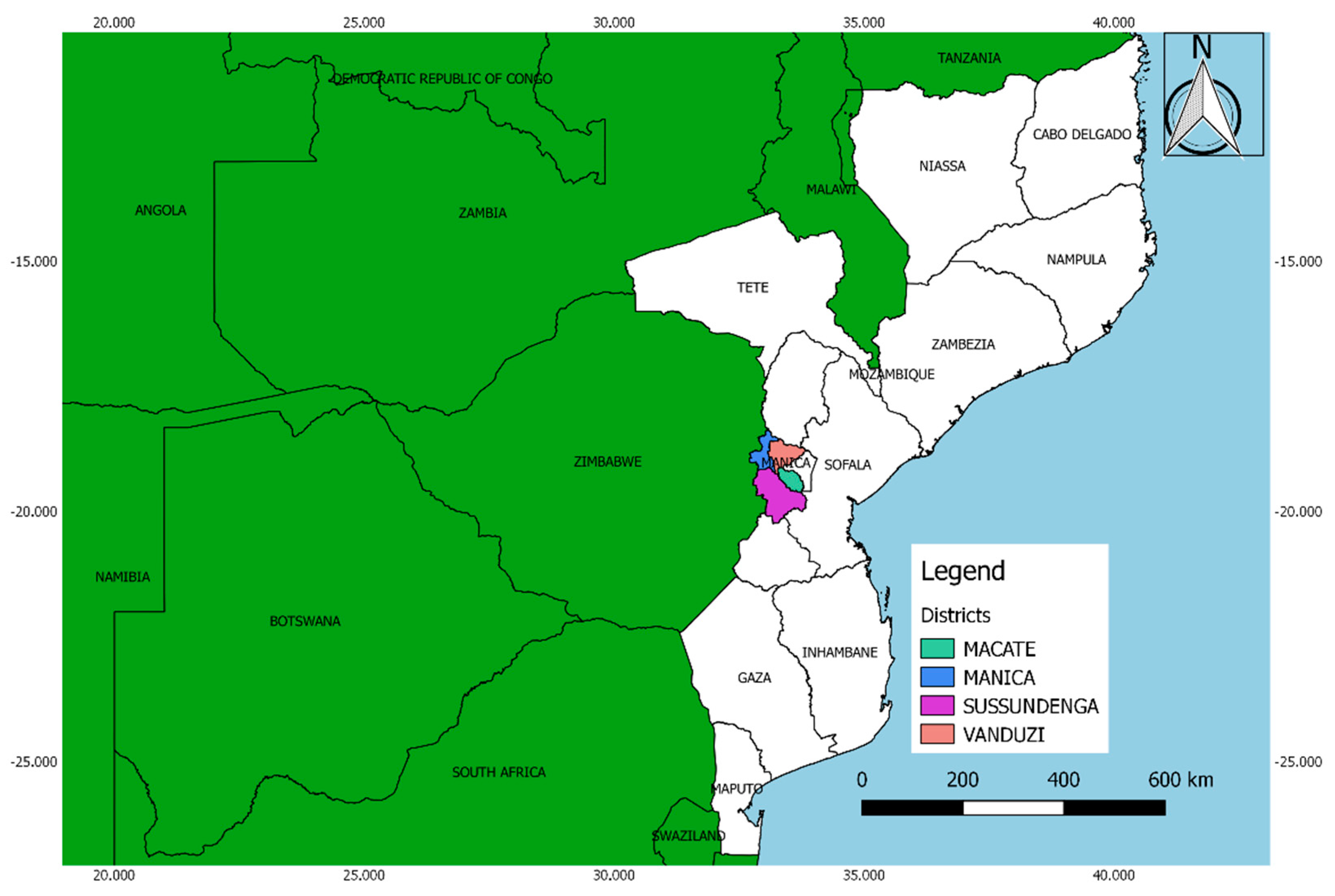
2.1. Description of the Study Area
2.2. Survey of Fall Armyworm
2.3. Variables
2.3.1. Percentage of Infested Fields
2.3.2. Percentage of Infested Plants
2.3.3. Percentage of Damaged Plants
2.3.4. Average Plant Damage
2.3.5. Number of FAW Egg Masses per Field
2.3.6. Number of FAW Larvae per Field
2.4. Meteorological Data
2.5. Data Analysis
3. Results
3.1. Infestation
3.2. Damage
3.3. Number of FAW Egg Masses and Larvae per Field
3.4. Temperature and Precipitation during the Survey
4. Discussion
5. Conclusions
Study Limitations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.; Paula-Moraes, S.; Peterson, J.A.; Hunt, T. Host Plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Molina-Ochoa, J.; Hamm, J.J.; Lezama-Gutierrez, R.; López-Edwards, M.; González-Ramírez, M.; Pescador-Rubio, A. A Survey of Fall Armyworm (Lepidoptera: Noctuidae) Parasitoids in the Mexican States of Michoacán, Colima, Jalisco, and Tamaulipas. Fla. Entomol. 2001, 84, 31–36. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Goergen, G.; Tounou, K.A.; Agboka, K.; Koffi, D.; Meagher, R.L. Analysis of strain distribution, migratory potential, and invasion history of fall armyworm populations in northern Sub-Saharan Africa. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First Report of Outbreaks of the Fall Armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a New Alien Invasive Pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, F.; Rieckmann, U.; Winter, S. The spread of the fall armyworm Spodoptera frugiperda in Africa—What should be done next? J. Plant Dis. Prot. 2019, 126, 97–101. [Google Scholar] [CrossRef]
- Cugala, D.; Agostinho, T.; Madogolele, N.; Simbine, A.; Lazaro, A.; Nhamuhave, A.; Vaz, A.; Pacho, D. Situação Actual da Lagarta do Funil de Milho, Spodoptera Frugiperda, em Moçambique. Maputo, 2017. Available online: https://www.ippc.int/static/media/files/pestreport/2017/04/17/FAM_survey_2017.pdf (accessed on 2 October 2018).
