Simultaneous Increase in CO2 and Temperature Alters Wheat Growth and Aphid Performance Differently Depending on Virus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material: Plants and Insects
2.2. Virus Isolate and Inoculation
2.3. Acclimation of Insects and Plants to Elevated CO2 and Temperature
2.4. Plant Traits
2.5. Rhopalosiphum Padi Fitness Experiment
2.6. Statistical Analysis
3. Results
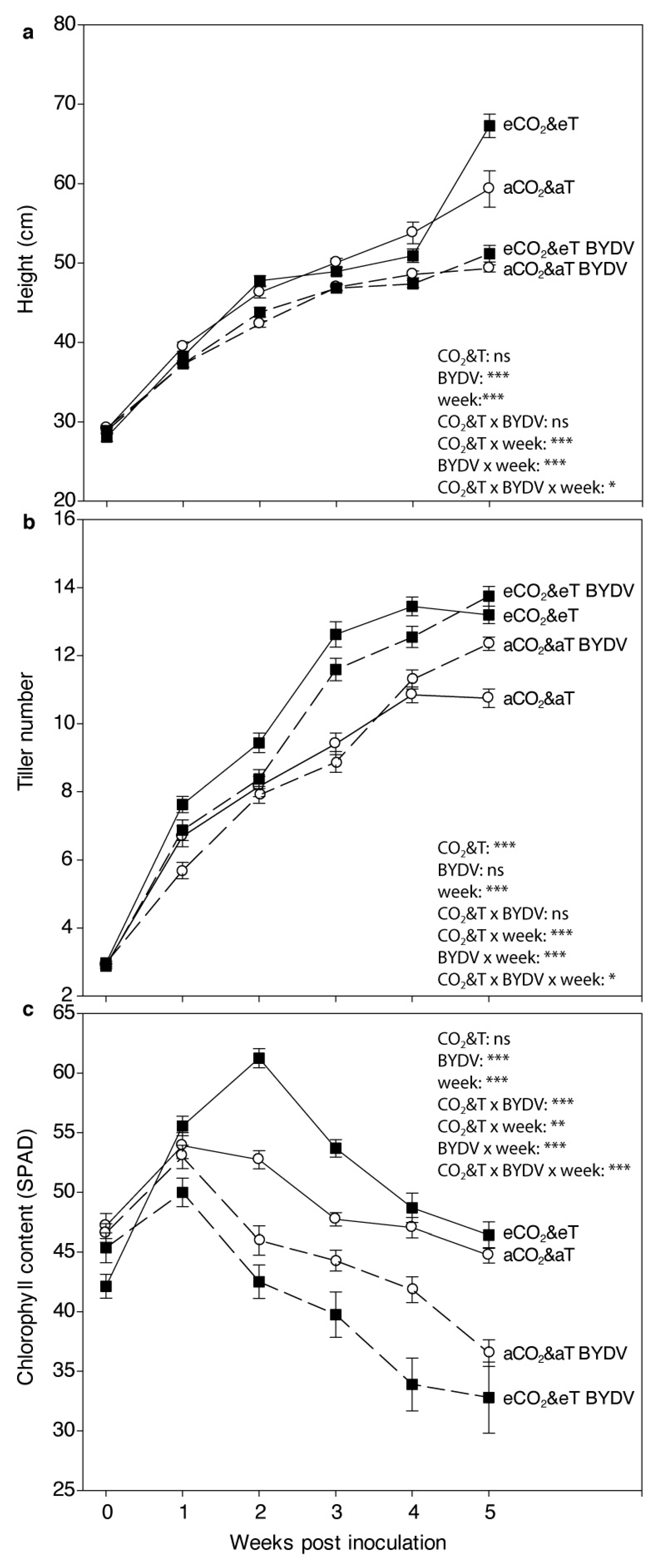
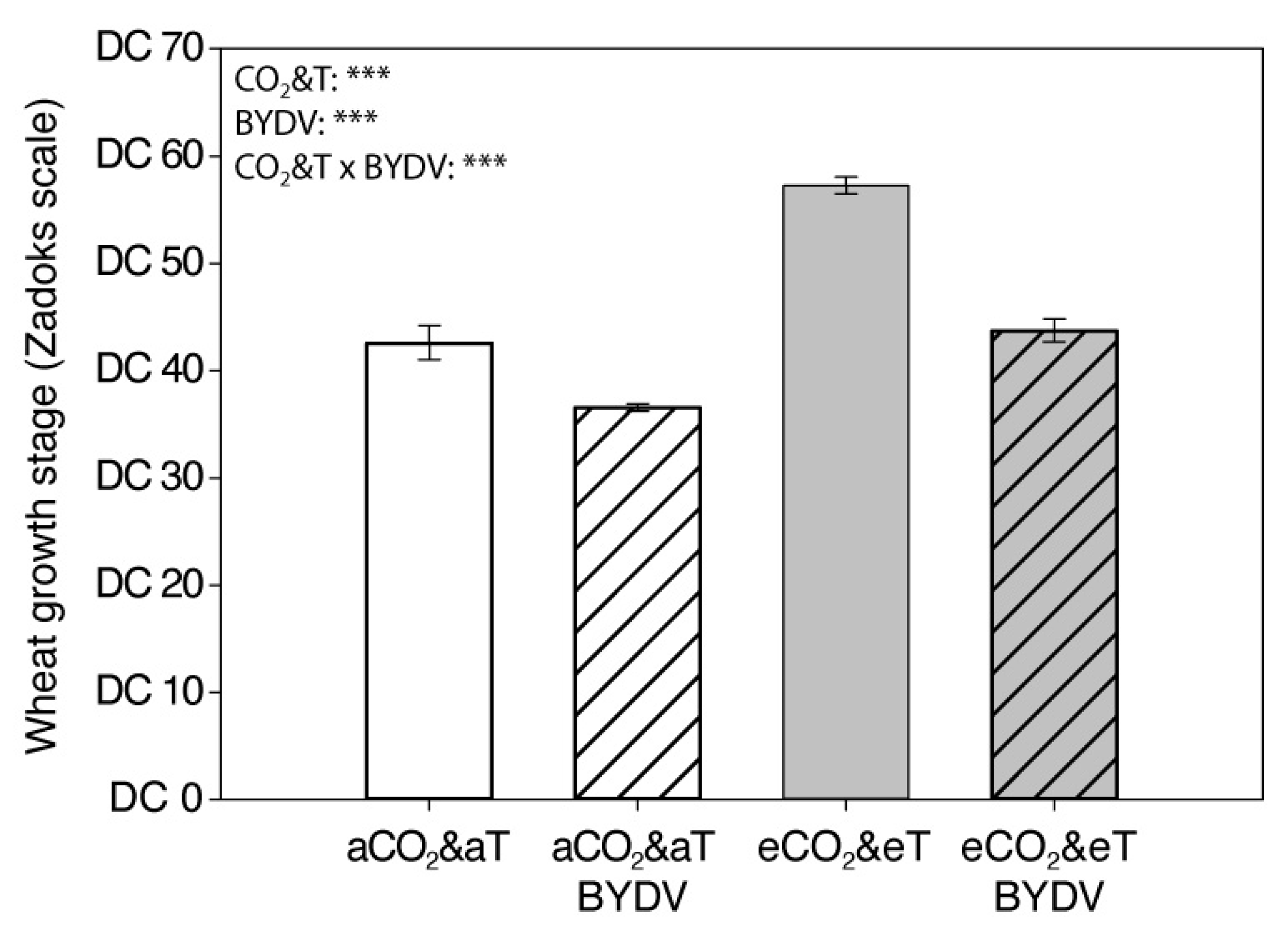
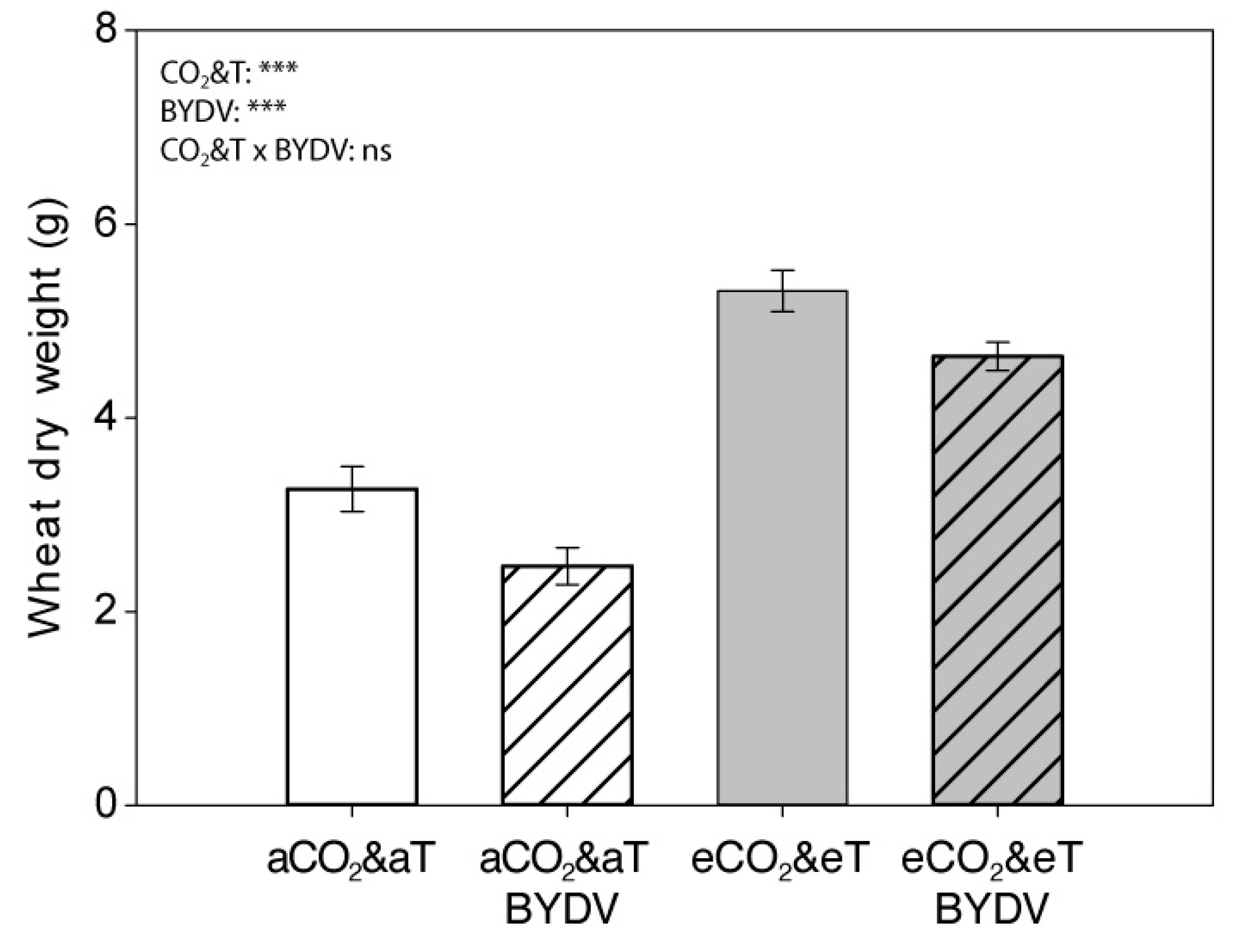
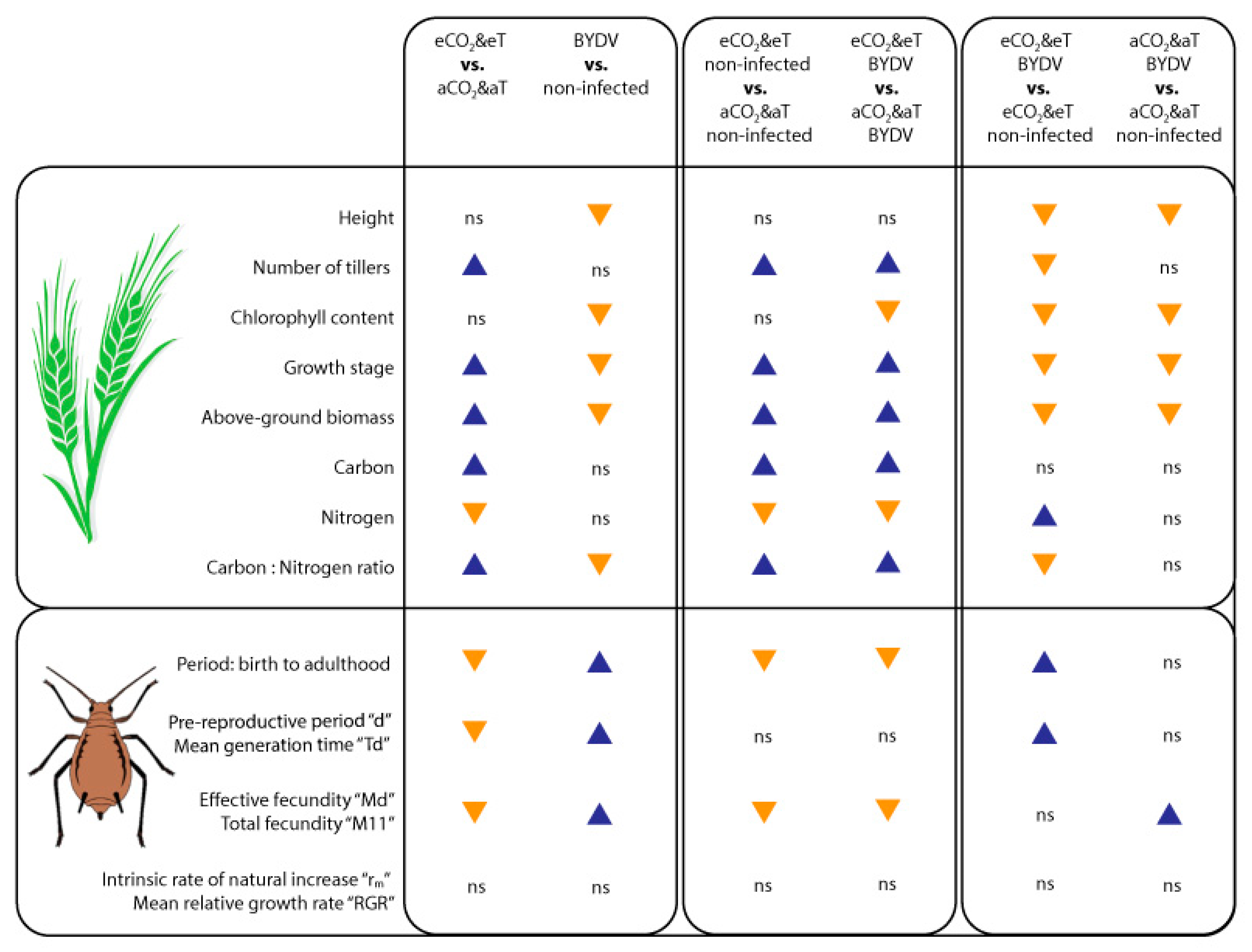
3.1. Plant Growth Parameters
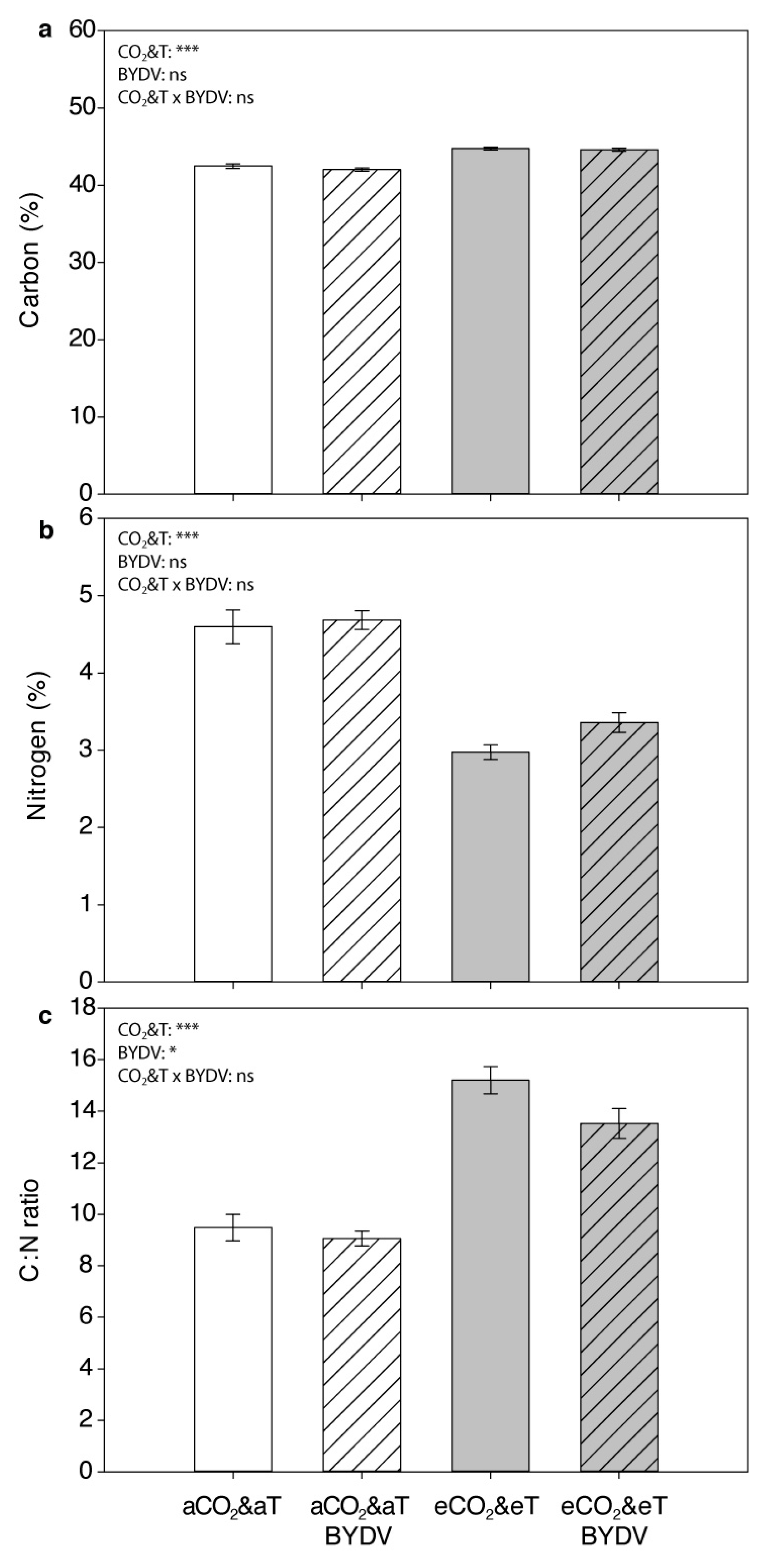
3.2. Plant Carbon and Nitrogen Content
3.3. Rhopalosiphum Padi Fitness Experiment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. (Eds.) IPCC Summary for Policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; ISBN 9781107415324. [Google Scholar]
- Facey, S.L.; Ellsworth, D.S.; Staley, J.T.; Wright, D.J.; Johnson, S.N. Upsetting the order: How climate and atmospheric change affects herbivore-enemy interactions. Curr. Opin. Insect Sci. 2014, 5, 66–74. [Google Scholar] [CrossRef]
- Trȩbicki, P.; Dáder, B.; Vassiliadis, S.; Fereres, A. Insect-plant-pathogen interactions as shaped by future climate: Effects on biology, distribution and implications for agriculture. Insect Sci. 2017, 24, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.S.; Smith, M.R.; Guth, S.; Golden, C.D.; Vaitla, B.; Mueller, N.D.; Dangour, A.D.; Huybers, P. Climate Change and Global Food Systems: Potential Impacts on Food Security and Undernutrition. Annu. Rev. Public Health 2017, 38, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, J.R.; Barzman, M.; Booij, K.; Boonekamp, P.; Desneux, N.; Huber, L.; Kudsk, P.; Langrell, S.R.H.; Ratnadass, A.; Ricci, P.; et al. Robust cropping systems to tackle pests under climate change. A review. Agron. Sustain. Dev. 2015, 35, 443–459. [Google Scholar] [CrossRef]
- Flood, J. The importance of plant health to food security. Food Secur. 2010, 2, 215–231. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture. Climate Change, Agriculture and Food Security; FAO: Rome, Italy, 2016; ISBN 9789251093740. [Google Scholar]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Ben-Yakir, D.; Fereres, A. The effects of UV radiation on arthropods: A review of recent publications (2010-2015). Acta Hortic. 2016, 1134, 335–342. [Google Scholar] [CrossRef]
- Trȩbicki, P.; Nancarrow, N.; Bosque-Pérez, N.A.; Rodoni, B.; Aftab, M.; Freeman, A.; Yen, A.; Fitzgerald, G.J. Virus incidence in wheat increases under elevated CO2: A 4-year study of yellow dwarf viruses from a free air carbon dioxide facility. Virus Res. 2017, 241, 137–144. [Google Scholar] [CrossRef]
