The Attraction of the Dung Beetle Anoplotrupes stercorosus (Coleoptera: Geotrupidae) to Volatiles from Vertebrate Cadavers
Abstract
1. Introduction
2. Materials and Methods
2.1. Piglet Cadaver Exposure and Dynamic Headspace Sampling of Cadaver Odor
2.2. Gas Chromatography with Electroantennographic Detection (GC–EAD)
2.3. Chemical Analyses (GC–MS)
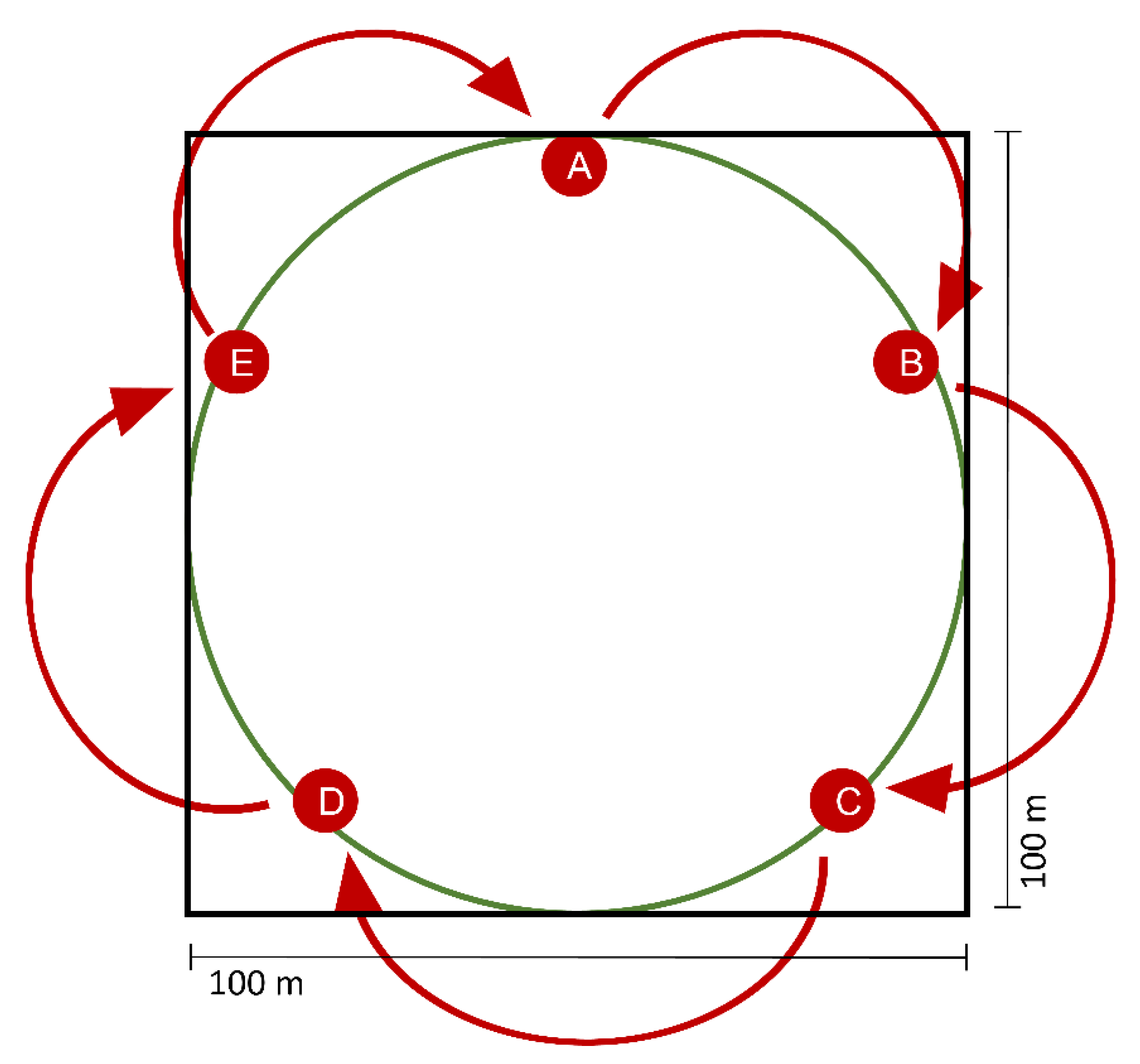
2.4. Field Experiment—Attracting Dung Beetles with Synthetic Cadaver Mixtures
- Treatment 1: a complete mix of all six EAD active compounds (benzaldehyde, DMTS, 3-octanone, 6-methyl-5-hepten-2-ol, nonanal, and dodecane), later described as “complete mixture”
- Treatment 2: three volatile EAD active compounds (benzaldehyde, DMTS, and 3-octanone), later described as “blend 2”
- Treatment 3: three volatile EAD active compounds (6-methyl-5-hepten-2-ol, nonanal, and dodecane), later described as “blend 3”
- Treatment P: positive control (a piece of piglet cadaver tissue (~1 cm3) in the post-bloating stage, previously cut from a decaying piglet and immediately frozen at −20 °C)
- Treatment N: negative control (empty tube).
