Trypanosomatids Detected in the Invasive Avian Parasite Philornis downsi (Diptera: Muscidae) in the Galapagos Islands
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Molecular Testing
3.2. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friend, M.; McLean, R.G.; Dein, J. Disease emergence in birds: Challenges for the twenty-first century. Auk 2001, 118, 290–303. [Google Scholar] [CrossRef]
- Parker, P.G.; Whiteman, N.K.; Miller, R.E. Conservation medicine on the Galápagos Islands: Partnerships among behavioral, population, and veterinary scientists. Auk 2006, 123, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.A. Parasitic diseases of wildlife and domestic animals: New trends of disease emergence. In Infectious and Parasitic Diseases of Livestock 1: General Considerations; Lefevre, P.C., Blancou, J., Chermette, R., Uilenberg, G., Eds.; Lavoisier: Paris, France, 2010; Volume 1, pp. 73–79. [Google Scholar]
- Thompson, R.C.A.; Lymbery, A.J.; Smith, A. Parasites, emerging disease and wildlife conservation. Int. J. Parasitol. 2010, 40, 1163–1170. [Google Scholar] [CrossRef]
- Loope, L.L.; Howarth, F.G.; Kraus, F.; Pratt, T.K. Newly emergent and future threats of alien species to pacific birds and ecosystems. Stud. Avian Biol. 2001, 22, 291–304. [Google Scholar]
- Wilkelski, M.; Foufopoulos, J.; Vargas, H.; Snell, H. Galapagos birds and diseases: Invasive pathogens as threats for island species. Ecol. Soc. 2004, 9, 5. [Google Scholar] [CrossRef]
- Simberloff, D. Invasive Species. In Conservation Biology for All; Sodhi, N.S., Ehrlich, P.R., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 131–152. [Google Scholar]
- Blackburn, T.M.; Ewen, J.G. Parasites as drivers and passengers of human-mediated biological invasions. EcoHealth 2016, 14, 61–73. [Google Scholar] [CrossRef]
- Hatcher, M.J.; Dick, J.T.A.; Dunn, A.M. Disease emergence and invasions. Funct. Ecol. 2012, 26, 1275–1287. [Google Scholar] [CrossRef]
- Wilfert, L.; Long, G.; Leggett, H.C.; Schmid-Hempel, P.; Butlin, R.; Martin, S.J.; Boots, M. Deformed wing virus is a recent global epidemic in honeybees driven by Varroa mites. Science 2016, 5, 594–597. [Google Scholar] [CrossRef]
- Wyatt, K.B.; Campos, P.F.; Gilbert, M.T.P.; Kolokotronis, S.-O.; Hynes, W.H.; DeSalle, R.; Daszak, P.; MacPhee, R.D.E.; Greenwood, A.D. Historical mammal extinction on Christmas Island (Indian Ocean) correlates with introduced infectious disease. PLoS ONE 2008, 3, e3602. [Google Scholar] [CrossRef]
- McNew, S.M.; Clayton, D.H. Alien invasion: Biology of Philornis flies highlighting Philornis downsi, an introduced parasite of Galapagos birds. Ann. Rev. Entomol. 2018, 63, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Causton, C.E.; Peck, S.B.; Sinclair, B.J.; Roque-Albelo, L.; Hodgson, C.J.; Landry, B. Alien insects: Threats and implications for conservation of Galápagos Islands. Ann. Entomol. Soc. Am. 2006, 99, 121–143. [Google Scholar] [CrossRef]