- Vassiliadis, S.; Plummer, K.M.; Powell, K.S.; Trȩbicki, P.; Luck, J.E.; Rochfort, S.J. The effect of elevated CO2 and virus infection on the primary metabolism of wheat. Funct. Plant Biol. 2016, 43, 892–902. [Google Scholar] [CrossRef]
- Dáder, B.; Fereres, A.; Moreno, A.; Trȩbicki, P. Elevated CO2 impacts bell pepper growth with consequences to Myzus persicae life history, feeding behaviour and virus transmission ability. Sci. Rep. 2016, 6, 19120. [Google Scholar] [CrossRef] [PubMed]
- Trȩbicki, P.; Vandegeer, R.K.; Bosque-Pérez, N.A.; Powell, K.S.; Dader, B.; Freeman, A.J.; Yen, A.L.; Fitzgerald, G.J.; Jo, E.; Calle, A.S.; et al. Virus infection mediates the effects of elevated CO2 on plants and vectors. Sci. Rep. 2016, 6, 22785. [Google Scholar] [CrossRef] [PubMed]
- Malmström, C.M.; Field, C.B. Virus-induced differences in the response of oat plants to elevated carbon dioxide. Plant Cell Environ. 1997, 20, 178–188. [Google Scholar] [CrossRef]
- Chung, B.N.; Koh, S.W.; Choi, K.S.; Joa, J.H.; Kim, C.H.; Selvakumar, G. Temperature and CO2 level influence Potato leafroll virus infection in Solanum tuberosum. Plant Pathol. J. 2017, 33, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Trebicki, P. Climate change and plant virus epidemiology. Virus Res. 2020, 286, 198059. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free air CO2 enrichment (FACE)? A meta-analytic review of the response of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Ode, P.J.; Johnson, S.N.; Moore, B.D. Atmospheric change and induced plant secondary metabolites—Are we reshaping the building blocks of multi-trophic interactions? Curr. Opin. Insect Sci. 2014, 5, 57–65. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Fitzgerald, G.J.; Tausz, M.; O’Leary, G.J.; Mollah, M.R.; Tausz-Posch, S.; Seneweera, S.; Mock, I.; Löw, M.; Partington, D.L.; Mcneil, D.; et al. Elevated atmospheric [CO2] can dramatically increase wheat yields in semi-arid environments and buffer against heat waves. Glob. Chang. Biol. 2016, 22, 2269–2284. [Google Scholar] [CrossRef]
- Chen, F.; Ge, F.; Parajulee, M.N. Impact of Elevated CO2 on Tri-Trophic Interaction of Gossypium hirsutum, Aphis gossypii, and Leis axyridis. Environ. Entomol. 2005, 34, 37–46. [Google Scholar] [CrossRef]
- Sun, Y.C.; Feng, L.; Gao, F.; Ge, F. Effects of elevated CO2 and plant genotype on interactions among cotton, aphids and parasitoids. Insect Sci. 2011, 18, 451–461. [Google Scholar] [CrossRef]
- Mattson, W.J. Herbivory in Relation To Plant Nitrogen Content. Annu. Rev. Ecol. Syst. 1980, 11, 119–161. [Google Scholar] [CrossRef]
- Stiling, P.; Cornelissen, T. How does elevated carbon dioxide (CO2) affect plant-herbivore interactions? A field experiment and meta-analysis of CO2- mediated changes on plant chemistry and herbivore performance. Glob. Chang. Biol. 2007, 13, 1823–1842. [Google Scholar] [CrossRef]
