In Vitro Protective Effect of Paste and Sauce Extract Made with Protaetia brevitarsis Larvae on HepG2 Cells Damaged by Ethanol
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PBL Koji, Paste, and Sauce
2.2. Preparation of Koji, Paste, and Sauce Extracts
2.3. Amino Nitrogen, Protease Activity, and Free Amino Acid Analyses
2.4. Cell Culture, EtOH-Damaged HepG2 Cells, and Cell Viability Assay
2.5. Aspartate Aminotransferase (AST) and Alanine Aminotransaminase (ALT) Analyses
2.6. Total RNA Extraction and Quantitative PCR Analysis
2.7. Glutathione (GSH) Activity and Superoxide Dismutase (SOD) Inhibition Rate Analyses
2.8. Statistical Analysis
3. Results
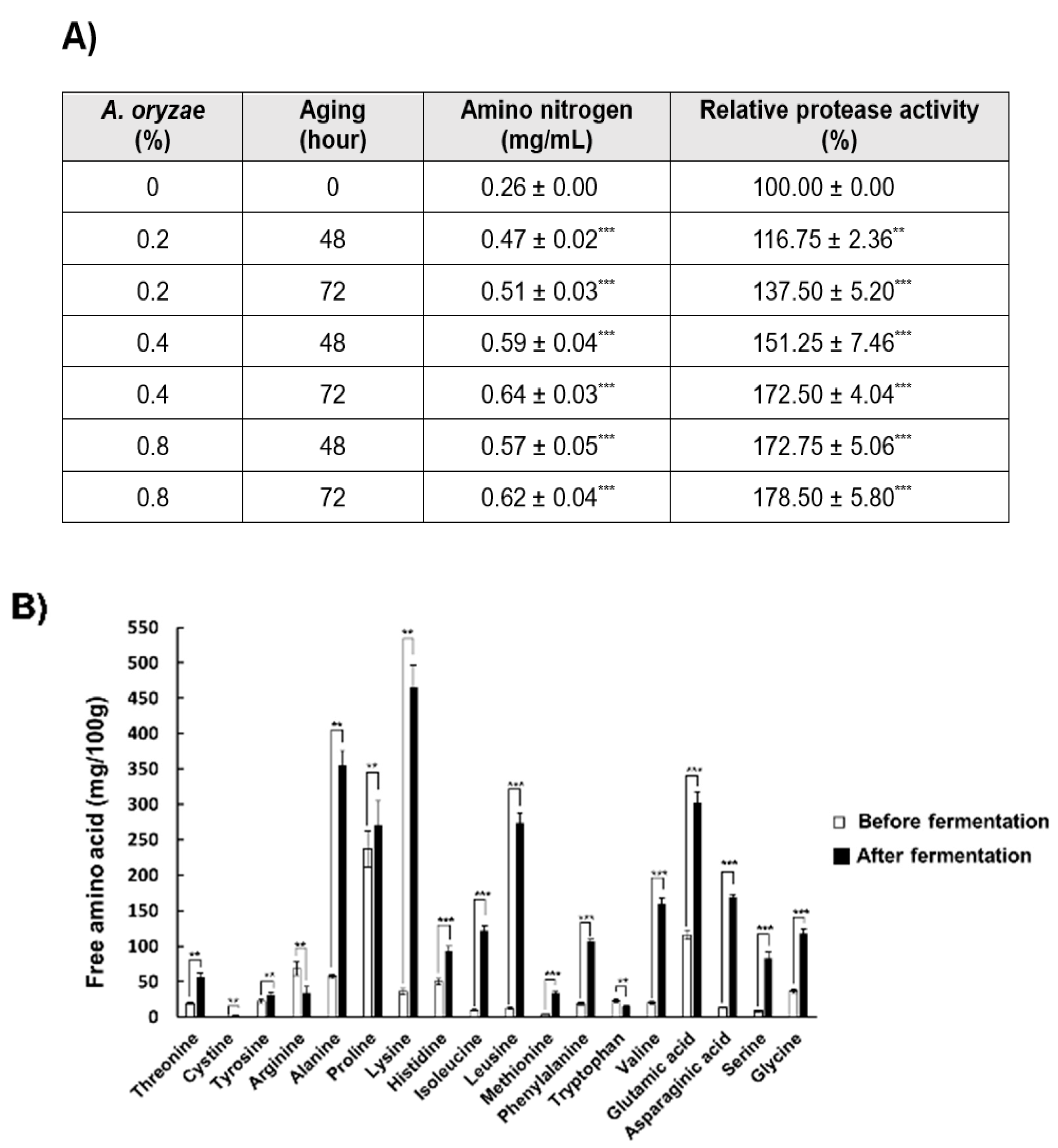
3.1. Evaluating the Fermentation of Koji Made with PBL
3.2. Evaluation of Aging on Paste and Sauce Made with PBL Koji
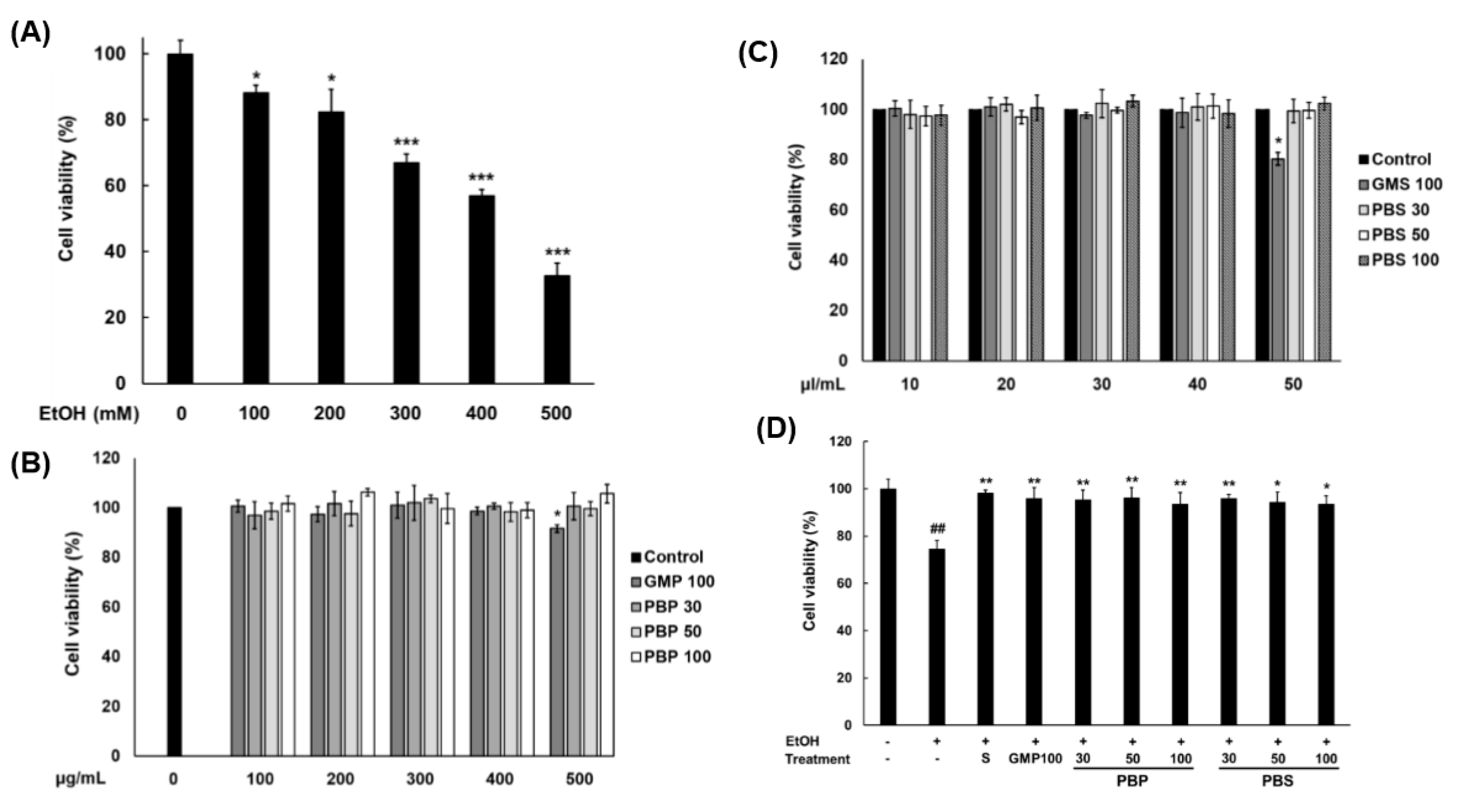
3.3. EtOH Concentration Causing Toxicity to Hepatocytes and Toxicity Assessment of PBL Paste and Sauce to Hepatocytes
3.4. Hepatoprotective Effect of Pretreatment with PBL Paste and Sauce on EtOH-Damaged Hepatocytes
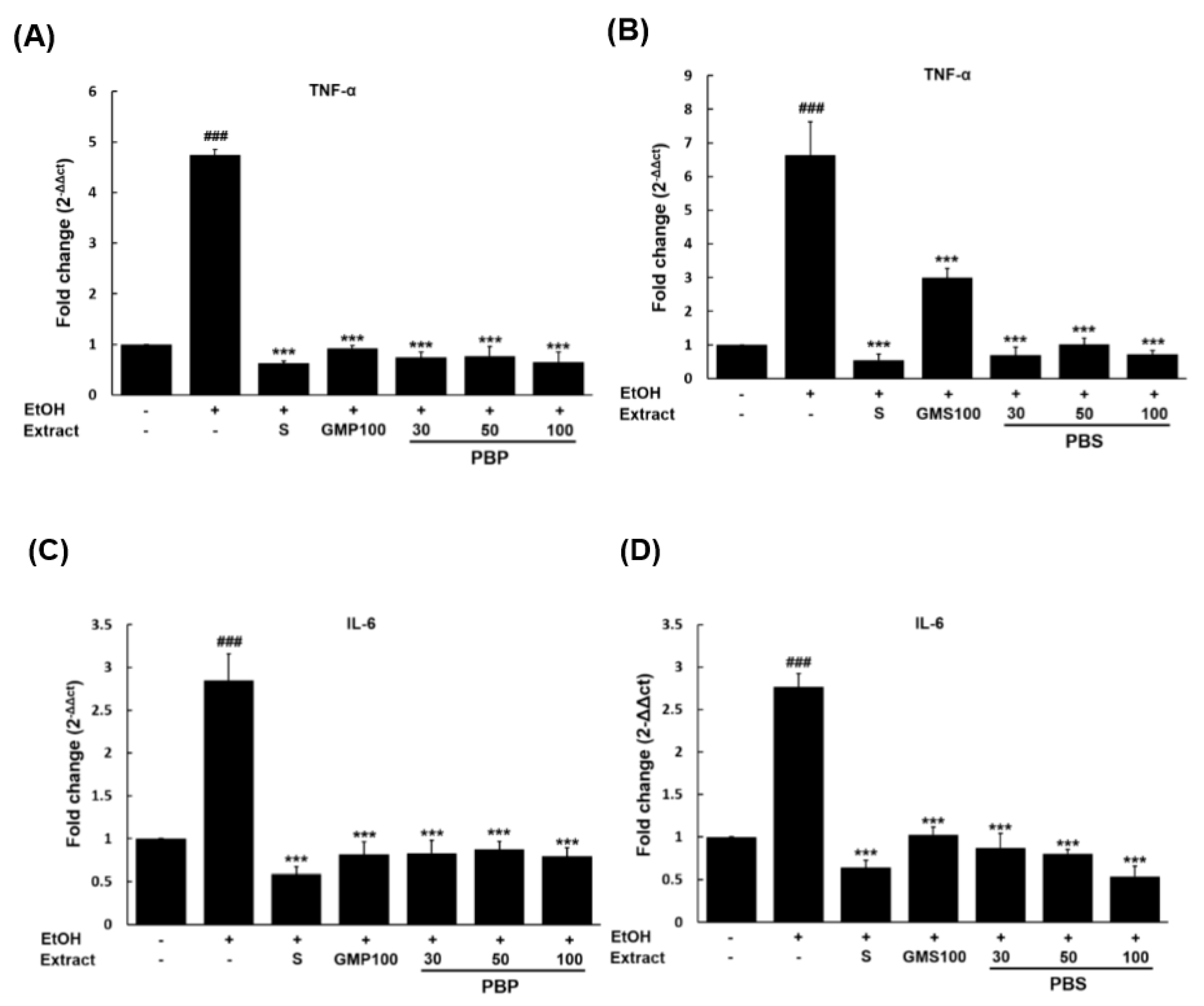
3.5. Evaluation of Anti-Inflammatory Potential of PBL Paste and Sauce in EtOH-Damaged Hepatocytes
3.6. Evaluation of Antioxidant Potential of PBL Paste and Sauce in EtOH-Damaged Hepatocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Scaraffia, P.Y.; Miesfeld, R.L. Encycclopedia of Biological Chemistry; Elsevier: Amsterdam, The Nederlands, 2013; pp. 590–595. [Google Scholar]
- Yi, C.; He, Q.; Wang, L.; Kuang, R. The utilization of insect resource in Chinese rural area. J. Agric. Sci. 2010, 2, 146–154. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.Y.; Kang, M.; Yoon, M.; Lee, Y.; Park, E. Antioxidant activity and safety evaluation of juice containing Protaetia brevitarsis. J. Korean Soc. Food Sci. Nutr. 2012, 41, 41–48. [Google Scholar] [CrossRef]
