Effect of Temperature on Life History and Parasitization Behavior of Trichogramma achaeae Nagaraja and Nagarkatti (Hym.: Trichogrammatidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Egg to Adult Developmental Time
2.3. Adult Longevity and Fecundity
2.4. Functional Response
2.5. Statistical Analysis
3. Results
3.1. Egg to Adult Developmental Time
3.2. Adult Longevity and Fecundity
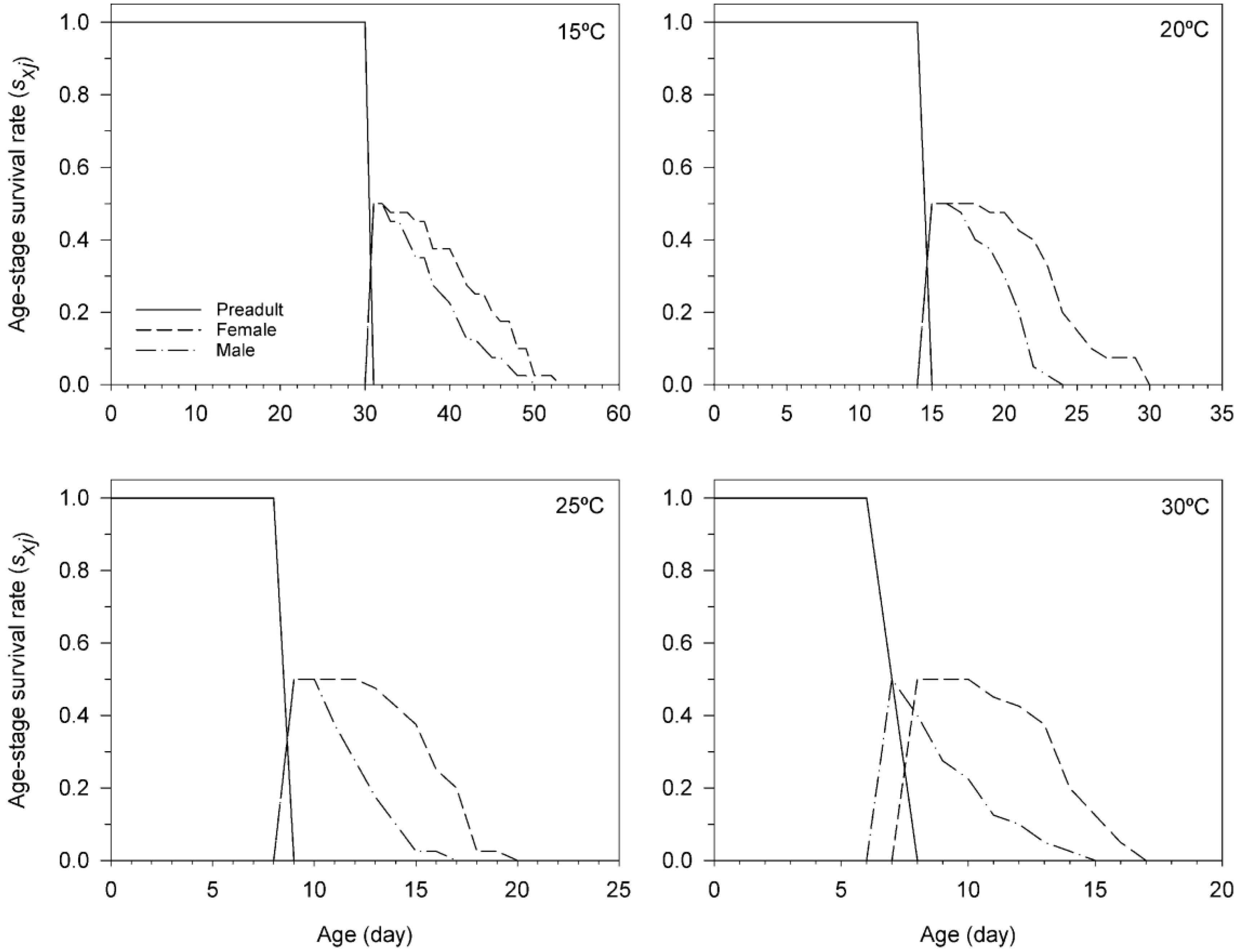
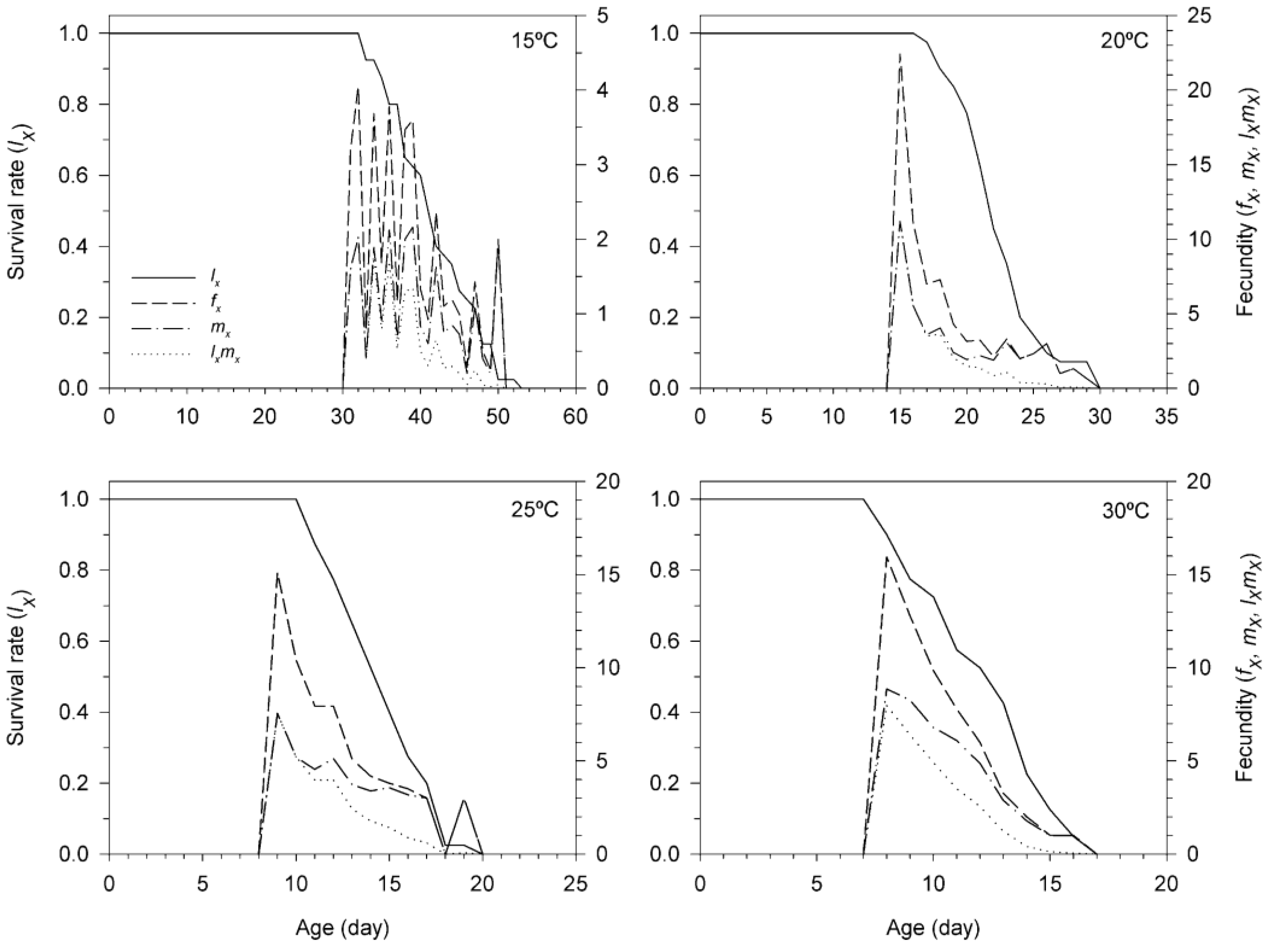
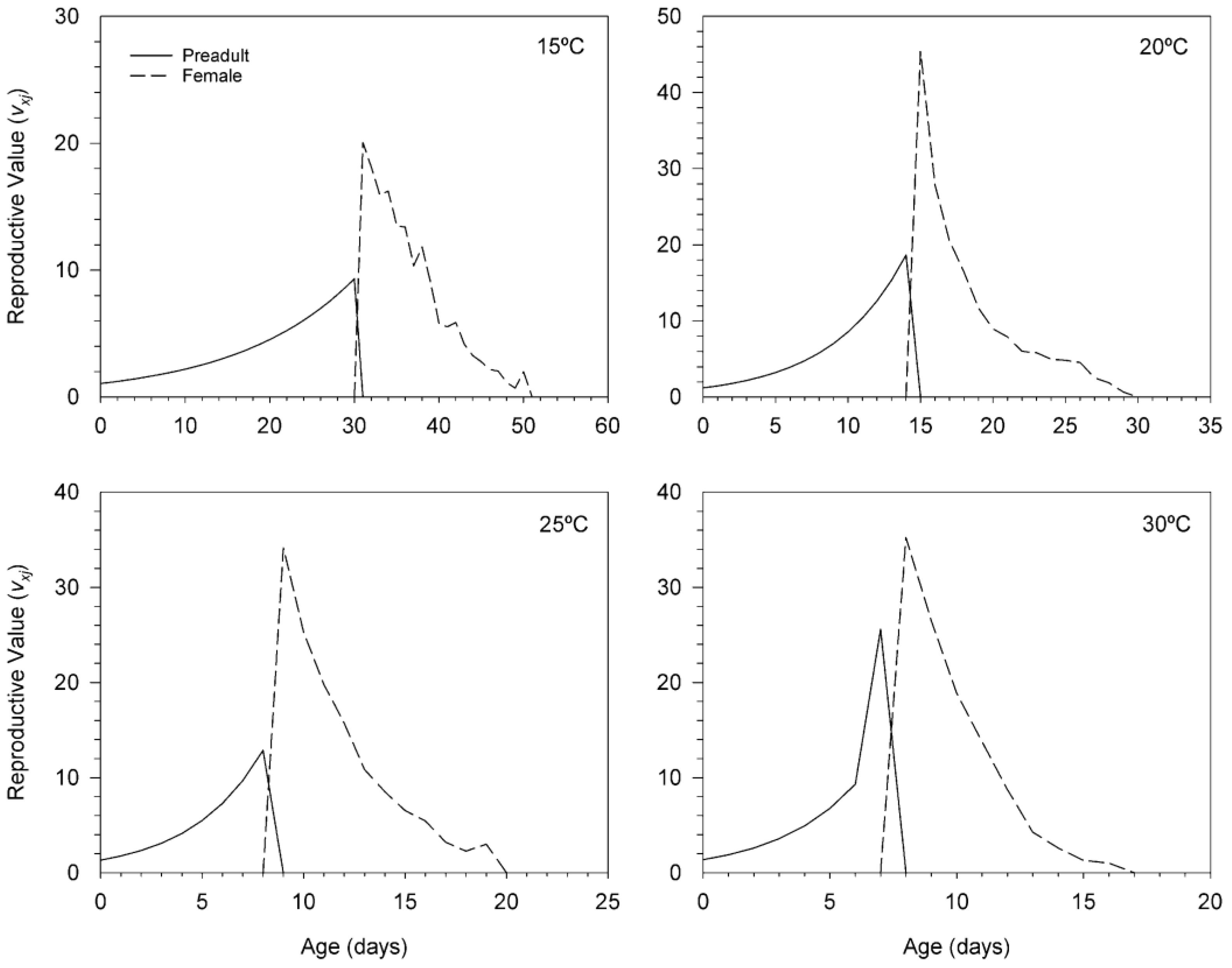
3.3. Age-Stage, Two-Sex Life Table of T. achaeae
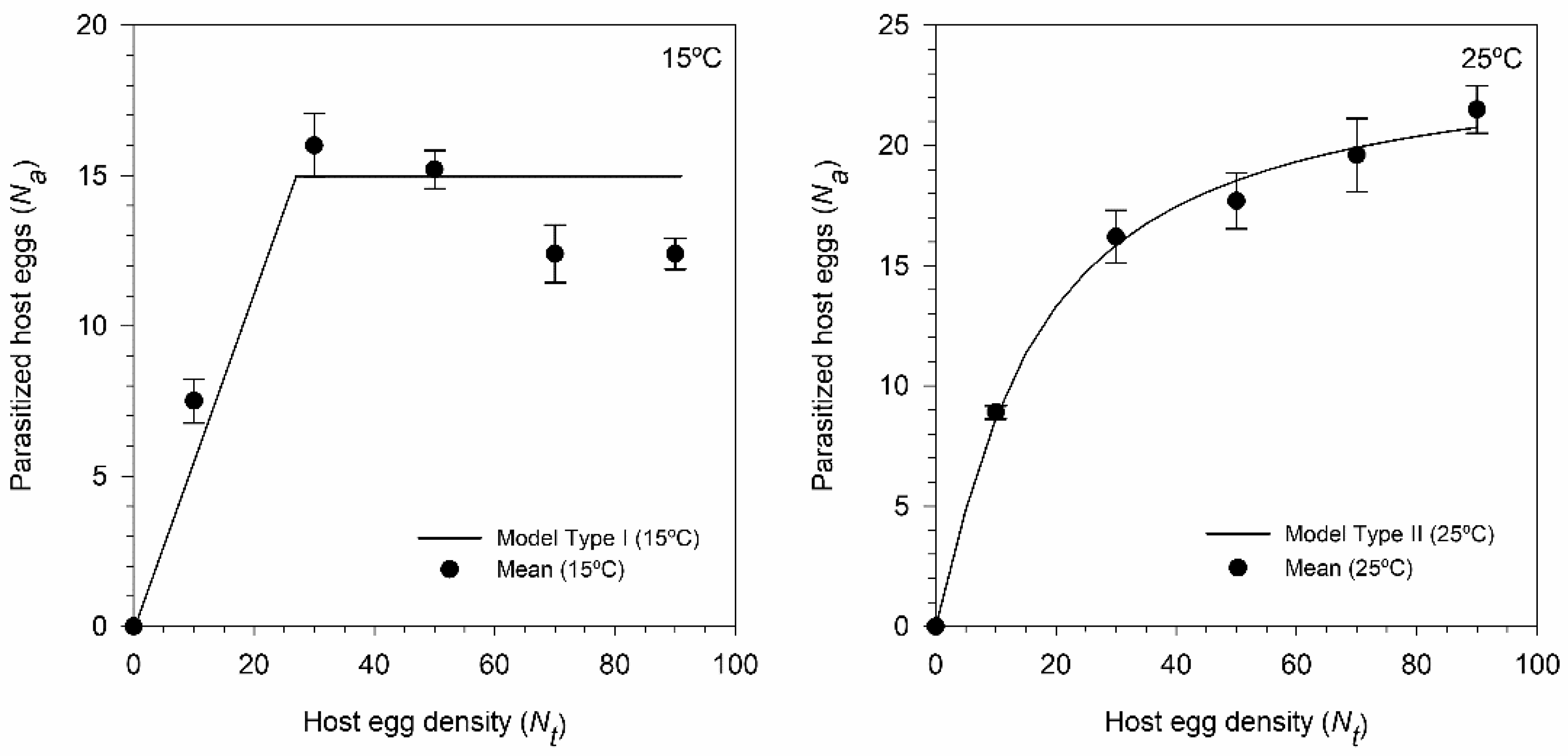
3.4. Functional Response of T. achaeae
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roques, A.; Auger-Rozenberg, M.A.; Blackburn, T.M.; Garnas, J.; Pyšek, P.; Rabitsch, W.; Richardson, D.M.; Wingfield, M.J.; Liebhold, A.M.; Duncan, R.P. Temporal