2.5. Preparation of Synthetic Cadaver Mixtures
2.6. Statistical Analyses
3. Results
3.1. Electrophysiology and Chemical Analyses
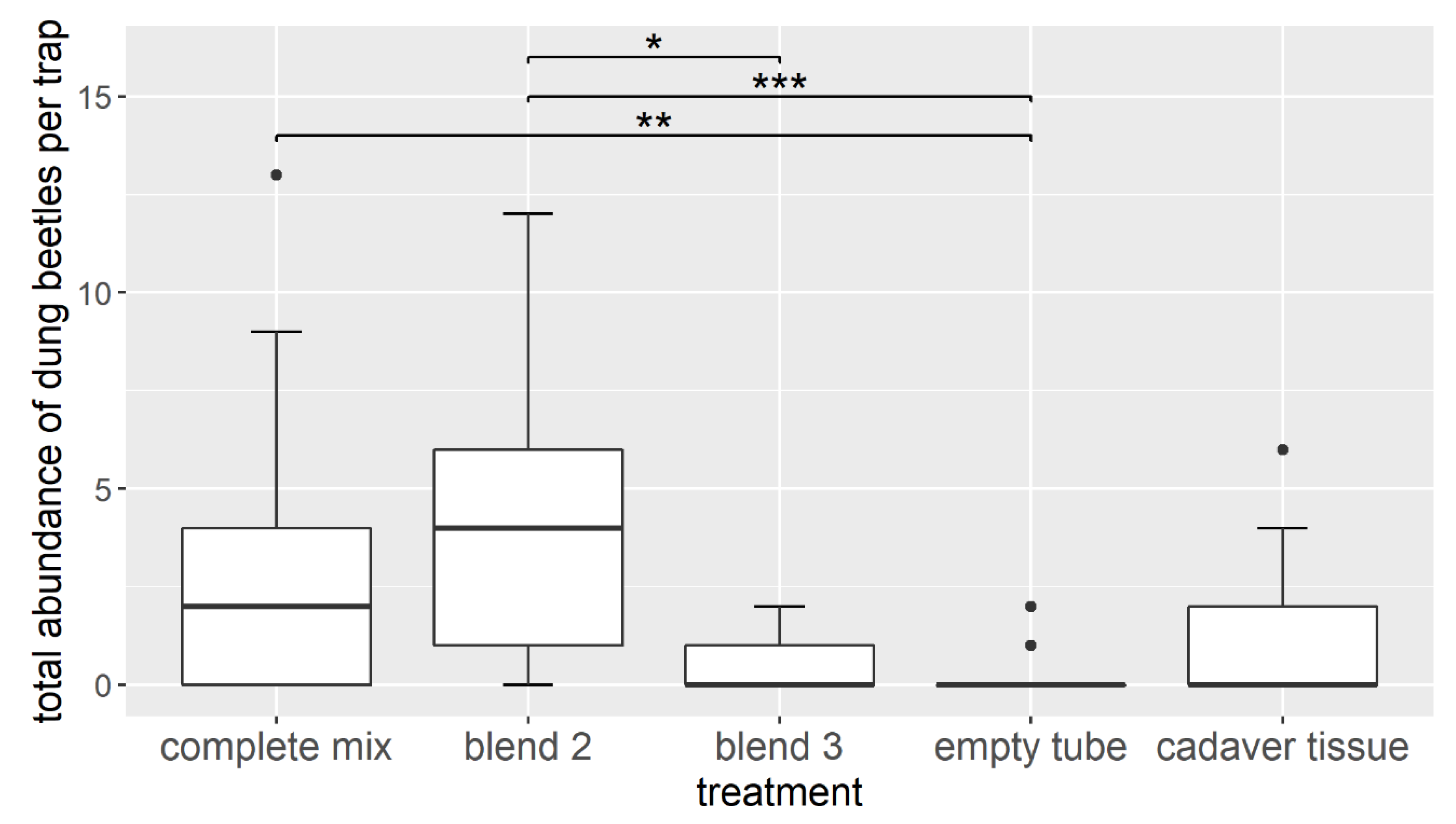
3.2. Field Experiment—Attracting Dung Beetles with Synthetic Cadaver Mixtures
4. Discussion
4.1. Perception of Volatile Carrion Odor Components
4.2. Attractiveness of Selected Carrion Odor Compounds in Field Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics Statement
References
- Parmenter, R.R.; MacMahon, J.A. Carrion decomposition and nutrient cycling in a semiarid shrub–steppe ecosystem. Ecol. Monogr. 2009, 79, 637–661. [Google Scholar] [CrossRef]
- Barton, P.S.; Cunningham, S.A.; Lindenmayer, D.B.; Manning, A.D. The role of carrion in maintaining biodiversity and ecological processes in terrestrial ecosystems. Oecologia 2013, 171, 761–772. [Google Scholar] [CrossRef]
- Carter, D.O.; Yellowlees, D.; Tibbett, M. Cadaver decomposition in terrestrial ecosystems. Naturwissenschaften 2007, 94, 12–24. [Google Scholar] [CrossRef]
- Paczkowski, S.; Schütz, S. Post-mortem volatiles of vertebrate tissue. Appl. Microbiol. Biotechnol. 2011, 91, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Cernosek, T.; Eckert, K.E.; Carter, D.O.; Perrault, K.A. Volatile organic compound profiling from postmortem microbes using gas chromatography-mass spectrometry. J. Forensic. Sci. 2019. [Google Scholar] [CrossRef]
- Verheggen, F.; Perrault, K.A.; Megido, R.C.; Dubois, L.M.; Francis, F.; Haubruge, E.; Forbes, S.L.; Focant, J.-F.; Stefanuto, P.-H. The odor of death: An overview of current knowledge on characterization and applications. BioScience 2017, 67, 600–613. [Google Scholar] [CrossRef]
- Pascual, J.; von Hoermann, C.; Rottler-Hoermann, A.-M.; Nevo, O.; Geppert, A.; Sikorski, J.; Huber, K.J.; Steiger, S.; Ayasse, M.; Overmann, J. Function of bacterial community dynamics in the formation of cadaveric semiochemicals during in situ carcass decomposition. Environ. Microbiol. 2017, 19, 3310–3322. [Google Scholar] [CrossRef] [PubMed]
- Dekeirsschieter, J.; Frederickx, C.; Lognay, G.; Brostaux, Y.; Verheggen, F.J.; Haubruge, E. Electrophysiological and behavioral responses of Thanatophilus sinuatus Fabricius (Coleoptera: Silphidae) to selected cadaveric volatile organic compounds. J. Forensic. Sci. 2013, 58, 917–923. [Google Scholar] [CrossRef]
- Davis, T.S.; Crippen, T.L.; Hofstetter, R.W.; Tomberlin, J.K. Microbial volatile emissions as insect semiochemicals. J. Chem. Ecol. 2013, 39, 840–859. [Google Scholar] [CrossRef]
- Von Hoermann, C.; Steiger, S.; Müller, J.K.; Ayasse, M. Too fresh is unattractive! The attraction of newly emerged Nicrophorus vespilloides females to odour bouquets of large cadavers at various stages of decomposition. PLoS ONE 2013, 8, e58524. [Google Scholar] [CrossRef]