- Sharanabasappa; Kalleshwaraswamy, C.M.; Asokan, R.; Mahadeva Swamy, H.M.; Maruthi, M.S.; Pavithra, H.B.; Hegde, K.; Navi, S.; Prabhu, S.T.; Goergen, G. First report of the fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), an alien invasive pest on maize in India. Pest Manag. Hortic. Ecosyst. 2018, 24, 23–29. [Google Scholar]
- Meagher, R.L.; Nagoshi, R.N.; Stuhl, C.; Mitchell, E.R. Larval development of fall armyworm (lepidoptera: Noctuidae) on different cover crop plants. Fla. Èntomol. 2004, 87, 454–460. [Google Scholar] [CrossRef]
- Kumela, T.; Simiyu, J.; Sisay, B.; Likhayo, P.; Mendesil, E.; Gohole, L.; Tefera, T. Farmers’ knowledge, perceptions, and management practices of the new invasive pest, fall armyworm (Spodoptera frugiperda) in Ethiopia and Kenya. Int. J. Pest Manag. 2018, 65, 1–9. [Google Scholar] [CrossRef]
- Toepfer, S.; Kuhlmann, U.; Kansiime, M.; Onyango, D.O.; Davis, T.; Cameron, K.; Day, R. Communication, information sharing, and advisory services to raise awareness for fall armyworm detection and area-wide management by farmers. J. Plant Dis. Prot. 2019, 126, 103–106. [Google Scholar] [CrossRef]
- Hailu, G.; Niassy, S.; Zeyaur, K.R.; Ochatum, N.; Subramanian, S. Maize-Legume Intercropping and Push-Pull for Management of Fall Armyworm, Stemborers, and Striga in Uganda. Agron. J. 2018, 110, 2513–2522. [Google Scholar] [CrossRef]
- Midega, C.; Pittchar, J.O.; Pickett, J.A.; Hailu, G.W.; Khan, Z.R. A climate-adapted push-pull system effectively controls fall armyworm, Spodoptera frugiperda (J E Smith), in maize in East Africa. Crop Prot. 2018, 105, 10–15. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Huesing, J.E.; Eddy, R.; Peschke, V.M. (Eds.) Fall Armyworm in Africa: A Guide for Integrated Pest Management, 1st ed.; USAID and CIMMYT: Mexico City, Mexico, 2018.