- Coviella, C.E.; Trumble, J.T. Effects of elevated atmospheric carbon dioxide on insect-plant interactions. Conserv. Biol. 1999, 13, 700–712. [Google Scholar] [CrossRef]
- Rosenblatt, A.E.; Schmitz, O.J. Climate Change, Nutrition, and Bottom-Up and Top-Down Food Web Processes. Trends Ecol. Evol. 2016, 31, 965–975. [Google Scholar] [CrossRef]
- Hughes, L.; Bazzaz, F.A. Effects of elevated CO2 on five plant-aphid interactions. Entomol. Exp. Appl. 2001, 99, 87–96. [Google Scholar] [CrossRef]
- Klaiber, J.; Najar-Rodriguez, A.J.; Piskorski, R.; Dorn, S. Plant acclimation to elevated CO2 affects important plant functional traits, and concomitantly reduces plant colonization rates by an herbivorous insect. Planta 2013, 237, 29–42. [Google Scholar] [CrossRef][Green Version]
- Klaiber, J.; Dorn, S.; Najar-Rodriguez, A.J. Acclimation to Elevated CO2 Increases Constitutive Glucosinolate Levels of Brassica Plants and Affects the Performance of Specialized Herbivores from Contrasting Feeding Guilds. J. Chem. Ecol. 2013, 39, 653–665. [Google Scholar] [CrossRef][Green Version]
- Hoover, J.K.; Newman, J.A. Tritrophic interactions in the context of climate change: A model of grasses, cereal Aphids and their parasitoids. Glob. Chang. Biol. 2004, 10, 1197–1208. [Google Scholar] [CrossRef]
- Ryan, G.D.; Shukla, K.; Rasmussen, S.; Shelp, B.J.; Newman, J.A. Phloem phytochemistry and aphid responses to elevated CO2, nitrogen fertilization and endophyte infection. Agric. For. Entomol. 2014, 16, 273–283. [Google Scholar] [CrossRef]
- Ryan, G.D.; Sylvester, E.V.A.; Shelp, B.J.; Newman, J.A. Towards an understanding of how phloem amino acid composition shapes elevated CO2-induced changes in aphid population dynamics. Ecol. Entomol. 2015, 40, 247–257. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.-K.; Song, Y.-Y.; Han, T.; Li, Z.; Wan, G.-J.; Chen, F.-J. Elevated CO2 and temperature alter specific-species population dynamic and interspecific competition of three wheat aphids. J. Appl. Entomol. 2018, 1–10. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, L.; Wang, W.; Wang, Z.; Ni, X.; Cai, W.; He, K. Changes in Life History Parameters of Rhopalosiphum maidis (Homoptera: Aphididae) Under Four Different Elevated Temperature and CO2 Combinations. J. Econ. Entomol. 2014, 107, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.N.; Ryalls, J.M.W.; Karley, A.J. Global climate change and crop resistance to aphids: Contrasting responses of lucerne genotypes to elevated atmospheric carbon dioxide. Ann. Appl. Biol. 2014, 165, 62–72. [Google Scholar] [CrossRef]
- Newman, J.A.; Gibson, D.J.; Parsons, A.J.; Thornley, J.H.M. How predictable are aphid population responses to elevated CO2? J. Anim. Ecol. 2003, 72, 556–566. [Google Scholar] [CrossRef]