- O’Connor, J.A.; Sulloway, F.J.; Robertson, J.; Kleindorfer, S. Philornis downsi parasitism is the primary cause of nestling mortality in the critically endangered Darwin’s medium tree finch (Camarhynchus pauper). Biodivers. Conserv. 2010, 19, 853–866. [Google Scholar] [CrossRef]
- Fessl, B.; Young, G.H.; Young, R.P.; Rodríguez-Matamoros, J.; Dvorak, M.; Tebbich, S.; Fa, J.E. How to save the rarest Darwin’s finch from extinction: The mangrove finch on Isabela Island. Philos. Trans. R. Soc. B 2010, 365, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Fessl, B.; Heimpel, G.E.; Causton, C.E. Invasion of an avian nest parasite, Philornis downsi, to the Galapagos Islands: Colonization history, adaptations to novel ecosystems, and conservation challenges. In Disease Ecology: Galapagos Birds and Their Parasites; Parker, P.G., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 213–268. [Google Scholar]
- Koop, J.A.H.; Huber, S.K.; Laverty, S.M.; Clayton, D.H. Experimental demonstration of the fitness consequences of an introduced parasite of Darwin’s finches. PLoS ONE 2011, 6, e19706. [Google Scholar] [CrossRef]
- Knutie, S.A.; McNew, S.M.; Bartlow, A.W.; Vargas, D.A.; Clayton, D.H. Darwin’s finches combat introduced nest parasites with fumigated cotton. Curr. Biol. 2014, 24, R355–R356. [Google Scholar] [CrossRef]
- Ben-Yosef, M.; Zaada, D.S.Y.; Dudaniec, R.Y.; Pasternak, Z.; Jurkevitch, E.; Smith, R.J.; Causton, C.E.; Lincango, M.P.; Tobe, S.S.; Mitchell, J.G.; et al. Host-specific associations affect the microbiome of Philornis downsi, an introduced parasite to the Galápagos Islands. Mol. Ecol. 2017, 26, 4644–4656. [Google Scholar] [CrossRef]
- Podlipaev, S. The more insect trypanosomatids under study - the more diverse Trypanosomatidae appears. Int. J. Parasitol. 2001, 31, 648–652. [Google Scholar] [CrossRef]
- Podlipaev, S.A. Insect trypanosomatids: The need to know more. Mem. Inst. Oswaldo Cruz 2000, 95, 517–522. [Google Scholar] [CrossRef]
- Undeen, A.H.; Vávra, J. Research methods for entomopathogenic protozoa. In Manual of Techniques in Insect Pathology, 1st ed.; Lacey, L., Ed.; Academic Press: London, UK, 1997; pp. 117–151. [Google Scholar]
- Lange, C.E.; Lord, J.C. Protistan entomopathogens. In Insect Pathology, 2nd ed.; Vega, F.E., Kaya, H.K., Eds.; Academic Press: London, UK, 2012; pp. 367–394. [Google Scholar]
- Erler, S.; Popp, M.; Wolf, S.; Lattorff, H.M.G. Sex, horizontal transmission, and multiple hosts prevent local adaptation of Crithidia bombi, a parasite of bumblebees (Bombus spp.). Ecol. Evol. 2012, 2, 930–940. [Google Scholar] [CrossRef]