- Harrison, R.D.; Thierfelder, C.; Baudron, F.; Chinwada, P.; Midega, C.; Schaffner, U.; Berg, J.V.D. Agro-ecological options for fall armyworm (Spodoptera frugiperda JE Smith) management: Providing low-cost, smallholder friendly solutions to an invasive pest. J. Environ. Manag. 2019, 243, 318–330. [Google Scholar] [CrossRef] [PubMed]
- MASA. Anuário de Estatísticas Agrárias 2015; MASA: Maputo, Mozambique, 2016. [Google Scholar]
- Mitchell, E.R. Monitoring Adult Populations of the Fall Armyworm. Fla. Èntomol. 1979, 62, 91–98. [Google Scholar] [CrossRef]
- Hruska, A.J.; Gladstone, S.M. Effect of Period and Level of Infestation of the Fall Armyworm, Spodoptera frugiperda, on Irrigated Maize Yield. Fla. Èntomol. 1988, 71, 249–254. [Google Scholar] [CrossRef]
- Hogg, D.B.; Pitre, H.N.; Anderson, R.E. Assessment of Early-Season Phenology of the Fall Armyworm (Lepidoptera: Noctuidae) in Mississippi 1. Environ. Èntomol. 1982, 11, 705–710. [Google Scholar] [CrossRef]
- Johnson, S.J. Migration and the life history strategy of the fall armyworm, Spodoptera frugiperda in the western hemisphere. Int. J. Trop. Insect Sci. 1987, 8, 543–549. [Google Scholar] [CrossRef]
- Westbrook, J.K.; Nagoshi, R.N.; Meagher, R.L.; Fleischer, S.J.; Jairam, S. Modeling seasonal migration of fall armyworm moths. Int. J. Biometeorol. 2015, 60, 255–267. [Google Scholar] [CrossRef]
- FAO and CABI. Community-Based Fall Armyworm Monitoring, Early Warning and Management: Training of Trainers Manual, 1st ed.; n.p. FAO & CABI: Rome, Italy, 2019. [Google Scholar]
- Tingle, F.C.; Mitchell, E.R. Seasonal Populations of Armyworms and Loopers at Hastings, Florida. Fla. Èntomol. 1977, 60, 115–122. [Google Scholar] [CrossRef]
- Simmons, A.M. Effects of Constant and Fluctuating Temperatures and Humidities on the Survival of Spodoptera frugiperda Pupae (Lepidoptera: Noctuidae). Fla. Èntomol. 1993, 76, 333–340. [Google Scholar] [CrossRef]
- Brown, E.S.; Betts, E.; Rainey, R.C. Seasonal changes in distribution of the African armyworm, Spodoptera exempta (Wlk.) (Lep., Noctuidae), with special reference to eastern Africa. Bull. Èntomol. Res. 1969, 58, 661–728. [Google Scholar] [CrossRef]
- García, A.G.; Godoy, W.A.C.; Thomas, J.M.G.; Nagoshi, R.N.; Meagher, R.L. Delimiting Strategic Zones for the Development of Fall Armyworm (Lepidoptera: Noctuidae) on Corn in the State of Florida. J. Econ. Èntomol. 2017, 111, 120–126. [Google Scholar] [CrossRef]
- Early, R.; González-Moreno, P.; Murphy, S.T.; Day, R.K. Forecasting the global extent of invasion of the cereal pest Spodoptera frugiperda, the fall armyworm. NeoBiota 2018, 40, 25–50. [Google Scholar] [CrossRef]
- Nboyine, J.; Kusi, F.; Abudulai, M.; Badii, B.; Zakaria, M.; Adu, G.; Haruna, A.; Seidu, A.; Osei, V.; Alhassan, S.; et al. A new pest, Spodoptera frugiperda (J.E. Smith), in tropical Africa: Its seasonal dynamics and damage in maize fields in northern Ghana. Crop Prot. 2020, 127, 104960. [Google Scholar] [CrossRef]
- Beckingham, C. Sweet Corn Growing; NSW Government-Department of Primary Industries, 2007. Available online: http://www.dpi.nsw.gov.au/agriculture/horticulture/vegetables/commodity-growing-guides/sweet-corn (accessed on 19 April 2019).