- Han, S.R.; Yun, E.Y.; Kim, J.Y.; Hwang, J.S.; Jeong, E.J.; Moon, K.S. Evaluation of genotoxicity and 28-day oral dose toxicity on freeze-dried powder of Tenebrio molitor Larvae (yellow mealworm). Toxicol. Res. 2014, 30, 121–130. [Google Scholar] [CrossRef]
- Chung, M.Y.; Hwang, J.S.; Goo, T.W.; Yun, E.Y. Analysis of general composition and harmful material of Protaetia brevitarsis. J. Life Sci. 2013, 23, 664–668. [Google Scholar] [CrossRef]
- Chon, J.W.; Kweon, H.Y.; Jo, Y.Y.; Yeo, J.H.; Lee, H.S. Protective effects of extracts of Protaetia brevitarsis on carbon tetrachloride-induced hepatotoxicity in the Mice. J. Seric. Entomol. Sci. 2012, 50, 93–100. [Google Scholar]
- Shin, M.K.; Park, N.K.; Kwon, T.Y.; Kim, H.G. A Herbalogical study on the holtricha. J. Trad. Korean Med. 1997, 7, 1–4. [Google Scholar]
- Yoo, Y.; Shin, B.H.; Hong, J.H.; Lee, J.; Chee, H.Y.; Song, K.S.; Lee, K.B. Isolation of fatty acids with anticancer activity from Protaetia brevitarsis Larva. Arch. Pharm. Res. 2007, 30, 361–365. [Google Scholar] [CrossRef]
- Yoon, W.; Lee, S.W.; Moon, H.K.; Kim, B.G.; Kim, B.J.; Kim, G.Y. Quality characteristic of traditional soybean paste (Doenjang) manufactured with mixed beans. J. East. Asian Soc. Dietary Life 2011, 21, 375–384. [Google Scholar]
- Kang, S.; Lee, S.; Ko, J.M.; Hwang, I. Comparison of the physicochemical characteristics of Korean traditional soy sauce with varying soybean seeding periods and regions of production. Korean J. Food Nutr. 2011, 24, 761–769. [Google Scholar]
- Park, K.Y.; Jeong, J.K.; Lee, Y.E.; Daily, J.W., 3rd. Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Guinee, T.P. The functionality of cheese as an ingredient: A review. Aust. J. Dairy Technol. 2002, 57, 79–91. [Google Scholar]