- von Hoermann, C.; Ruther, J.; Ayasse, M. Volatile organic compounds of decaying piglet cadavers perceived by Nicrophorus vespilloides. J. Chem. Ecol. 2016, 42, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Dekeirsschieter, J.; Verheggen, F.J.; Gohy, M.; Hubrecht, F.; Bourguignon, L.; Lognay, G.; Haubruge, E. Cadaveric volatile organic compounds released by decaying pig carcasses (Sus domesticus L.) in different biotopes. Forensic. Sci. Int. 2009, 189, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Amendt, J.; Krettek, R.; Zehner, R. Forensic entomology. Naturwissenschaften 2004, 91, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, S.; Bajerlein, D.; Konwerski, S.; Szpila, K. Insect succession and carrion decomposition in selected forests of Central Europe. Part 2: Composition and residency patterns of carrion fauna. Forensic. Sci. Int. 2010, 195, 42–51. [Google Scholar] [CrossRef]
- Matuszewski, S.; Bajerlein, D.; Konwerski, S.; Szpila, K. Insect succession and carrion decomposition in selected forests of Central Europe. Part 3: Succession of carrion fauna. Forensic. Sci. Int. 2011, 207, 150–163. [Google Scholar] [CrossRef]
- Iancu, L.; Dean, D.E.; Purcarea, C. Temperature influence on prevailing necrophagous diptera and bacterial taxa with forensic implications for postmortem interval estimation: A review. J. Med. Entomol. 2018, 55, 1369–1379. [Google Scholar] [CrossRef]
- Feddern, N.; Mitchell, E.A.D.; Amendt, J.; Szelecz, I.; Seppey, C.V.W. Decomposition and insect colonization patterns of pig cadavers lying on forest soil and suspended above ground. Forensic Sci. Med. Pathol. 2019, 15, 1–10. [Google Scholar] [CrossRef]
- Martin, C.; Minchilli, D.; Francis, F.; Verheggen, F. Behavioral and electrophysiological responses of the fringed larder beetle Dermestes frischii to the smell of a cadaver at different decomposition stages. Insects 2020, 11, 238. [Google Scholar] [CrossRef]
- von Hoermann, C.; Ruther, J.; Ayasse, M. The attraction of virgin female hides beetles (Dermestes maculatus) to cadavers by a combination of decomposition odour and male sex pheromones. Front. Zool. 2012, 9, 18. [Google Scholar] [CrossRef]
- Schmitt, T.; Krell, F.-T.; Linsenmair, K.E. Quinone mixture as attractant for necrophagous dung beetles specialized on dead millipedes. J. Chem. Ecol. 2004, 30, 731–740. [Google Scholar] [CrossRef]
- Halffter, G.; Halffter, V. Why and where coprophagous beetles (Coleoptera: Scarabaeinae) eat seeds, fruits or vegetable detritus. Boletín Soc. Etimologica Aragonesa 2009, 45, 1–22. [Google Scholar]
- Larsen, T.H.; Lopera, A.; Forsyth, A.; Génier, F. From coprophagy to predation: A dung beetle that kills millipedes. Biol. Lett. 2009, 5, 152–155. [Google Scholar] [CrossRef]
- Simmons, L.W.; Ridsdill-Smith, J. Ecology and Evolution of Dung Beetles; John Wiley and Sons: Chichester, UK, 2011. [Google Scholar]