- Trȩbicki, P.; Nancarrow, N.; Cole, E.; Bosque-Pérez, N.A.; Constable, F.E.; Freeman, A.J.; Rodoni, B.; Yen, A.L.; Luck, J.E.; Fitzgerald, G.J. Virus disease in wheat predicted to increase with a changing climate. Glob. Chang. Biol. 2015, 21, 3511–3519. [Google Scholar] [CrossRef]
- Trębicki, P.; Finlay, K. Pests and Diseases under Climate Change; Its Threat to Food Security. In Food Security and Climate Change; Yadav, S.S., Redden, R.J., Hatfield, J.L., Ebert, A.W., Hunter, D., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 229–249. ISBN 9781119180661. [Google Scholar]
- Zvereva, E.L.; Kozlov, M.V. Consequences of simultaneous elevation of carbon dioxide and temperature for plant-herbivore interactions: A metaanalysis. Glob. Chang. Biol. 2006, 12, 27–41. [Google Scholar] [CrossRef]
- Ryalls, J.M.W.; Moore, B.D.; Riegler, M.; Bromfield, L.M.; Hall, A.A.G.; Johnson, S.N. Climate and atmospheric change impacts on sap-feeding herbivores: A mechanistic explanation based on functional groups of primary metabolites. Funct. Ecol. 2016, 31, 161–171. [Google Scholar] [CrossRef]
- Ma, G.; Rudolf, V.H.W.; Ma, C.-S. Extreme temperature events alter demographic rates, relative fitness, and community structure. Glob. Chang. Biol. 2015, 21, 1794–1808. [Google Scholar] [CrossRef]
- Nancarrow, N.; Constable, F.; Finlay, K.J.; Freeman, A.; Rodoni, B.; Trȩbicki, P.; Vassiliadis, S.; Yen, A.; Luck, J. The effect of elevated temperature on Barley yellow dwarf virus-PAV in wheat. Virus Res. 2014, 186, 97–103. [Google Scholar] [CrossRef]
- Ryalls, J.M.W.; Harrington, R. Climate and atmospheric change impacts on aphids as vectors of plant diseases. In Global Climate Change and Terrestrial Invertebrates; Johnson, S.N., Jones, T.H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 148–175. ISBN 9781119070894. [Google Scholar]
- Rosenblatt, A.E.; Smith-Ramesh, L.M.; Schmitz, O.J. Interactive effects of multiple climate change variables on food web dynamics: Modeling the effects of changing temperature, CO2, and water availability on a tri-trophic food web. Food Webs 2017, 13, 98–108. [Google Scholar] [CrossRef]
- Finlay, K.J.; Luck, J.E. Response of the bird cherry-oat aphid (Rhopalosiphum padi) to climate change in relation to its pest status, vectoring potential and function in a crop-vector-virus pathosystem. Agric. Ecosyst. Environ. 2011, 144, 405–421. [Google Scholar] [CrossRef]
- Fu, X.; Ye, L.; Kang, L.; Ge, F. Elevated CO2 shifts the focus of tobacco plant defences from cucumber mosaic virus to the green peach aphid. Plant Cell Environ. 