- Park, J.W.; Lee, Y.J.; Yoon, S. Total flavonoids and phenolics in fermented soy products and their effects on antioxidant activities determined by different assays. Korean J. Food Cul. 2007, 22, 353–358. [Google Scholar]
- Kim, H.S.; Lim, J.M.; Kwon, H.J.; Yoo, J.Y.; Park, P.S.; Choi, Y.H.; Choi, J.H.; Park, S.Y. Antioxidant activity and quality characteristics on the maturation period of the soy sauce containing Astragalus membranaceus and oak mushroom (Lentinus edodes). Korean J. Food Preserv. 2013, 20, 467–474. [Google Scholar] [CrossRef][Green Version]
- Lim, S.Y.; Park, K.Y.; Rhee, S.H. Anticancer effect of Doenjang in vitro sulforhodamine B (SRB) assay. J. Korean Soc. Food Sci. Nutr. 1999, 28, 240–245. [Google Scholar]
- Kwon, H.J.; Kim, H.S.; Choi, Y.H.; Choi, J.H.; Choi, H.S.; Song, J.; Park, S.Y. Antioxidant activity and quality characteristics on the maturation period of the soy sauce with Gastrodia elata and oak mushroom (Lentinus edodes). Korean J. Food Preserv. 2014, 21, 231–238. [Google Scholar] [CrossRef]
- Shin, Y.J.; Lee, C.K.; Kim, H.J.; Kim, H.S.; Seo, H.G.; Lee, S.C. Preparation and characteristics of low-salt soy sauce with anti-hypertensive activity by addition of miduduk tunic, mulberry, and onion extracts. J. Korean Soc. Food Sci. Nutr. 2014, 43, 854–858. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.; Park, S.; Kim, I.; Jeong, Y.; Yu, S.; Shin, S.C.; Kim, M. Antioxidant activity of Korean traditional soy sauce fermented in Korean earthenware, Onggi, from different regions. J. Korean Soc. Food Sci. Nutr. 2015, 44, 847–853. [Google Scholar] [CrossRef]
- Faithong, N.; Benjakul, S. Changes in antioxidant activities and physicochemical properties of Kapi, a fermented shrimp paste, during fermentation. J. Food Sci. Technol. 2014, 51, 2463–2471. [Google Scholar] [CrossRef]