- Karimbumkara, S.N.; Priyadarsanan, D.R. Report of dung beetles (Scarabaeidae: Scarabaeinae) attracted to unconventional resources, with the description of three new species. Entomon 2016, 41, 265–282. [Google Scholar]
- Scholtz, C.H.; Davis, A.L.V.; Kryger, U. Evolutionary Biology and Conservation of Dung Beetles; Pensoft: Sofia, Bulgaria, 2009. [Google Scholar]
- Hanski, I.; Cambefort, Y. Dung Beetle Ecology; Princeton University Press: Princeton, NJ, USA, 1991. [Google Scholar]
- Braack, L. Arthropods associated with carcasses in the Northern Kruger National Park. South Afr. J. Wildl. Res. 1986, 16, 91–98. [Google Scholar]
- Kočárek, P. Decomposition and Coleoptera succession on exposed carrion of small mammal in Opava, the Czech Republic. Eur. J. Soil Biol. 2003, 39, 31–45. [Google Scholar] [CrossRef]
- Byk, A. Abundance and composition of Geotrupidae (Coleoptera: Scarabaeoidea) in the developmental cycle of pine stands in Człuchów Forest (NW Poland). Balt. J. Coleopt. 2011, 11, 171–186. [Google Scholar]
- Byk, A. The structure and seasonal dynamics of coprophagous Scarabaeoidea (Coleoptera) communities in later developmental stages of pine stands in NW Poland. J. Entomol. Res. Soc. 2015, 17, 39–57. [Google Scholar]
- Marczak, D. Habitat selection by two species of dung beetle, Anoplotrupes stercorosus (Scriba) and Trypocopris vernalis (L.) (Coleoptera: Geotrupidae), changes with stand age in a fresh pine forest. For. Res. Pap. 2013, 74, 227–232. [Google Scholar] [CrossRef]
- Frank, K.; Hülsmann, M.; Assmann, T.; Schmitt, T.; Blüthgen, N. Land use affects dung beetle communities and their ecosystem service in forests and grasslands. Agric. Ecosyst. Environ. 2017, 243, 114–122. [Google Scholar] [CrossRef]
- Freude, H.; Harde, K.W.; Lohse, G.A. Die Käfer Mitteleuropas: Teredilia, Heteromera, Lamellicornia; Goecke and Evers: Krefeld, Germany, 1964. [Google Scholar]
- von Hoermann, C.; Weithmann, S.; Deißler, M.; Ayasse, M.; Steiger, S. Forest habitat parameters influence abundance and diversity of cadaver-visiting dung beetles in Central Europe. R. Soc. Open Sci. 2020, 7, 191722. [Google Scholar] [CrossRef]
- Dormont, L.; Rapior, S.; McKey, D.B.; Lumaret, J.-P. Influence of dung volatiles on the process of resource selection by coprophagous beetles. Chemoecology 2007, 17, 23–30. [Google Scholar] [CrossRef]
- Dormont, L.; Epinat, G.; Lumaret, J.-P. Trophic preferences mediated by olfactory cues in dung beetles colonizing cattle and horse dung. Environ. Entomol. 2004, 33, 370–377. [Google Scholar] [CrossRef]
- Warnke, G. Experimentelle Untersuchungen über den Geruchssinn von Geotrupes silvaticus Panz. und Geotrupes vernalis Lin. J. Comp. Physiol. 1931, 14, 121–199. [Google Scholar] [CrossRef]