and interspecific variation in rates of spread for insect species invading Europe during the last 200 years. Biol. Invasions 2016, 18, 907–920. [Google Scholar] [CrossRef]
- Lopez-Vaamonde, C.; Agassiz, D.; Augustin, S.; De Prins, J.; De Prins, W.; De Prins, W.; Gomboc, S.; Ivinskis, P.; Karsholt, O.; Koutroumpas, A.; et al. Alien terrestrial arthropods of Europe. Lepidoptera. Chapter 11. BioRisk 2010, 4, 603–668. [Google Scholar] [CrossRef]
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.G.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A.; González-Cabrera, J.; Catalán-Ruescas, D.; Tabone, E.; Frandon, J.; et al. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest. Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Campos, M.R.; Biondi, A.; Adiga, A.; Guedes, R.N.C.; Desneux, N. From the Western Palaearctic region to beyond: Tuta absoluta 10 years after invading Europe. J. Pest Sci. 2017, 90, 787–796. [Google Scholar] [CrossRef]
- Biondi, A.; Guedes, R.N.C.; Wan, F.H.; Desneux, N. Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: Past, present, and future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef]
- Sparks, T.H.; Dennis, R.L.H.; Croxton, P.J.; Cade, M. Increased migration of Lepidoptera linked to climate change. Eur. J. Entomol. 2007, 104, 139–143. [Google Scholar] [CrossRef]
- Collins, L.; Korycinska, A.; Baker, R. Rapid Pest Risk Analysis for Crysodeixis chalcites. In Food and Environment Research Agency, United Kingdom; 2014. Available online: http://www.fera.defra.gov.uk/plants/plantHealth/pestsDiseases/documents/chrysodeixisChalcites.pdf (accessed on 1 June 2020).