- Cho, J.H.; Zhao, H.L.; Kim, J.S.; Kim, S.H.; Chung, C.H. Characteristics of fermented seasoning sauces using Tenebrio molitor larvae. Inno. Food Sci. Emerg. Technol. 2018, 45, 186–195. [Google Scholar] [CrossRef]
- Hwang, D.; Lee, W.S.; Goo, T.W.; Park, S.W.; Yun, E.Y. Effect of pretreatment with paste and sauce extract made using Tenebrio molitor larvae on ethanol-damaged HepG2 cells. Entomol. Res. 2019, 49, 509–518. [Google Scholar] [CrossRef]
- Bruha, R.; Dvorak, K.; Dousa, M.; Petrtyl, J.; Svestka, T. Alcoholic liver disease. Prague Med. Rep. 2009, 110, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, K.; Mohan, C.V.R.; Gobianand, K.; Karthikeyan, S. Silymarin modulates the oxidant-antioxidant imbalance during diethylnitrosamaine induced oxidative stress in rats. Eur. J. Phamcol. 2007, 560, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Rainone, F. Milk thistle. Am. Fam. Physician 2005, 72, 1285–1292. [Google Scholar]
- Lucena, M.I.; Andrade, R.J.; Cruz, J.P.; Rodriguez-Medizabal, M.; Blanco, E.; Sánchez de la Cuesta, F. Effects of silymarin MZ-80 on oxidative stress in patients with alcoholic cirrhosis. Results of a randomized, double-blind, placebo-controlled clinical study. Int. J. Clin. Pharmacol. Ther. 2002, 40, 2–8. [Google Scholar] [CrossRef]
- Body-Malapel, M.; Dharancy, S.; Berrebi, D.; Louvet, A.; Hugot, J.P.; Phillpott, D.J.; Giovannini, A.; Chareyre, F.; Pages, G.; Gantier, E.; et al. NOD2: A potential target for regulation liver injury. Lab. Investig. 2008, 88, 318–327. [Google Scholar] [CrossRef]