2010, 33, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.N.; Canto, T.; Tenllado, F.; Choi, K.S.; Joa, J.H.; Ahn, J.J.; Kim, C.H.; Do, K.S. The effects of high temperature on infection by Potato virus Y, Potato virus A, and Potato leafroll virus. Plant Pathol. J. 2016, 32, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Ryalls, J.M.W.; Moore, B.D.; Riegler, M.; Gherlenda, A.; Johnson, S.N. Amino acid-mediated impacts of elevated carbon dioxide and simulated root herbivory on aphids are neutralized by increased air temperatures. J. Exp. Bot. 2015, 66, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Srinavasa Rao, M.; Shaila, O.; Abdul Khadar, B.; Mani Manjari, D.; Vennila, S.; Vanaja, M.; Srinavasa Rao, C. Impact of elevated CO2 and temperature on aphids—A review. Bioscan 2016, 11, 2055–2062. [Google Scholar]
- Lapierre, H.; Signoret, P.A. Viruses and Virus Diseases of Poaceae (Gramineae); Lapierre, H., Signoret, P.A., Eds.; INRA Editions: Paris, France, 2004; ISBN 2738010881. [Google Scholar]
- Miller, W.A.; Rasochová, L. Barley Yellow Dwarf Viruses. Annu. Rev. Phytopathol. 1997, 35, 167–190. [Google Scholar] [CrossRef]
- Nancarrow, N.; Aftab, M.; Freeman, A.; Rodoni, B.; Hollaway, G.; Trȩbicki, P. Prevalence and Incidence of Yellow Dwarf Viruses Across a Climatic Gradient: A Four-Year Field Study in Southeastern Australia. Plant Dis. 2018, 102, 2465–2472. [Google Scholar] [CrossRef]
- Chrpová, J.; Veškrna, O.; Palicová, J.; Kundu, J.K. The evaluation of wheat cultivar resistance and yield loss thresholds in response to barley yellow dwarf virus-PAV infection. Agriculture 2020, 10, 20. [Google Scholar] [CrossRef]
- Jarošová, J.; Chrpová, J.; Šíp, V.; Kundu, J.K. A comparative study of the Barley yellow dwarf virus species PAV and PAS: Distribution, accumulation and host resistance. Plant Pathol. 2013, 62, 436–443. [Google Scholar] [CrossRef]
- Gray, S.; Cilia, M.; Ghanim, M. Circulative, “Nonpropagative” virus transmission: An orchestra of virus-, insect-, and plant-derived instruments. In Advances in Virus Research; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 89, pp. 141–199. ISBN 9780128001721. [Google Scholar]
- Dietzgen, R.G.; Mann, K.S.; Johnson, K.N. Plant virus-insect vector interactions: Current and potential future research directions. Viruses 2016, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.E.; Thresh, J.M. Epidemiology of Barley Yellow Dwarf: A Study in Ecological Complexity. Annu. Rev. Phytopathol. 1990, 28, 393–424. [Google Scholar] [CrossRef]
- Blackman, R.L.; Eastop, V.F. Taxonomic Issues. In Aphids as Crop Pests; Van Emden, H.F., Harrington, R., Eds.; CAB International: Oxfordshire, UK, 2017; pp. 1–36. [Google Scholar]
- Klaiber, J.; Najar-Rodriguez, A.J.; Dialer, E.; Dorn, S. Elevated carbon dioxide impairs the performance of a specialized parasitoid of an aphid host feeding on Brassica plants. Biol. Control 2013, 66, 49–55. [Google Scholar] [CrossRef]