- del Pino, M.; Cabello, T.; Hernández-Suárez, E. Age-Stage, Two-Sex Life Table of Chrysodeixis chalcites (Lepidoptera: Noctuidae) at constant temperatures on semi-synthetic diet. Environ. Entomol. 2020, nvaa050. [Google Scholar] [CrossRef]
- Suckling, D.M.; Conlong, D.E.; Carpenter, J.E.; Bloem, K.A.; Rendon, P.; Vreysen, M.J.B. Global range expansion of pest Lepidoptera requires socially acceptable solutions. Biol. Invasions 2017, 19, 1107–1119. [Google Scholar] [CrossRef]
- Campos, M.R.; Silva, T.B.M.; Silva, W.M.; Silva, J.E.; Siqueira, H.A.A. Spinosyn resistance in the tomato borer Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). J. Pest Sci. 2015, 88, 405–412. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Gallego, J.R.; Guerrero-Manzano, J.; Fernández-Maldonado, F.J.; Cabello, T. Susceptibility of the egg parasitoid Trichogramma achaeae (Hymenoptera: Trichogrammatidae) to selected insecticides used in tomato greenhouses. Spanish J. Agric. Res. 2019, 17, e1009. [Google Scholar] [CrossRef]
- del Pino, M.; Carnero, A.; Hernández-Suárez, E.; Cabello, T. Bases para la gestión integrada de Chrysodeixis chalcites (Lep.: Noctuidae) en cultivos de platanera de Canarias. Phytoma 2015, 271, 40–46. [Google Scholar]
- Smith, S.M. Biological control with Trichogramma: Advances, successes, and potential of their use. Annu. Rev. Entomol. 1996, 41, 375–406. [Google Scholar] [CrossRef] [PubMed]
- Mills, N. Egg parasitoids in biological control and integrated pest management, Chapter 15. In Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma; Consoli, F.L., Parra, J.R.P., Zucchi, R.A., Eds.; Springer: New York, NY, USA, 2010; pp. 389–412. [Google Scholar]
- Wang, Z.Y.; He, K.L.; Zhang, F.; Lu, X.; Babendreier, D. Mass rearing and release of Trichogramma for biological control of insect pests of corn in China. Biol. Control 2014, 68, 136–144. [Google Scholar] [CrossRef]
- Kumar, P.; Sekhar, J.C.; Kaur, J. Trichogrammatids: Integration with other methods of pest control. In Biological Control of Insect Pests Using Egg Parasitoids; Sithanantham, S., Ballal, C.R., Jalali, S.K., Bakthavatsalam, N., Eds.; Springer: New Delhi, India, 2013; pp. 191–208. [Google Scholar]
- Polaszek, A.; Rugman-Jones, P.F.; Stouthamer, R.; Hernández-Suarez, E.; Cabello, T.; del Pino-Pérez, M. Molecular and morphological diagnoses of five species of Trichogramma: Biological control agents of Chrysodeixis chalcites (Lepidoptera: Noctuidae) and Tuta absoluta (Lepidoptera: Gelechiidae) in the Canary Islands. Biocontrol 2012, 57, 21–35. [Google Scholar] [CrossRef]
- Polaszek, A. Biodiversity and host associations of Trichogramma in Eurasia, Chapter 9. In Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma; Consoli, F.L., Parra, J.R.P., Zucchi, R.A., Eds.; Springer: New York, NY, USA, 2010; pp. 237–266. [Google Scholar]
- Wright, M.G.; Stouthamer, R. First report of Trichogramma achaeae (Hymenoptera: Trichogrammatidae) from Hawaii. Proc. Hawaii. Entomol. Soc. 2011, 43, 67. [Google Scholar]
- del Pino, M.; Rugman-Jones, P.F.; Hernández-Suárez, E.; Polaszek, A.; Stouthamer, R. Rapid molecular identification of five species of Trichogramma occurring in the Canary Islands with notes on their distribution in banana groves. Biocontrol 2013, 58, 515–524. [Google Scholar] [CrossRef]
- van Lenteren, J.C.; Bolckmans, K.; Kohl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59. [Google Scholar] [CrossRef]
- Cabello, T.; Gallego, J.R.; Fernández, F.J.; Gámez, M.; Vila, E.; del Pino, M.; Hernández-Suárez, E. Biological control strategies for the south American tomato moth Tuta absoluta (Lepidoptera: Gelechiidae) in greenhouse tomatoes. J. Econ. Entomol. 2012, 105, 2085–2096. [Google Scholar] [CrossRef]
- Chailleux, A.; Desneux, N.; Seguret, J.; Khanh, H.D.T.; Maignet, P.; Tabone, E. Assessing European egg parasitoids as a mean of controlling the invasive South American tomato pinworm Tuta absoluta. PLoS ONE 2012, 7, e48068. [Google Scholar] [CrossRef]
- Giorgini, M.; Guerrieri, E.; Cascone, P.; Gontijo, L. Current strategies and future outlook for managing the neotropical tomato pest Tuta absoluta (Meyrick) in the Mediterranean basin. Neotrop. Entomol. 2019, 48, 1–17. [Google Scholar] [CrossRef]