- Lee, H.C.; Hwang, S.G.; Kang, Y.K.; Sohn, H.O.; Moon, J.Y.; Lim, H.B.; Jeon, B.H.; Lee, D.W. Influence of Protatia brevitarsis extract on liver damage induced by carbon tetrachloride and ethanol in rats. Korean J. Life Sci. 2001, 11, 405–414. [Google Scholar]
- Krishnan, H.B.; Nelson, R.L. Proteomic analysis of high protein soybean (Glycine Max) accessions demonstrates the contribution of novel glycine subunits. J. Agric. Food Chem. 2011, 59, 2432–2439. [Google Scholar] [CrossRef]
- Huang, X.J.; Choi, Y.K.; Im, H.S.; Yarimaga, O.; Yoon, E.; Kim, H.S. Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) detection techniques. Sensors 2006, 6, 756–782. [Google Scholar] [CrossRef]
- Jung, M.E. Biochemical and histological effects of Phellinus linteus methanol extract on liver lipid metabolism of rats CCl4 and high fat. J. Korean Soc. Food Sci. Nutr. 2001, 30, 331–337. [Google Scholar]
- Kim, K.A. Undestanding and application of liver function tests. Korean J. Med. 2009, 76, 163–168. [Google Scholar]
- Choi, R.Y.; Ham, J.R.; Ryu, H.S.; Park, K.W.; Kang, K.Y.; Lee, M.K. The effects of defatted Tenebrio molitor larva ferment extract on CCl4–induced liver damage in mice. J. Korean Soc. Food Sci. Nutr. 2019, 48, 501–508. [Google Scholar] [CrossRef]
- Pascual, C.; Gonz, R.; Armesto, J.; Muriel, P. Effect of silymarin and silybinin on oxygen radicals. Drug Devel. Res. 1993, 29, 73–77. [Google Scholar] [CrossRef]
- Arafa, H.M.M. Uroprotective effects of curcumin in cyclophos¬phamide-induced haemorrhagic cystitis paradigm. Basic Clin. Pharmacol. Toxicol. 2009, 104, 393–399. [Google Scholar] [CrossRef]
- Avci, H.; Tunca, R.; Epikmen, E.T.; Birincioğlu, S.S.; Sekkin, S.; Boyacioglu, M.; Akşit, H. Protective and antigenotoxic effects of Silymarin and Curcumin in experimental cyclophosphamide intoxication in rats. Kafkas Univ. Vet. Fak. Derg. 2016, 22, 693–701. [Google Scholar]