- Olsen, O.W. Animal Parasites: Their Life Cycles and Ecology, 3rd ed.; Dover Publications: Mineola, NY, USA, 1986; pp. 21–24. [Google Scholar]
- Dias, F.A.; Vasconcellos, L.R.C.; Romeiro, A.; Attias, M.; Souto-Padrón, T.C.; Lopes, A.H. Transovum transmission of trypanosomatid cysts in the Milkweed Bug, Oncopeltus fasciatus. PLoS ONE 2014, 9, e108746. [Google Scholar] [CrossRef][Green Version]
- Frolov, A.O.; Malysheva, M.N.; Ganyukova, A.I.; Yurchenko, V.; Kostygov, A.Y. Life cycle of Blastocrithidia papi sp. n. (Kinetoplastea, Trypanosomatidae) in Pyrrhocoris apterus (Hemiptera, Pyrrhocoridae). Eur. J. Protistol. 2017, 57, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Conchon, I.; Campaner, M.; Sbravate, C.; Camargo, E.P. Trypanosomatids, other than Phytomonas spp. isolated and cultured from fruit. J. Protozool. 1989, 36, 412–414. [Google Scholar] [CrossRef]
- Schaub, G.A.; Jensen, C. Developmental time and mortality of the reduviid bug Triatoma infestans with differential exposure to coprophagic infections with Blastocrithidia triatomae (Trypanosomatidae). J. Invertebr. Pathol. 1990, 55, 17–27. [Google Scholar] [CrossRef]
- Brown, M.J.F.; Schmid-Hempel, R.; Schmid-Hempel, P. Strong context-dependent virulence in a host-parasite system: Reconciling genetic evidence with theory. J. Anim. Ecol. 2003, 72, 994–1002. [Google Scholar] [CrossRef]
- Ibraham, E.A.; Molyneux, D.H. Pathogenicity of Crithidia fasciculata in the haemocoele of Glossina. Acta Trop. 1987, 44, 13–22. [Google Scholar]
- Arnquist, G.; Mäki, M. Infection rates and pathogenicity of trypanosomatid gut parasites in the water strider Gerris odontogaster (Zett.) (Heteroptera: Gerridae). Oecologia 1990, 84, 194–198. [Google Scholar] [CrossRef]
- Tanada, Y.; Kaya, H.K. Protozoan infections: Zoomastigina, rhizopoda, and ciliophoran. In Insect Pathology, 1st ed.; Tanada, Y., Kaya, H., Eds.; Academic Press: San Diego, CA, USA, 1993; pp. 388–397. [Google Scholar]
- Lipa, J.J.; Carl, K.P.; Valentine, E.W. Blastocrithidia caliroae sp. n., a flagellate parasite of Caliroa cerasi (L.) (Hymenoptera: Tenthredinidae) and notes on its epizootics in host field populations. Acta Protozool. 1977, 16, 121–129. [Google Scholar]
- Bartlett-Healy, K.; Crans, W.; Gaugler, R. Vertebrate hosts and phylogenetic relationships of amphibian trypanosomes from a potential invertebrate vector, Culex territans Walker (Diptera: Culicidae). J. Parasitol. 2009, 95, 381–387. [Google Scholar] [CrossRef]
- Bennett, G.F. On the specificity and transmission of some avian trypanosomes. Can. J. Zool. 1961, 39, 17–33. [Google Scholar] [CrossRef]