- Yadav, D.N. Egg parasitoids in cotton ecosystem. In Biological Control of Insect Pests Using Egg Parasitoids; Sithanantham, S., Ballal, C.R., Jalali, S.K., Bakthavatsalam, N., Eds.; Springer: New Delhi, India, 2013; pp. 301–315. [Google Scholar]
- de Freitas Bueno, R.C.O.; Parra, J.R.P.; de Freitas Bueno, A. Trichogramma pretiosum parasitism of Pseudoplusia includens and Anticarsia gemmatalis eggs at different temperatures. Biol. Control 2012, 60, 154–162. [Google Scholar] [CrossRef]
- Coelho, A.; Rugman-Jones, P.F.; Reigada, C.; Stouthamer, R.; Parra, J.R.P. Laboratory performance predicts the success of field releases in inbred lines of the egg parasitoid Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). PLoS ONE 2016, 11, e0146153. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.M.; de Oliveira, J.V.; Barbosa, D.R.S.; Breda, M.O.; de França, S.M.; Duarte, B.L.R. Biological parameters and thermal requirements of Trichogramma pretiosum for the management of the tomato fruit borer (Lepidoptera: Crambidae) in tomatoes. Crop Prot. 2017, 99, 39–44. [Google Scholar] [CrossRef]
- Maceda, A.; Hohmann, C.L.; Santos, H.R. Temperature effects on Trichogramma pretiosum Riley and Trichogrammatoidea annulata De Santis. Brazilian Arch. Biol. Technol. 2003, 46, 27–32. [Google Scholar] [CrossRef]
- Pratissoli, D.; Parra, J.R.P. Fertility life table of Trichogramma pretiosum (Hym., Trichogrammatidae) in eggs of Tuta absoluta and Phthorimaea operculella (Lep., Gelechiidae) at different temperatures. J. Appl. Entomol. 2000, 124, 339–342. [Google Scholar] [CrossRef]
- Andrade, G.S.; Pratissoli, D.; Dalvi, L.P.; Desneux, N.; dos Santos, H.J.G. Performance of four Trichogramma species (Hymenoptera: Trichogrammatidae) as biocontrol agents of Heliothis virescens (Lepidoptera: Noctuidae) under various temperature regimes. J. Pest Sci. 2011, 84, 313–320. [Google Scholar] [CrossRef]
- Parra, J.R.P. Egg Parasitoids Commercialization in the New World. Chapter 14. In Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma; Consoli, F.L., Parra, J.R.P., Zucchi, R.A., Eds.; Springer: New York, NY, USA, 2010; pp. 373–388. [Google Scholar]
- Schöller, M.; Hassan, S.A. Comparative biology and life tables of Trichogramma evanescens and T. cacoeciae with Ephestia elutella as host at four constant temperatures. Entomol. Exp. Appl. 2001, 98, 35–40. [Google Scholar] [CrossRef]
- Foerster, M.R.; Foerster, L.A. Effects of temperature on the immature development and emergence of five species of Trichogramma. Biocontrol 2009, 54, 445–450. [Google Scholar] [CrossRef]
- Özder, N.; Kara, G. Comparative biology and life tables of Trichogramma cacoeciae, T. brassicae and T. evanescens (Hymenoptera: Trichogrammatidae) with Ephestia kuehniella and Cadra cautella (Lepidoptera: Pyralidae) as hosts at three constant temperatures. Biocontrol Sci. Technol. 2010, 20, 245–255. [Google Scholar] [CrossRef]
- Berryman, A.A. The theoretical foundations of biological control. In Theoretical Approaches to Biological Control; Hawkins, B.A., Cornell, H.V., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 3–21. [Google Scholar]
- Solomon, M.E. The natural control of animal populations. J. Anim. Ecol. 1949, 18, 1–35. [Google Scholar] [CrossRef]
- Oaten, A.; Murdoch, W.W. Functional response and stability in predator-prey systems. Am. Nat. 1975, 109, 289–298. [Google Scholar] [CrossRef]
- Holling, C.S. The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can. Entom. 1959, 91, 293–320. [Google Scholar] [CrossRef]
- Hassell, M.P. The Spatial and Temporal Dynamics of Insect Host–Parasitoid Interactions; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Mills, N.J.; Lacan, I. Ratio dependence in the functional response of insect parasitoids: Evidence from Trichogramma minutum foraging for eggs in small host patches. Ecol. Entomol. 2004, 29, 208–216. [Google Scholar] [CrossRef]
- Cabello, T.; Vargas, P. Response of Trichogramma cordubensis and T. pintoi to different densities of alternative host eggs. Les Colloques de l’INRA 1988, 9, 165–172. [Google Scholar]