- Jarmusz, M.; Bajerlein, D. Anoplotrupes stercorosus (Scr.) and Trypocopris vernalis (L.) (Coleoptera: Geotrupidae) visiting exposed pig carrion in forests of Central Europe: Seasonality, habitat preferences and influence of smell of decay on their abundances. Entomol. Gen. 2015, 35, 213–228. [Google Scholar] [CrossRef]
- Fischer, M.; Bossdorf, O.; Gockel, S.; Hänsel, F.; Hemp, A.; Hessenmöller, D.; Korte, G.; Nieschulze, J.; Pfeiffer, S.; Prati, D.; et al. Implementing large-scale and long-term functional biodiversity research: The biodiversity exploratories. Basic Appl. Ecol. 2010, 11, 473–485. [Google Scholar] [CrossRef]
- Payne, J.A. A summer carrion study of the baby pig Sus scrofa Linnaeus. Ecology 1965, 46, 592–602. [Google Scholar] [CrossRef]
- Dötterl, S.; Wolfe, L.M.; Jürgens, A. Qualitative and quantitative analyses of flower scent in Silene latifolia. Phytochemistry 2005, 66, 203–213. [Google Scholar] [CrossRef]
- Charabidze, D.; Colard, T.; Vincent, B.; Pasquerault, T.; Hedouin, V. Involvement of larder beetles (Coleoptera: Dermestidae) on human cadavers: A review of 81 forensic cases. Int. J. Legal Med. 2014, 128, 1021–1030. [Google Scholar] [CrossRef]
- Von Hoermann, C.; Ruther, J.; Reibe, S.; Madea, B.; Ayasse, M. The importance of carcass volatiles as attractants for the hide beetle Dermestes maculatus (De Geer). Forensic Sci. Int. 2011, 212, 173–179. [Google Scholar] [CrossRef]
- Nizio, K.D.; Ueland, M.; Stuart, B.H.; Forbes, S.L. The analysis of textiles associated with decomposing remains as a natural training aid for cadaver-detection dogs. Forensic Chem. 2017, 5, 33–45. [Google Scholar] [CrossRef]
- Perrault, K.; Stuart, B.; Forbes, S. A longitudinal study of decomposition odour in soil using sorbent tubes and solid phase microextraction. Chromatography 2014, 1, 120–140. [Google Scholar] [CrossRef]
- Rendine, M.; Fiore, C.; Bertozzi, G.; de Carlo, D.; Filetti, V.; Fortarezza, P.; Riezzo, I. Decomposing human blood: Canine detection odor signature and volatile organic compounds. J. Forensic Sci. 2019, 64, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Dekeirsschieter, J.; Stefanuto, P.-H.; Brasseur, C.; Haubruge, E.; Focant, J.-F. Enhanced characterization of the smell of death by comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry (GCxGC-TOFMS). PLoS ONE 2012, 7, e39005. [Google Scholar] [CrossRef] [PubMed]
- Ayasse, M.; Schiestl, F.P.; Paulus, H.F.; Ibarra, F.; Francke, W. Pollinator attraction in a sexually deceptive orchid by means of unconventional chemicals. Proc. Biol. Sci. 2003, 270, 517–522. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Pohlert, T. The Pairwise Multiple Comparison of Mean Ranks Package (PMCMR); R Package; 2016; Available online: http://CRAN.R-project.org/package=PMCMR (accessed on 24 July 2020).