- Debaeke, P.; Rouet, P.; Justes, E. Relationship between the normalized SPAD index and the nitrogen nutrition index: Application to durum wheat. J. Plant Nutr. 2006, 29, 75–92. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Wyatt, I.J.; White, P.F. Simple estimation of intrinsic increase rates for aphids and tetranychid mites. J. Appl. Ecol. 1977, 14, 757–766. [Google Scholar] [CrossRef]
- Dixon, A.F.G. Parthenogenetic reproduction and the rate of increase in aphids. In Aphids: Their Biology, Natural Enemies and Control; Minks, A.K., Harrewinjn, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 269–287. [Google Scholar]
- Wang, Z.; Goonewardene, L.A. The use of MIXED models in the analysis of animal experiments with repeated measures data. Can. J. Anim. Sci. 2004, 84, 1–11. [Google Scholar] [CrossRef]
- Bosquee, E.; Boullis, A.; Bertaux, M.; Francis, F.; Verheggen, F.J. Dispersion of Myzus persicae and transmission of Potato virus Y under elevated CO2 atmosphere. Entomol. Exp. Appl. 2018, 166, 380–385. [Google Scholar] [CrossRef]
- Vassiliadis, S.; Plummer, K.M.; Powell, K.S.; Rochfort, S.J. Elevated CO2 and virus infection impacts wheat and aphid metabolism. Metabolomics 2018, 14, 133. [Google Scholar] [CrossRef]
- O’Leary, G.J.; Christy, B.; Nuttall, J.; Huth, N.; Cammarano, D.; Stöckle, C.; Basso, B.; Shcherbak, I.; Fitzgerald, G.J.; Luo, Q.; et al. Response of wheat growth, grain yield and water use to elevated CO2 under a Free-Air CO2 Enrichment (FACE) experiment and modelling in a semi-arid environment. Glob. Chang. Biol. 2015, 21, 2670–2686. [Google Scholar] [CrossRef]
- Kimball, B.A.; Idso, S.B. Increasing atmospheric CO2: Effects on crop yield, water use and climate. Agric. Water Manag. 1983, 7, 55–72. [Google Scholar] [CrossRef]
- Batts, G.R.; Morison, J.K.L.; Ellis, R.H.; Hadley, P.; Wheeler, T.R. Effects of CO2 and temperature on growth and yield of crops of winter wheat over four seasons. Eur. J. Agron. 1997, 7, 43–52. [Google Scholar] [CrossRef]
- Mitchell, R.A.; Mitchell, V.; Driscoll, S.; Franklin, J.; Lawlor, D. Effects of increased CO2 concentration and temperature on growth and yield of winter wheat at two levels of nitrogen application. Plant Cell Environ. 1993, 16, 521–529. [Google Scholar] [CrossRef]
- Poorter, H.; Pérez-Soba, M. The growth response of plants to elevated CO2 under non-optimal environmental conditions. Oecologia 2001, 129, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, D.R.; Nasreen, A.; Moffat, C.E.; Clarke, P.; Roitberg, B.D. Effects of simulated heat waves on an experimental community of pepper plants, green peach aphids and two parasitoid species. Oikos 2012, 121, 149–159. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, L.; Ma, C. Sen Sporadic short temperature events cannot be neglected in predicting impacts of climate change on small insects. J. Insect Physiol. 2019, 112, 48–56. [Google Scholar] [CrossRef]