- Faria, C.A.; Torres, J.B.; Farias, A.M.I. Resposta funcional de Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae) parasitando ovos de Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae): Efeito da idade do hospedeiro. An. Soc. Entomológica Bras. 2000, 29, 85–93. [Google Scholar] [CrossRef][Green Version]
- Kalyebia, A.; Overholtb, W.A.; Schulthessa, F.; Muekec, J.M.; Hassan, S.A.; Sithananthama, S. Functional response of six indigenous trichogrammatid egg parasitoids in Kenya: Influence of temperature and relative humidity. Biol. Control 2005, 32, 164–171. [Google Scholar] [CrossRef]
- Cabello, T.; Gámez, M.; Torres, A.; Garay, J. Possible effects of inter-specific competition on the coexistence of two parasitoid species: Trichogramma brassicae Bezdenko and Chelonus oculator (F.) (Hymenoptera: Trichogrammatidae, Braconidae). Community Ecol. 2011, 12, 78–88. [Google Scholar] [CrossRef]
- Wang, B.; Ferro, D.N. Functional responses of Trichogramma ostriniae to Ostrinia nubilalis under laboratory and field conditions. Environ. Entomol. 1998, 27, 752–758. [Google Scholar] [CrossRef]
- Moezipour, M.; Kafil, M.; Allahyari, H. Functional response of Trichogramma brassicae at different temperatures and relative humidities. Bull. Insectol. 2008, 61, 245–250. [Google Scholar]
- Reay-Jones, F.P.F.; Rochat, J.; Goebel, R.; Tabone, E. Functional response of Trichogramma chilonis to Galleria mellonella and Chilo sacchariphagus eggs. Entomol. Exp. Appl. 2006, 118, 229–236. [Google Scholar] [CrossRef]
- Varma, G.C. Growth of population of Trichogramma achaeae Nagaraja and Nagarkatti (Trichogrammatidae: Hymenoptera). J. Res.-Punjab Agric. Univ. 1981, 18, 101–103. [Google Scholar]
- Ghosh, E.; Ballal, C.R.; Verghese, A. Temperature based differences in biological parameters of some potential species/strains of Trichogramma. J. Biol. Control 2017, 31, 82–89. [Google Scholar] [CrossRef]
- Manohar, T.N.; Sharma, P.L.; Verma, S.C.; Chandel, R.S. Demographic parameters of the indigenous egg parasitoids, Trichogramma spp., parasitizing the invasive tomato leafminer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Egypt J. Biol. Pest Control 2019, 29, 1–9. [Google Scholar] [CrossRef]
- Jalali, S.K.; Singh, S.P. Differential response of four Trichogramma species to low temperatures for short term storage. Entomophaga 1992, 37, 159–165. [Google Scholar] [CrossRef]
- Singhamuni, S.A.A.; Hemachandra, K.S.; Sirisena, U.G.A.I. Potential for mass rearing of the egg parasitoids, Trichogramma chilonis and Tricogramma achaeae (Hymenoptera: Trichogrammatidae) on Corcyra cephalonica eggs. Trop. Agric. Res. 2016, 27, 1–12. [Google Scholar] [CrossRef]
- Ghosh, E.; Ballal, C.R. Short-term storage of the egg parasitoids, Trichogramma and Trichogrammatoidea. Egypt J. Biol. Pest Control 2018, 28, 34. [Google Scholar] [CrossRef]
- Cascone, P.; Carpenito, S.; Slotsbo, S.; Iodice, L.; Givskov, J.; Holmstrup, M.; Guerrieri, E. Improving the efficiency of Trichogramma achaeae to control Tuta absoluta. BioControl 2015, 60, 761–771. [Google Scholar] [CrossRef]
- Cerutti, F.; Bigler, F.; Eden, G.; Bosshart, S. Optimal larval density and quality control aspects in mass rearing of the Mediterranean flour moth, Ephestia kuehniella Zell. (Lep.: Phycitidae). J. Appl. Entomol. 1992, 114, 353–361. [Google Scholar] [CrossRef]
- Cabello, T.; Vargas, P. The effect of temperature on the bionomic of Trichogramma cordubensis Vargas and Cabello (Hym.: Trichogrammatidae). In Trichogramma and Other Egg Parasites; Voegele, J., Waage, J., van Lenteren, J., Eds.; I.N.R.A. Pub.: Paris, France, 1988; pp. 155–164. [Google Scholar]
- IBM. IBM SPSS Statistics for Windows, Version 22.0; IBM Corp.: Armonk, NY, USA, 2013. [Google Scholar]
- Ikemoto, T.; Takai, K. A new linearized formula for the law of total effective temperature and the evaluation of line fitting methods with both variables subject to error. Environ. Entomol. 2000, 29, 671–682. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: Computer Program for Age Stage, Two-Sex Life Table Analysis; Laboratory of Theoretical Ecology: Taichung, Taiwan, 2013; Available online: http://140.120.197.173/ecology/ (accessed on 1 June 2020).