- Murúa, G.; Molina-Ochoa, J.; Coviella, C. Population dynamics of the fall armyworm, spodoptera frugiperda (lepidoptera: Noctuidae) and its parasitoids in northwestern argentina. Fla. Èntomol. 2006, 89, 175–182. [Google Scholar] [CrossRef]
- Cammell, M.; Knight, J. Effects of Climatic Change on the Population Dynamics of Crop Pests. Adv. Ecol. Res. 1992, 22, 117–162. [Google Scholar] [CrossRef]
- van Huis, A.; Nauta, R.S.; Vulto, M.E. Traditional Pest Management in Maize in NICARAGUA: A Survey; Meded. Landbouwhogeschool Wageningen, 1982; Volume 82-6, p. 1-43. [Google Scholar]
- Waddill, V.H.; Mitchell, E.R.; Denton, W.H.; Poe, S.L.; Schuster, D.J. Seasonal Abundance of the Fall Armyworm and Velvetbean Caterpillar (Lepidoptera: Noctuidae) at Four Locations in Florida. Fla. Èntomol. 1982, 65, 350–354. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Meagher, R.L. Seasonal Distribution of Fall Armyworm (Lepidoptera: Noctuidae) Host Strains in Agricultural and Turf Grass Habitats. Environ. Èntomol. 2004, 33, 881–889. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; O’Neil, R.J. Population dynamics of Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) and associated arthropod natural enemies in Honduran subsistence maize. Crop Prot. 2006, 25, 1180–1190. [Google Scholar] [CrossRef]
- Cruz, I.; Turpin, F.T. Yield Impact of Larval Infestations of the Fall Armyworm (Lepidoptera: Noctuidae) to Midwhorl Growth Stage of Corn. J. Econ. Èntomol. 1983, 76, 1052–1054. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Chalfant, R.B.; Young, J.R. Comparison of Sugarline Sampling and Pheromone Trapping for Monitoring Adult Populations of Corn Earworm and Fall Armyworm (Lepidoptera: Noctuidae) in Sweet Corn. Environ. Èntomol. 1987, 16, 1241–1243. [Google Scholar] [CrossRef]
- Stadelbacher, E.A.; Laster, M.L.; Pfrimmer, T.R. Seasonal Occurrence of Populations of Bollworm and Tobacco Budworm Moths 1 in the Central Delta of Mississippi 2, 3. Environ. Èntomol. 1972, 1, 318–323. [Google Scholar] [CrossRef]
- Pair, S.D.; Raulston, J.R.; Sparks, A.N.; Westbrook, J.K.; Douce, G.K. Fall Armyworm Distribution and Population Dynamics in the Southeastern States. Fla. Èntomol. 1986, 69, 468–487. [Google Scholar] [CrossRef]
- Silvain, J.F.; Ti-A-Hing, J. Prediction of Larval Infestation in Pasture Grasses by Spodoptera frugiperda (Lepidoptera: Noctuidae from Estimates of Adult Abundance. Fla. Èntomol. 1985, 68, 686–691. [Google Scholar] [CrossRef]
- Kennedy, G.G.; Storer, N.P. Life Systems of Polyphagous Arthropod Pests in Temporally Unstable Cropping Systems. Annu. Rev. Èntomol. 2000, 45, 467–493. [Google Scholar] [CrossRef] [PubMed]