- Dixon, A.F.G.; Wellings, P.W.; Carter, C.; Nichols, J.F.A. The role of food quality and competition in shaping the seasonal cycle in the reproductive activity of the sycamore aphid. Oecologia 1993, 95, 89–92. [Google Scholar] [CrossRef]
- Xing, G.; Zhang, J.; Liu, J.; Zhang, X.; Wang, G.; Wang, Y. Impacts of Atmospheric CO2 Concentrations and Soil Water on the Population Dynamics, Fecundity and Development of the Bird Cherry-Oat Aphid Rhopalosiphum padi. Phytoparasitica 2003, 31, 499–514. [Google Scholar] [CrossRef]
- Moreno-Delafuente, A.; Garzo, E.; Moreno, A.; Fereres, A. A Plant Virus Manipulates the Behavior of Its Whitefly Vector to Enhance Its Transmission Efficiency and Spread. PLoS ONE 2013, 8, e61543. [Google Scholar] [CrossRef]
- Ingwell, L.L.; Eigenbrode, S.D.; Bosque-Pérez, N.A. Plant viruses alter insect behavior to enhance their spread. Sci. Rep. 2012, 2, 578. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Bosque-pérez, N.A.; Davis, T.S. Insect-Borne Plant Pathogens and Their Vectors: Ecology, Evolution, and Complex Interactions. Annu. Rev. Entomol. 2018, 63, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Mauck, K.E.; Chesnais, Q.; Shapiro, L.R. Evolutionary Determinants of Host and Vector Manipulation by Plant Viruses. In Advances in Virus Research; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 101, pp. 189–250. [Google Scholar]
- Fereres, A.; Lister, R.M.; Araya, J.E.; Foster, J.E. Development and reproduction of the English grain aphid (Homoptera: Aphididae) on wheat cultivars infected with Barley yellow dwarf virus. Environ. Entomol. 1989, 18, 388–393. [Google Scholar] [CrossRef]
- Bosque-Pérez, N.A.; Eigenbrode, S.D. The influence of virus-induced changes in plants on aphid vectors: Insights from luteovirus pathosystems. Virus Res. 2011, 159, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Martínez, E.S.; Bosque-Pérez, N.A.; Berger, P.H.; Zemetra, R.S.; Ding, H.; Eigenbrode, S.D. Volatile Cues Influence the Response of Rhopalosiphum padi (Homoptera: Aphididae) to Barley Yellow Dwarf Virus–Infected Transgenic and Untransformed Wheat. Environ. Entomol. 2004, 33, 1207–1216. [Google Scholar] [CrossRef]
- Ajayi, O.; Dewar, A.M. The effect of barley yellow dwarf virus on field populations of the cereal aphids, Sitobion avenae and Metopolophium dirhodum. Ann. Appl. Biol. 1983, 103, 1–11. [Google Scholar] [CrossRef]
- Guo, H.; Huang, L.; Sun, Y.C.; Guo, H.; Ge, F. The contrasting effects of elevated CO2 on TYLCV infection of tomato genotypes with and without the resistance gene, Mi-1.2. Front. Plant Sci. 2016, 7, 1680. [Google Scholar] [CrossRef]
- Smyrnioudis, I.N.; Harrington, R.; Hall, M.; Katis, N.; Clark, S.J. The effect of temperature on variation in transmission of a BYDV PAV-like isolate by clones of Rhopalosiphum padi and Sitobion avenae. Eur. J. Plant Pathol. 2001, 107, 167–173. [Google Scholar] [CrossRef]