- Ramos, B.; Urdaneta-Morales, S. Hematophagous insects as vectors for frog trypanosomes. Rev. Biol. Trop. 1978, 25, 209–217. [Google Scholar]
- Wamwiri, F.N.; Changasi, R.E. Tsetse flies (Glossina) as vectors of human African trypanosomiasis: A review. Biomed. Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hartman, D. Galapagos Mosquitoes as Avian Disease Vectors. Master’s Thesis, University of Missouri-St. Louis, St. Louis, MO, USA, 2014. [Google Scholar]
- Wallace, F.G. The trypanosomatid parasites of insects and arachnids. Exp. Parasitol. 1966, 18, 124–193. [Google Scholar] [CrossRef]
- Bulat, S.A.; Mokrousov, I.V.; Podlipaev, S.A. Classification of trypanosomatids from insects and plants by the UP-PCR (Universally Primed PCR) technique and cross dot blot hybridization of PCR products. Eur. J. Protistol. 1999, 35, 319–326. [Google Scholar] [CrossRef]
- Podlipaev, S.A.; Sturm, N.R.; Fiala, I.; Fernandes, O.; Westenberger, S.J.; Dollet, M.; Campbell, D.A.; Lukeš, J. Diversity of insect trypanosomatids assessed from the spliced leader RNA and 5S rRNA genes and intergenic regions. J. Eukaryot. Microbiol. 2004, 51, 283–290. [Google Scholar] [CrossRef]
- Causton, C.E.; Moon, R.D.; Cimadom, A.; Boulton, R.A.; Cedeño, D.; Lincango, M.P.; Tebbich, S.; Ulloa, A. Population dynamics of an invasive bird parasite, Philornis downsi (Diptera: Muscidae), in the Galapagos Islands. PLoS ONE 2019, 14, e0224125. [Google Scholar] [CrossRef]
- Maslov, D.A.; Lukeš, J.; Jirku, M.; Simpson, L. Phylogeny of trypanosomes as inferred from the small and large subunit rRNAs: Implication for the evolution of parasitism in the trypanosomatid protozoa. Mol. Biochem. Parasitol. 1996, 75, 197–205. [Google Scholar] [CrossRef]
- Votýpka, J.; Szabová, J.; Rádrová, J.; Zídková, L.; Svobodová, M. Trypanosoma culicavium sp. nov., an avian trypanosome transmitted by Culex mosquitoes. Int. J. Syst. Evol. Microbiol. 2012, 62, 745–754. [Google Scholar] [CrossRef]
- Sehgal, R.M.N.; Jones, H.I.; Smith, T.B. Host specificity and incidence of Trypanosoma in some African rainforest birds: A molecular approach. Mol. Ecol. 2001, 10, 2319–2327. [Google Scholar] [CrossRef]
- Nzelu, C.O.; Kato, H.; Puplampu, N.; Desewu, K.; Odoom, K.; Wilson, M.D.; Sakurai, T.; Katakura, K.; Boakye, D. First detection of Leishmania tropica DNA and Trypanosoma species in Sergentomyia sand flies (Diptera: Psychodidae) from an outbreak area of cutaneous leishmaniasis in Ghana. PLoS Negl. Trop. Dis. 2014, 8, e2630. [Google Scholar] [CrossRef]