| District | % of Infested Fields | % of Infested Plants Per Field (Mean ± SD) | ||
|---|---|---|---|---|
| Dry Season | Rainy Season | Dry Season | Rainy Season | |
| Macate | 60.00 | 16.15 | 31.00 ± (38.94) Ba | 2.62 ± (7.02) Bb |
| Manica | 82.76 | 23.36 | 48.45 ± (35.36) ABa | 5.62 ± (14.49) Bb |
| Sussundenga | 81.48 | 14.18 | 66.48 ± (37.95) Aa | 3.23 ± (9.64) Bb |
| Vanduzi | 71.19 | 34.25 | 42.63 ± (38.43) Ba | 11.99 ± (21.03) Ab |
| District | % of Damaged Plants Per Field (Mean ± SD) | Plant Damage Score Per Field (Scale 0–5) (Mean ± SD) | ||
|---|---|---|---|---|
| Dry Season | Rainy Season | Dry Season | Rainy Season | |
| Macate | 62.4 ± (40.03) Aa | 19.35 ± (38.47) ABb | 1.33 ± (1.16) Ba | 0.33 ± (0.66) Ab |
| Manica | 79.14 ± (35.71) Aa | 18.61 ± (33.40) ABb | 1.62 ± (0.95) Ba | 0.34 ± (0.63) Ab |
| Sussundenga | 81.48 ± (31.31) Aa | 11.88 ± (28.43) Bb | 2.88 ± (5.04) Aa | 0.25 ± (0.61) Ab |
| Vanduzi | 80.59 ± (30.39) Aa | 30.27 ± (42.34) Ab | 1.51 ± (0.90) Ba | 0.69 ± (1.03) Ab |
| District | Number of Egg Masses (mean ± SD) | Number of Larvae (Mean ± SD) | ||
|---|---|---|---|---|
| Dry Season | Rainy Season | Dry Season | Rainy Season | |
| Macate | 0.16 ± (0.62) Aa | 0.03 ± (0.35) Aa | 7.92 ± (10.36) Ba | 0.52 ± (1.40) Bb |
| Manica | 0.69 ± (1.63) Aa | 0.01 ± (0.09) Aa | 11.76 ± (9.75) Ba | 1.25 ± (3.33) Bb |
| Sussundenga | 1 ± (2.56) Aa | 0 ± (0.0) Aa | 26.19 ± (24.73) Aa | 0.74 ± (2.32) Bb |
| Vanduzi | 0.44 ± (1.60) Aa | 0 ± (0.0) Aa | 10.56 ± (11.16) Ba | 2.75 ± (5.59) Ab |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caniço, A.; Mexia, A.; Santos, L. Seasonal Dynamics of the Alien Invasive Insect Pest Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) in Manica Province, Central Mozambique. Insects 2020, 11, 512. https://doi.org/10.3390/insects11080512
Caniço A, Mexia A, Santos L. Seasonal Dynamics of the Alien Invasive Insect Pest Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) in Manica Province, Central Mozambique. Insects. 2020; 11(8):512. https://doi.org/10.3390/insects11080512
Chicago/Turabian StyleCaniço, Albasini, António Mexia, and Luisa Santos. 2020. "Seasonal Dynamics of the Alien Invasive Insect Pest Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) in Manica Province, Central Mozambique" Insects 11, no. 8: 512. https://doi.org/10.3390/insects11080512
APA StyleCaniço, A., Mexia, A., & Santos, L. (2020). Seasonal Dynamics of the Alien Invasive Insect Pest Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) in Manica Province, Central Mozambique. Insects, 11(8), 512. https://doi.org/10.3390/insects11080512