- Luck, J.; Spackman, M.; Freeman, A.; TreBicki, P.; Griffiths, W.; Finlay, K.; Chakraborty, S. Climate change and diseases of food crops. Plant Pathol. 2011, 60, 113–121. [Google Scholar] [CrossRef]

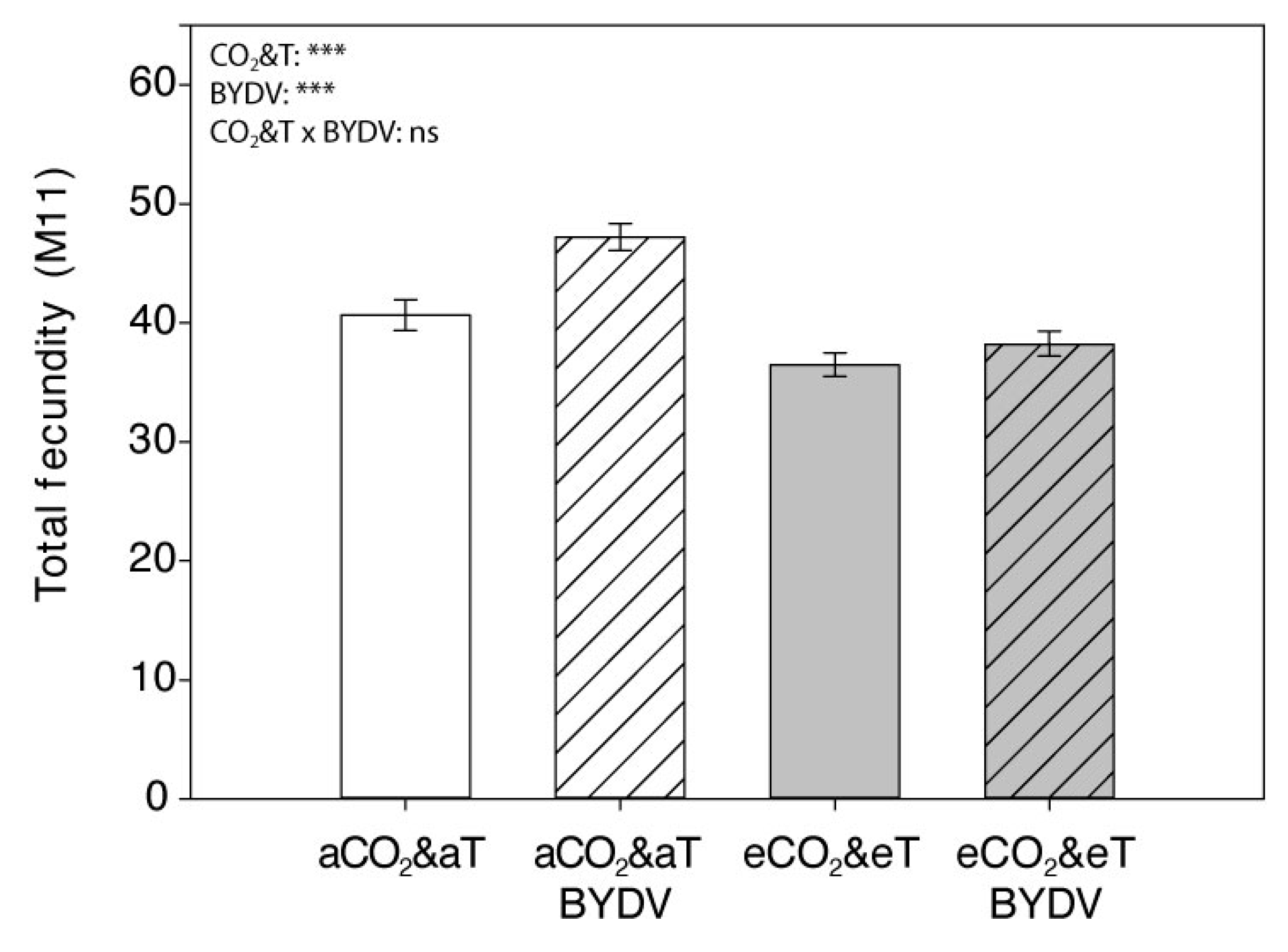
| Aphid Parameter | CO2&T | Virus Infection | Mean | SEM | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| Non-Infected | BYDV-PAV Infected | CO2&T | Virus | CO2&T × Virus | ||||
| Period from birth to adulthood (days) | aCO2&aT | 7.250 | 7.474 | 7.362 A | 0.103 | <0.001 *** | 0.001 *** | 0.182 |
| eCO2&eT | 6.650 | 7.150 | 6.900 B | |||||
| Mean | 6.950 b | 7.312 a | ||||||
| Pre-reproductive period “d” (days) | aCO2&aT | 8.300 | 8.579 | 8.439 A | 0.121 | 0.048 * | 0.006 ** | 0.558 |
| eCO2&eT | 8.000 | 8.400 | 8.200 B | |||||
| Mean | 8.150 b | 8.489 a | ||||||
| Effective fecundity “Md” | aCO2&aT | 35.250 | 41.111 | 38.181 A | 1.089 | <0.001 *** | <0.001 *** | 0.190 |
| eCO2&eT | 30.600 | 33.579 | 32.089 B | |||||
| Mean | 32.925 b | 37.345 a | ||||||
| Mean generation time “Td” (days) | aCO2&aT | 11.247 | 11.625 | 11.436 A | 0.164 | 0.048 * | 0.006 ** | 0.556 |
| eCO2&eT | 10.840 | 11.382 | 11.111 B | |||||
| Mean | 11.043 b | 11.503 a | ||||||
| Intrinsic rate of natural increase “rm” | aCO2&aT | 0.316 | 0.305 | 0.311 | 0.013 | 0.674 | 0.194 | 0.687 |
| eCO2&eT | 0.316 | 0.295 | 0.305 | |||||
| Mean | 0.316 | 0.300 | ||||||
| Mean relative growth rate “RGR” | aCO2&aT | 0.368 | 0.355 | 0.361 | 0.015 | 0.674 | 0.194 | 0.687 |
| eCO2&eT | 0.368 | 0.343 | 0.355 | |||||
| Mean | 0.368 | 0.349 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Delafuente, A.; Viñuela, E.; Fereres, A.; Medina, P.; Trębicki, P. Simultaneous Increase in CO2 and Temperature Alters Wheat Growth and Aphid Performance Differently Depending on Virus Infection. Insects 2020, 11, 459. https://doi.org/10.3390/insects11080459
Moreno-Delafuente A, Viñuela E, Fereres A, Medina P, Trębicki P. Simultaneous Increase in CO2 and Temperature Alters Wheat Growth and Aphid Performance Differently Depending on Virus Infection. Insects. 2020; 11(8):459. https://doi.org/10.3390/insects11080459
Chicago/Turabian StyleMoreno-Delafuente, Ana, Elisa Viñuela, Alberto Fereres, Pilar Medina, and Piotr Trębicki. 2020. "Simultaneous Increase in CO2 and Temperature Alters Wheat Growth and Aphid Performance Differently Depending on Virus Infection" Insects 11, no. 8: 459. https://doi.org/10.3390/insects11080459
APA StyleMoreno-Delafuente, A., Viñuela, E., Fereres, A., Medina, P., & Trębicki, P. (2020). Simultaneous Increase in CO2 and Temperature Alters Wheat Growth and Aphid Performance Differently Depending on Virus Infection. Insects, 11(8), 459. https://doi.org/10.3390/insects11080459