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman & Hall: New York, NY, USA, 1993. [Google Scholar]
- Huang, Y.B.; Chi, H. Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci. 2012, 19, 263–273. [Google Scholar] [CrossRef]
- Cabello, T.; Gámez, M.; Varga, Z. An improvement of the holling type III functional response in entomophagous species model. J. Biol. Syst. 2007, 15, 515–524. [Google Scholar] [CrossRef]
- García-Martin, M.; Gámez, M.; Torres-Ruiz, A.; Cabello, T. Functional response of Chelonus oculator according to temperature. Community Ecol. 2008, 9, 45–51. [Google Scholar] [CrossRef]
- Jandel Scientific. Table Curve 2D—User´s manual. Version 2.0; Jandel Scientific: San Rafael, CA, USA, 1994. [Google Scholar]
- Motulsky, H.; Christopoulos, A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting; GraphPad Software Inc.: San Diego, CA, USA, 2003. [Google Scholar]
- Samara, R.; Monje, J.C.; Zebitz, C.P.W.; Qubbaj, T. Comparative biology and life tables of Trichogramma aurosum on Cydia pomonella at constant temperatures. Phytoparasitica 2011, 39, 109–119. [Google Scholar] [CrossRef]
- Bueno, R.C.O.d.F.; Parra, J.R.P.; Bueno, A.D.F. Biological characteristics and thermal requirements of a Brazilian strain of the parasitoid Trichogramma pretiosum reared on eggs of Pseudoplusia includens and Anticarsia gemmatalis. Biol. Control 2009, 51, 355–361. [Google Scholar] [CrossRef]
- Altoé, T.; Pratissoli, D.; De Carvalho, J.R.; Dos Santos, H.J.G.; Paes, J.P.P.; Bueno, R.C.O.D.F.; Bueno, A.D.F. Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) parasitism of Trichoplusia ni (Lepidoptera: Noctuidae) eggs under different temperatures. Ann. Entomol. Soc. Am. 2012, 105, 82–89. [Google Scholar] [CrossRef]
- Kishani Farahani, H.; Ashouri, A.; Zibaee, A.; Abroon, P.; Alford, L. The effect of host nutritional quality on multiple components of Trichogramma brassicae fitness. Bull. Entomol. Res. 2016, 106, 633–641. [Google Scholar] [CrossRef]
- Foerster, M.R.; Marchioro, C.A.; Foerster, L.A. Temperature dependent parasitism, survival, and longevity of five species of Trichogramma Westwood (Hymenoptera: Trichogrammatidae) associated with Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae). Neotrop. Entomol. 2014, 43, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.J.L.A.L. Dynamics Study and Temperature Effect on Biological Traits of TWO TRICHOGRAMMA SPECIES (Hymenoptera, Trichogrammatidae) of S. Miguel Island. Master’s Thesis, Universidade dos Açores, Açores, Portugal, 2011. [Google Scholar]
- Mashal, S.; Agamy, E.; Abou-bakr, H.; El-Wahab, T.E.A.; El Behery, H. Effect of honeybee products, as food supplements, on the biological activities of three Trichogramma species (Hymenoptera: Trichogrammatidae). Egypt J. Biol. Pest Control 2019, 29, 46. [Google Scholar] [CrossRef]
- Pratissoli, D.; Fernandes, O.A.; Zanuncio, J.C.; Pastori, P.L. Fertility life table of Trichogramma pretiosum and Trichogramma acacioi (Hymenoptera: Trichogrammatidae) on Sitotroga cerealella (Lepidoptera: Gelechiidae) eggs at different constant temperatures. Ann. Entomol. Soc. Am. 2004, 97, 729–731. [Google Scholar] [CrossRef]
- Cakmak, T.; Piedra-Buena, A.; Hernández-Suárez, E.; Alvarez-Acosta, C. Chrysodeixis chalcites (Esper) (Lepidoptera: Noctuidae) oviposition preferences on different growing stages of banana (Musa acuminate Colla, Musaceae) plants. Phytoparasitica 2019, 47, 485–498. [Google Scholar] [CrossRef]
- Fuentes, E.G.; Hernández-Suárez, E.; Simón, O.; Williams, T.; Caballero, P. Chrysodeixis chalcites, a pest of banana crops on the Canary Islands: Incidence, economic losses and current control measures. Crop Prot. 2018, 108, 137–145. [Google Scholar] [CrossRef]
- Kfir, R. Functional response to host density by the egg parasite Trichogramma Pretiosum. Entomophaga 1983, 28, 345–353. [Google Scholar] [CrossRef]
- Jeschke, J.M.; Kopp, M.; Tollrian, R. Consumer-food systems: Why type I functional responses are exclusive to filter feeders. Biol. Rev. 2004, 79, 337–349. [Google Scholar] [CrossRef]