- Kalinová, B.; Podskalská, H.; Růzicka, J.; Hoskovec, M. Irresistible bouquet of death—How are burying beetles (Coleoptera: Silphidae: Nicrophorus) attracted by carcasses. Naturwissenschaften 2009, 96, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B.S.; Babcock, T.; Gries, R.; Benn, A.; Gries, G. Acquired smell? Mature females of the common green bottle fly shift semiochemical preferences from feces feeding sites to carrion oviposition sites. J. Chem. Ecol. 2016, 42, 40–50. [Google Scholar] [CrossRef]
- Zito, P.; Sajeva, M.; Raspi, A.; Dötterl, S. Dimethyl disulfide and dimethyl trisulfide: So similar yet so different in evoking biological responses in saprophilous flies. Chemoecology 2014, 24, 261–267. [Google Scholar] [CrossRef]
- Inouchi, J.; Shibuya, T.; Hatanaka, T. Food odor responses of single antennal olfactory cells in the Japanese dung beetle, Geotrupes auratus (Coleoptera: Geotrupidae). Appl. Entomol. Zool. 1988, 23, 167–174. [Google Scholar] [CrossRef]
- Jürgens, A.; Wee, S.-L.; Shuttleworth, A.; Johnson, S.D. Chemical mimicry of insect oviposition sites: A global analysis of convergence in angiosperms. Ecol. Lett. 2013, 16, 1157–1167. [Google Scholar] [CrossRef]
- Venkateshwarlu, G.; Chandravadana, M.V.; Tewari, P.P. Volatile flavour components of some edible mushrooms (Basidiomycetes). Flavour Frag. J. 1999, 14, 191–194. [Google Scholar] [CrossRef]
- Stavert, J.; Drayton, B.; Beggs, J.; Gaskett, A. The volatile organic compounds of introduced and native dung and carrion and their role in dung beetle foraging behaviour. Ecol. Entomol. 2014, 39, 556–565. [Google Scholar] [CrossRef]
- Johansen, H.; Solum, M.; Knudsen, G.K.; Hagvar, E.B.; Norli, H.R.; Aak, A. Blow fly responses to semiochemicals produced by decaying carcasses. Med. Vet. Entomol. 2014, 28, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Marneweck, C.; Jürgens, A.; Shrader, A.M. Temporal variation of white rhino dung odours. J. Chem. Ecol. 2017, 43, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Dormont, L.; Jay-Robert, P.; Bessière, J.-M.; Rapior, S.; Lumaret, J.-P. Innate olfactory preferences in dung beetles. J. Exp. Biol. 2010, 213, 3177–3186. [Google Scholar] [CrossRef]