- Van Dyken, M.; Bolling, B.G.; Moore, C.G.; Blair, C.D.; Beaty, B.J.; Black, W.C., IV; Foy, B.D. Molecular evidence for trypanosomatids in Culex mosquitoes collected during a West Nile virus survey. Int. J. Parasitol. 2006, 36, 1015–1023. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vey016. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, R.R.; Drummond, A.J. Bmodeltest: Bayesian phylogenetic site model averaging and model comparison. BMC Evol. Biol. 2017, 17, 42. [Google Scholar] [CrossRef]
- Stöver, B.C.; Müller, K.F. TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinform. 2010, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Pike, C.; Lincango, P.; Causton, C.; Parker, P. Sequence Alignment Data for Trypanosomatidae Parasites Found in Philornis downsi from the Galapagos Islands; Version 2; Mendeley Repository: Amsterdam, The Netherlands, 2020; Available online: http://dx.doi.org/10.17632/c6wsxd2jz5.2 (accessed on 19 March 2020).
- Jukes, T.H.; Cantor, C.R. Evolution of protein molecules. In Mammalian Protein Metabolism; Munro, H.N., Ed.; Academic Press: New York, NY, USA, 1969; pp. 21–132. [Google Scholar]
- Solter, L.F.; Becnel, J.J.; Vavra, J. Research methods for entomopathogenic microsporidia and other protists. In Manual of Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L.A., Ed.; Elsevier: San Diego, CA, USA, 2012; pp. 329–371. [Google Scholar]
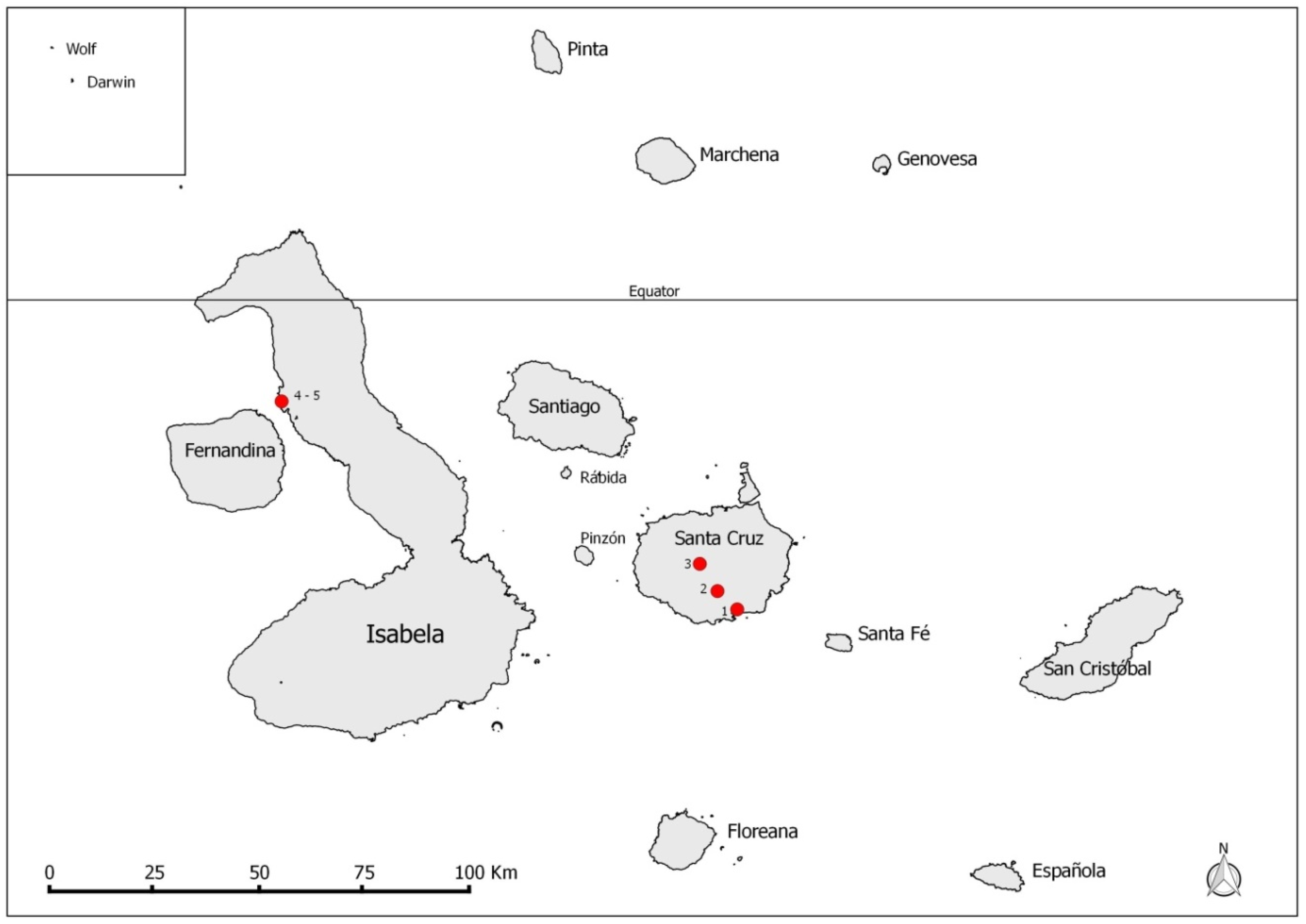

| Island | Site | Total Sample Pools (# Total Flies) | Molecular Tests (# Positive Pools/# Total Tested Pools) | MIR (%) | |
|---|---|---|---|---|---|
| [44] 1 | [45] 1 | ||||
| Santa Cruz | Lowland, dry (El Barranco) | 165 (376) | 138/165 | 19/40 | 36.7 |
| Agricultural zone (Los Guayabillos) | 2 (2) | 2/2 | 0/0 | 100.0 | |
| Highland, humid (Los Gemelos) | 110 (267) | 109/110 | 18/43 | 40.8 | |
| Isabela | Mangrove forest (Playa Tortuga Negra) | 17 (45) | 14/17 | 3/9 | 31.1 |
| Lava field (Playa Tortuga Negra) | 3 (4) | 3/3 | 0/3 | 75.0 | |
| Total | 297 (694) | 267/297 | 40/95 | 38.5 | |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Trypanosomatidae sp. P041 | 0.17 | 0.14 | 0.30 | 0.32 | 0.19 | 0.29 | 0.43 | 1.58 | 1.44 | 1.58 | 1.35 | 1.41 | 1.44 | 1.54 | 1.48 | 1.46 | |
| 2 | Trypanosomatidae sp. P034 | 0.32 | 0.11 | 0.32 | 0.34 | 0.26 | 0.34 | 0.49 | 1.53 | 1.42 | 1.53 | 1.34 | 1.41 | 1.41 | 1.51 | 1.44 | 1.43 | |
| 3 | Trypanosomatidae sp. P057 | 0.21 | 0.11 | 0.34 | 0.35 | 0.24 | 0.32 | 0.46 | 1.53 | 1.42 | 1.53 | 1.34 | 1.41 | 1.41 | 1.51 | 1.44 | 1.42 | |
| 4 | Trypanosomatidae sp. P120 | 0.86 | 0.97 | 1.08 | 0.21 | 0.37 | 0.41 | 0.50 | 1.57 | 1.40 | 1.57 | 1.32 | 1.37 | 1.45 | 1.52 | 1.48 | 1.47 | |
| 5 | Blastocrithidia miridarum | 0.97 | 1.08 | 1.19 | 0.43 | 0.39 | 0.42 | 0.54 | 1.57 | 1.40 | 1.57 | 1.31 | 1.38 | 1.43 | 1.53 | 1.47 | 1.46 | |
| 6 | Crithidia bombi | 0.32 | 0.65 | 0.54 | 1.19 | 1.30 | 0.36 | 0.50 | 1.57 | 1.45 | 1.57 | 1.35 | 1.42 | 1.46 | 1.54 | 1.50 | 1.48 | |