- Manohar, T.N.; Sharma, P.L.; Verma, S.C.; Sharma, K.C.; Chandel, R.S. Functional response of indigenous Trichogramma spp. to invasive tomato leafminer, Tuta absoluta (Meyrick) under laboratory conditions. Int. J. Trop. Insect Sci. 2020, 40, 101–107. [Google Scholar] [CrossRef]

| Parameters | Temperature (°C) | |||
|---|---|---|---|---|
| 15 | 20 | 25 | 30 | |
| Total development time (days) | 31.08 ± 0.14 a | 14.52 ± 0.08 b | 8.83 ± 0.05 c | 7.55 ± 0.07 d |
| Female longevity (days) | 12.75 ± 1.22 a | 9.55 ± 0.67 b | 7.10 ± 0.35 bc | 5.55 ± 0.40 c |
| Male longevity (days) | 9.12 ± 1.04 a | 5.65 ± 0.42 b | 3.95 ± 0.38 bc | 3.40 ± 0.47 c |
| APOP (days) | 2.15 ± 0.49 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| TPOP (days) | 33.15 ± 0.49 a | 14.49 ± 0.11 b | 8.82 ± 0.06 c | 7.66 ± 0.10 d |
| Oviposition period (days) | 10.95 ± 1.13 a | 8.60 ± 0.69 ab | 7.50 ± 0.39 bc | 5.70 ± 0.37 c |
| Fecundity (eggs/female) | 28.45 ± 2.83 b | 64.05 ± 4.57 a | 55.75 ± 3.60 a | 54.30 ± 4.57 a |
| Emergence (%) | 77.52 ± 2.21 c | 86.31 ± 1.88 b | 89.88 ± 1.65 b | 95.43 ± 0.64 a |
| Sex offspring (% female) | 68.10 ± 1.90 a | 63.15 ± 1.66 ab | 59.64 ± 1.69 b | 48.41 ± 0.91 c |
| Sex offspring (% male) | 31.90 ± 1.90 c | 36.85 ± 1.66 bc | 40.36 ± 1.69 b | 51.59 ± 0.91 a |
| Sex ratio (F:M) | 2.13:1 | 1.71:1 | 1.48:1 | 0.94:1 |
| Parameters | Temperature (°C) | |||
|---|---|---|---|---|
| 15 | 20 | 25 | 30 | |
| R0 (offspring) | 14.075 ± 2.591 c | 32.000 ± 5.519 a | 27.875 ± 4.756 b | 27.150 ± 4.843 b |
| r (day−1) | 0.072 ± 0.005 c | 0.195 ± 0.010 b | 0.284 ± 0.015 a | 0.319 ± 0.017 a |
| λ (day−1) | 1.075 ± 0.006 c | 1.215 ± 0.012 b | 1.328 ± 0.020 a | 1.375 ± 0.024 a |
| T (day) | 36.707 ± 0.397 a | 17.761 ± 0.132 b | 11.728 ± 0.156 c | 10.357 ± 0.100 d |
| GRR (offspring) | 23.070 ± 3.908 c | 46.210 ± 6.713 a | 42.280 ± 5.817 b | 41.520 ± 5.861 b |
| Egg Density | Temperature (°C) | Average | |
|---|---|---|---|
| 15 | 25 | ||
| 10 | 7.50 ± 0.73 c | 8.90 ± 0.28 c | 8.20 ± 0.41 |
| 30 | 16.00 ± 1.06 a | 16.20 ± 1.10 b | 16.10 ± 0.75 |
| 50 | 15.20 ± 0.63 ab | 17.70 ± 1.16 ab | 16.45 ± 0.70 |
| 70 | 12.40 ± 0.96 b | 19.60 ± 1.53 ab | 16.00 ± 1.21 |
| 90 | 12.40 ± 0.96 b | 21.50 ± 0.99 a | 16.95 ± 1.18 |
| Average | 12.70 ± 0.55 | 16.78 ± 0.77 | |
| Temperature (°C) | Type | Parameters 1 | Statistical Parameters | ||||
|---|---|---|---|---|---|---|---|
| Th | a’ | α | AICc | df | r2 | ||
| I | - | 0.8097 | - | 4.0076 | 3 | 0.9342 | |
| 15 | II | 0.0706 | 9.9374 | - | 11.259 | 5 | 0.7695 |
| III | 0.0629 | - | 2.3843 | 11.440 | 5 | 0.7612 | |
| I | - | 0.8557 | - | 8.8033 | 3 | 0.8325 | |
| 25 | II | 0.0414 | 11.834 | - | −2.5839 | 5 | 0.9923 |
| III | 0.0422 | - | 15.548 | −2.3435 | 5 | 0.9901 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Pino, M.; Gallego, J.R.; Hernández Suárez, E.; Cabello, T. Effect of Temperature on Life History and Parasitization Behavior of Trichogramma achaeae Nagaraja and Nagarkatti (Hym.: Trichogrammatidae). Insects 2020, 11, 482. https://doi.org/10.3390/insects11080482
del Pino M, Gallego JR, Hernández Suárez E, Cabello T. Effect of Temperature on Life History and Parasitization Behavior of Trichogramma achaeae Nagaraja and Nagarkatti (Hym.: Trichogrammatidae). Insects. 2020; 11(8):482. https://doi.org/10.3390/insects11080482
Chicago/Turabian Styledel Pino, Modesto, Juan Ramón Gallego, Estrella Hernández Suárez, and Tomás Cabello. 2020. "Effect of Temperature on Life History and Parasitization Behavior of Trichogramma achaeae Nagaraja and Nagarkatti (Hym.: Trichogrammatidae)" Insects 11, no. 8: 482. https://doi.org/10.3390/insects11080482
APA Styledel Pino, M., Gallego, J. R., Hernández Suárez, E., & Cabello, T. (2020). Effect of Temperature on Life History and Parasitization Behavior of Trichogramma achaeae Nagaraja and Nagarkatti (Hym.: Trichogrammatidae). Insects, 11(8), 482. https://doi.org/10.3390/insects11080482