- Wurmitzer, C.; Blüthgen, N.; Krell, F.-T.; Maldonado, B.; Ocampo, F.; Müller, J.K.; Schmitt, T. Attraction of dung beetles to herbivore dung and synthetic compounds in a comparative field study. Chemoecology 2017, 27, 75–84. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Stahl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Kesselmeier, J.; Straudt, M. Biogenic volatile organic compounds (VOC): An overview on emission, physiology and ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Frank, K.; Brückner, A.; Blüthgen, N.; Schmitt, T. In search of cues: Dung beetle attraction and the significance of volatile composition of dung. Chemoecology 2018, 28, 145–152. [Google Scholar] [CrossRef]
- Holighaus, G.; Rohlfs, M. Volatile and non-volatile fungal oxylipins in fungus-invertebrate interactions. Fungal Ecol. 2019, 38, 28–36. [Google Scholar] [CrossRef]
- Fu, X.; Guo, J.; Finkelbergs, D.; He, J.; Zha, L.; Guo, Y.; Cai, J. Fungal succession during mammalian cadaver decomposition and potential forensic implications. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Veselova, M.A.; Plyuta, V.A.; Khmel, I.A. Volatile compounds of bacterial origin: Structure, biosynthesis, and biological activity. Microbiology 2019, 88, 261–274. [Google Scholar] [CrossRef]
- Bedoussac, L.; Favila, M.E.; López, R.M. Defensive volatile secretions of two diplopod species attract the carrion ball roller scarab Canthon morsei (Coleoptera: Scarabaeidae). Chemoecology 2007, 17, 163–167. [Google Scholar] [CrossRef]
- Cruise, A.; Watson, D.W.; Schal, C. Ecological succession of adult necrophilous insects on neonate Sus scrofa domesticus in central North Carolina. PLoS ONE 2018, 13, e0195785. [Google Scholar] [CrossRef] [PubMed]
- Paczkowski, S.; Maibaum, F.; Paczkowska, M.; Schütz, S. Decaying mouse volatiles perceived by Calliphora vicina Rob.-Desv. J. Forensic Sci. 2012, 57, 1497–1506. [Google Scholar] [CrossRef]
- Paczkowski, S.; Nicke, S.; Ziegenhagen, H.; Schütz, S. Volatile emission of decomposing pig carcasses (Sus scrofa domesticus L.) as an indicator for the postmortem interval. J. Forensic. Sci. 2015, 60, S130–S137. [Google Scholar] [CrossRef]
- El-Sayed, A.M. The Pherobase: Database of Pheromones and Semiochemicals. Available online: http://www.pherobase.com (accessed on 24 July 2020).