- Schuppan, D.; Jia, J.D.; Brinkhaus, B.; Hahn, E.G. Herbal products for liver diseases: A therapeutic challenge for the new millennium. Hepatology 1999, 30, 1099–1104. [Google Scholar] [CrossRef]
- Lee, J.H.; Baek, M.; Lee, H.J.; Kim, I.W.; Kim, S.Y.; Seo, M.; Kim, M.A.; Kim, S.H.; Hwang, J.S. Anti-inflammatory activity of antimicrobial peptide protaetiamycine 2 derived from the Protaetia brevitarsis seulensis. J. Life Sci. 2019, 29, 1218–1226. [Google Scholar]
- Lee, H.J.; Seo, M.; Lee, J.H.; Kim, I.W.; Kim, S.Y.; Hwang, J.S.; Kim, M.A. Inhibitory effect of Protaetia brevitarsis seulensis ethanol extract on neuroinflammation in LPS-stimulated BV-2 microglia. J. Life Sci. 2019, 29, 1096–1103. [Google Scholar]
- Kaplowitz, N.; Aw, T.Y.; Ookhtens, M. The regulation of hepatic glutathione. Ann. Rev. Pharmacol. Toxicol. 1985, 25, 715–744. [Google Scholar] [CrossRef]
| Samples | Amino Nitrogen (mg/mL) | Relative Protease Activity (%) |
|---|---|---|
| GMP 100 | 0.38 ± 0.01 | 100.00 ± 0.00 |
| PBP 30 | 0.36 ± 0.02 | 96.73 ± 1.94 |
| PBP 50 | 0.43 ± 0.01 *** | 119.93 ± 2.98 *** |
| PBP 100 | 0.45 ± 0.01 *** | 129.10 ± 3.44 *** |
| GMS 100 | 3.18 ± 0.13 | 100.00 ± 0.00 |
| PBS 30 | 3.05 ± 0.10 | 108.03 ± 1.00 |
| PBS 50 | 3.23 ± 0.18 | 115.19 ± 2.39 ** |
| PBS 100 | 3.88 ± 0.06 *** | 133.53 ± 6.76 *** |
| EtOH | Extract | AST (IU/L) | ALT (IU/L) |
|---|---|---|---|
| − | − | 23.23 ± 3.19 | 25.67 ± 2.20 |
| + | − | 56.45 ± 2.20 ## | 34.46 ± 2.02 ## |
| + | S | 23.35 ± 5.38 ** | 18.24 ± 0.76 *** |
| + | GMP 100 | 36.11 ± 4.12 ** | 24.59 ± 5.99 ** |
| + | PBP 30 | 37.35 ± 3.20 ** | 27.62 ± 0.49 ** |
| + | PBP 50 | 29.69 ± 1.87 ** | 21.27 ± 4.54 ** |
| + | PBP 100 | 25.26 ± 1.43 ** | 19.75 ± 0.88 *** |
| + | GMS 100 | 36.78 ± 4.23 ** | 26.43 ± 0.79 ** |
| + | PBS 30 | 35.18 ± 1.44 ** | 25.66 ± 6.48 ** |