| 7 | Crithidia confusa | 0.75 | 1.08 | 0.97 | 1.52 | 1.63 | 1.08 | 0.53 | 1.59 | 1.43 | 1.59 | 1.34 | 1.37 | 1.41 | 1.51 | 1.44 | 1.42 | |
| 8 | Crithidia dedva | 1.75 | 2.08 | 1.97 | 2.19 | 2.53 | 2.08 | 2.42 | 1.54 | 1.43 | 1.54 | 1.37 | 1.47 | 1.41 | 1.46 | 1.46 | 1.47 | |
| 9 | Leptomonas collosoma | 15.12 | 14.81 | 14.81 | 15.12 | 15.27 | 14.81 | 15.12 | 14.96 | 1.56 | 0.15 | 1.50 | 1.50 | 1.48 | 1.52 | 1.43 | 1.40 | |
| 10 | Leptomonas mirabilis | 14.20 | 14.05 | 14.05 | 13.75 | 14.05 | 14.50 | 14.20 | 14.35 | 16.52 | 1.55 | 1.10 | 1.11 | 1.42 | 1.40 | 1.42 | 1.41 | |
| 11 | Leptomonas rigidus | 15.12 | 14.81 | 14.81 | 15.12 | 15.27 | 14.81 | 15.12 | 14.81 | 0.21 | 16.36 | 1.49 | 1.49 | 1.48 | 1.52 | 1.43 | 1.40 | |
| 12 | Leptomonas samueli | 13.16 | 13.01 | 13.01 | 12.86 | 12.86 | 13.16 | 13.31 | 13.75 | 15.74 | 9.49 | 15.58 | 0.80 | 1.54 | 1.62 | 1.55 | 1.55 | |
| 13 | Leptomonas costoris | 14.50 | 14.50 | 14.50 | 13.90 | 14.20 | 14.50 | 14.20 | 15.27 | 15.89 | 9.49 | 15.74 | 5.61 | 1.49 | 1.53 | 1.47 | 1.46 | |
| 14 | Trypanosoma avium | 13.90 | 13.75 | 13.60 | 13.90 | 13.75 | 14.20 | 13.45 | 13.75 | 14.96 | 15.42 | 14.96 | 16.05 | 15.74 | 0.80 | 0.63 | 0.62 | |
| 15 | Trypanosoma bennetti | 15.12 | 14.96 | 14.96 | 14.81 | 14.96 | 15.12 | 14.66 | 14.66 | 15.58 | 14.96 | 15.58 | 16.84 | 16.52 | 5.37 | 0.82 | 0.84 | |
| 16 | Trypanosoma corvi | 14.50 | 14.35 | 14.20 | 14.50 | 14.35 | 14.81 | 13.90 | 14.35 | 14.50 | 15.74 | 14.50 | 16.52 | 16.36 | 3.93 | 5.49 | 0.18 | |
| 17 | Trypanosoma culicavium | 14.20 | 14.05 | 13.90 | 14.20 | 14.05 | 14.50 | 13.60 | 14.50 | 14.05 | 15.74 | 14.05 | 16.52 | 16.36 | 4.04 | 5.86 | 0.32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pike, C.L.; Lincango, M.P.; Causton, C.E.; Parker, P.G. Trypanosomatids Detected in the Invasive Avian Parasite Philornis downsi (Diptera: Muscidae) in the Galapagos Islands. Insects 2020, 11, 422. https://doi.org/10.3390/insects11070422
Pike CL, Lincango MP, Causton CE, Parker PG. Trypanosomatids Detected in the Invasive Avian Parasite Philornis downsi (Diptera: Muscidae) in the Galapagos Islands. Insects. 2020; 11(7):422. https://doi.org/10.3390/insects11070422
Chicago/Turabian StylePike, Courtney L., María Piedad Lincango, Charlotte E. Causton, and Patricia G. Parker. 2020. "Trypanosomatids Detected in the Invasive Avian Parasite Philornis downsi (Diptera: Muscidae) in the Galapagos Islands" Insects 11, no. 7: 422. https://doi.org/10.3390/insects11070422
APA StylePike, C. L., Lincango, M. P., Causton, C. E., & Parker, P. G. (2020). Trypanosomatids Detected in the Invasive Avian Parasite Philornis downsi (Diptera: Muscidae) in the Galapagos Islands. Insects, 11(7), 422. https://doi.org/10.3390/insects11070422