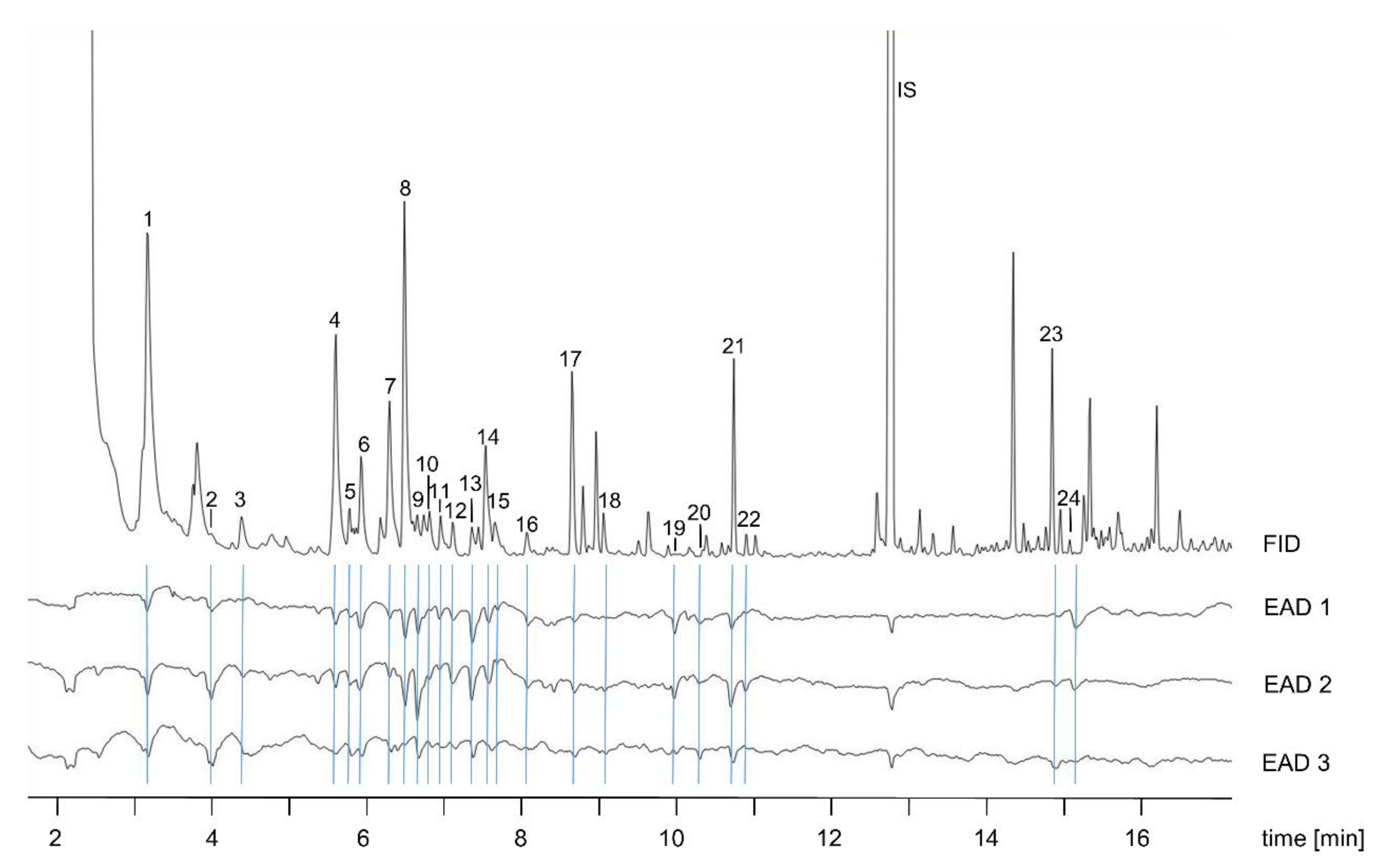
| No. | Compound Name | RI | Relative Amount (%) in Post-Bloating Decay (6 days p.m.) |
|---|---|---|---|
| 1 | unknown (artifact) | - | 22.77 |
| 2 | unknown | - | 6.4 |
| 3 | unknown | - | 1.27 |
| 4 | methyl propyl disulfide | 927 | 11.31 |
| 5 | α-pinene | 931 | 0.83 |
| 6 | camphene | 948 | 5.23 |
| 7 | benzaldehyde * | 960 | 7.08 |
| 8 | dimethyl trisulfide * | 971 | 14.34 |
| 9 | 3-octanone * | 984 | 1.88 |
| 10 | 6-methyl-5-hepten-2-ol * | 991 | 1.89 |
| 11 | decane | 1000 | 1.92 |
| 12 | 3-carene | 1008 | 1.4 |
| 13 | 1-methoxy-4-methylbenzene | 1019 | 1.01 |
| 14 | limonene | 1027 | 1.4 |
| 15 | benzyl alcohol | 1032 | 5.29 |
| 16 | butylbenzene | 1055 | 0.93 |
| 17 | methyl pentyl disulfide | 1084 | 3.04 |
| 18 | nonanal * | 1104 | 1.23 |
| 19 | camphor | 1151 | 0.23 |
| 20 | ethyl pentyl disulfide | 1167 | 0.44 |
| 21 | dodecene 1 | 1192 | 4.52 |
| 22 | dodecane * | 1200 | 0.64 |
| tridecane 2 | 1300 | ||
| 23 | unknown | 1423 | 4.62 |
| 24 | unknown | 1437 | 0.34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weithmann, S.; von Hoermann, C.; Schmitt, T.; Steiger, S.; Ayasse, M. The Attraction of the Dung Beetle Anoplotrupes stercorosus (Coleoptera: Geotrupidae) to Volatiles from Vertebrate Cadavers. Insects 2020, 11, 476. https://doi.org/10.3390/insects11080476
Weithmann S, von Hoermann C, Schmitt T, Steiger S, Ayasse M. The Attraction of the Dung Beetle Anoplotrupes stercorosus (Coleoptera: Geotrupidae) to Volatiles from Vertebrate Cadavers. Insects. 2020; 11(8):476. https://doi.org/10.3390/insects11080476
Chicago/Turabian StyleWeithmann, Sandra, Christian von Hoermann, Thomas Schmitt, Sandra Steiger, and Manfred Ayasse. 2020. "The Attraction of the Dung Beetle Anoplotrupes stercorosus (Coleoptera: Geotrupidae) to Volatiles from Vertebrate Cadavers" Insects 11, no. 8: 476. https://doi.org/10.3390/insects11080476
APA StyleWeithmann, S., von Hoermann, C., Schmitt, T., Steiger, S., & Ayasse, M. (2020). The Attraction of the Dung Beetle Anoplotrupes stercorosus (Coleoptera: Geotrupidae) to Volatiles from Vertebrate Cadavers. Insects, 11(8), 476. https://doi.org/10.3390/insects11080476