| + | PBS 50 | 33.47 ± 0.11 ** | 20.55 ± 2.29 *** |
| + | PBS 100 | 28.23 ± 2.97 ** | 19.22 ± 1.51 *** |
| EtOH | Extract | GSH Activity (%) | SOD Inhibition Rate (%) |
|---|---|---|---|
| − | − | 100.00 ± 0.00 | 100.00 ± 0.00 |
| + | − | 68.70 ± 3.40 ### | 87.78 ± 2.17 ## |
| + | S | 160.08 ± 5.00 *** | 161.77 ± 1.40 *** |
| + | GMP 100 | 149.84 ± 1.90 *** | 138.38 ± 3.22 *** |
| + | PBP 30 | 149.81 ± 1.01 *** | 149.80 ± 2.99 *** |
| + | PBP 50 | 150.40 ± 1.67 *** | 155.76 ± 2.53 *** |
| + | PBP 100 | 151.77 ± 1.35 *** | 155.42 ± 1.19 *** |
| + | GMS 100 | 145.03 ± 4.13 *** | 145.24 ± 3.47 *** |
| + | PBS 30 | 144.63 ± 3.41 *** | 144.05 ± 2.01 *** |
| + | PBS 50 | 153.85 ± 1.20 *** | 155.16 ± 0.53 *** |
| + | PBS 100 | 154.14 ± 4.57 *** | 158.10 ± 2.02 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, D.; Goo, T.-W.; Yun, E.-Y. In Vitro Protective Effect of Paste and Sauce Extract Made with Protaetia brevitarsis Larvae on HepG2 Cells Damaged by Ethanol. Insects 2020, 11, 494. https://doi.org/10.3390/insects11080494
Hwang D, Goo T-W, Yun E-Y. In Vitro Protective Effect of Paste and Sauce Extract Made with Protaetia brevitarsis Larvae on HepG2 Cells Damaged by Ethanol. Insects. 2020; 11(8):494. https://doi.org/10.3390/insects11080494
Chicago/Turabian StyleHwang, Dooseon, Tae-Won Goo, and Eun-Young Yun. 2020. "In Vitro Protective Effect of Paste and Sauce Extract Made with Protaetia brevitarsis Larvae on HepG2 Cells Damaged by Ethanol" Insects 11, no. 8: 494. https://doi.org/10.3390/insects11080494
APA StyleHwang, D., Goo, T.-W., & Yun, E.-Y. (2020). In Vitro Protective Effect of Paste and Sauce Extract Made with Protaetia brevitarsis Larvae on HepG2 Cells Damaged by Ethanol. Insects, 11(8), 494. https://doi.org/10.3390/insects11080494





