Landscape Vegetation Productivity Influences Population Dynamics of Key Pests in Small Avocado Farms in Kenya
Abstract
1. Introduction
2. Materials and Methods
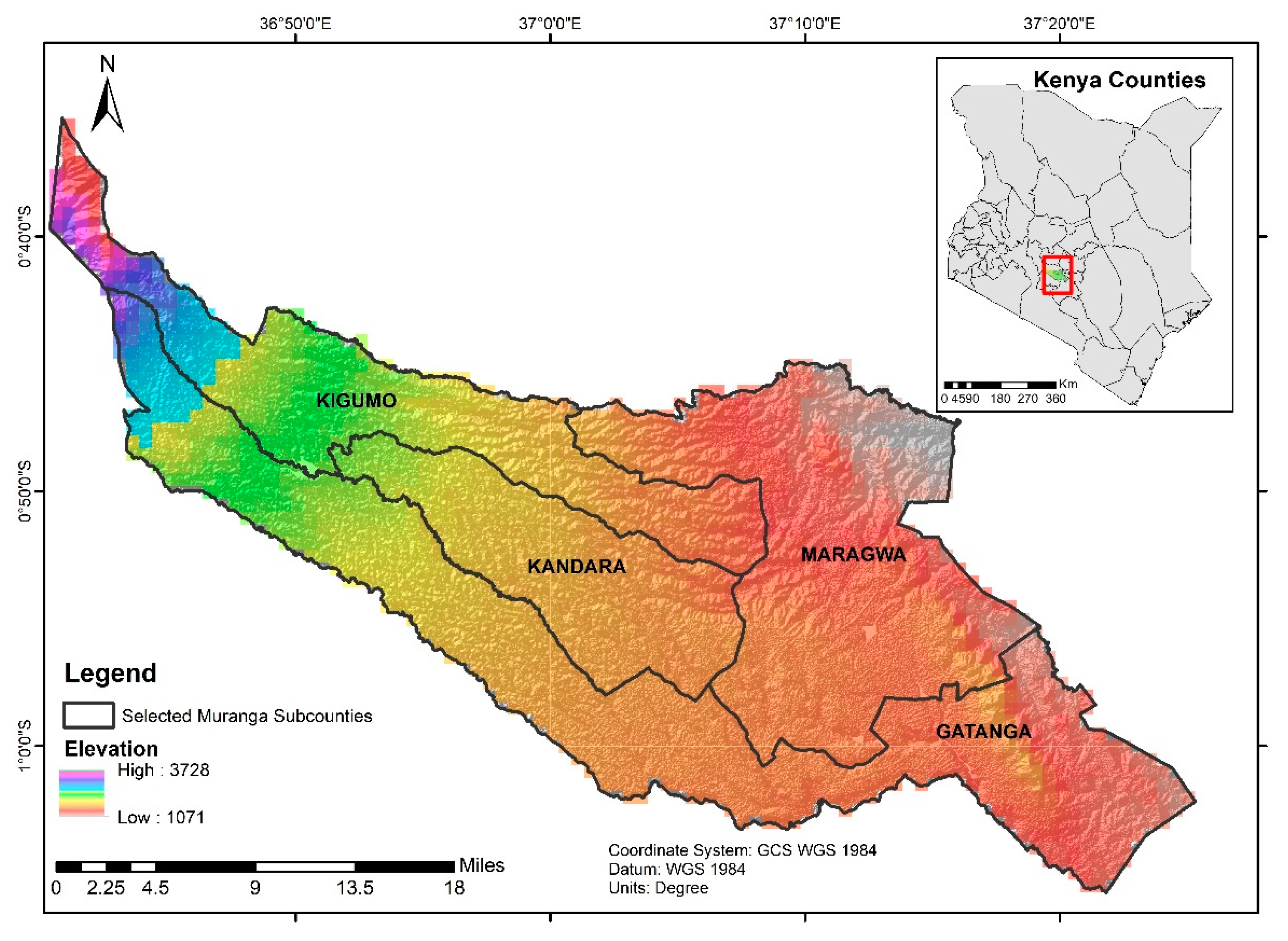
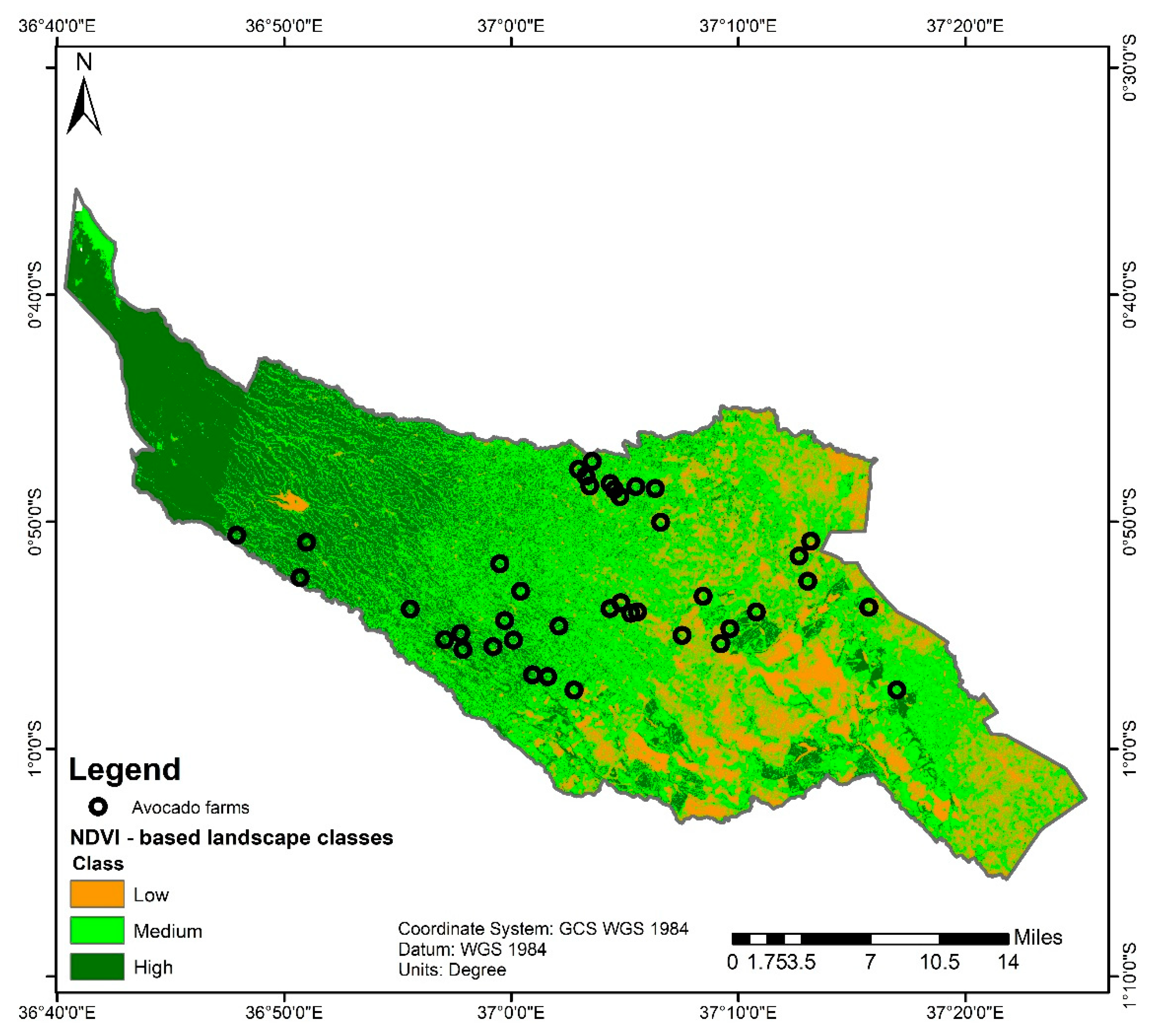
2.1. Study Site and Characterization of the Landscape
2.2. Trapping of the Oriental Fruit Fly and False Codling Moth
2.3. Rearing of Pests and Parasitoids from Fruit
2.4. Data Analyses
3. Results
3.1. Farm Characteristics and Overview of Pests
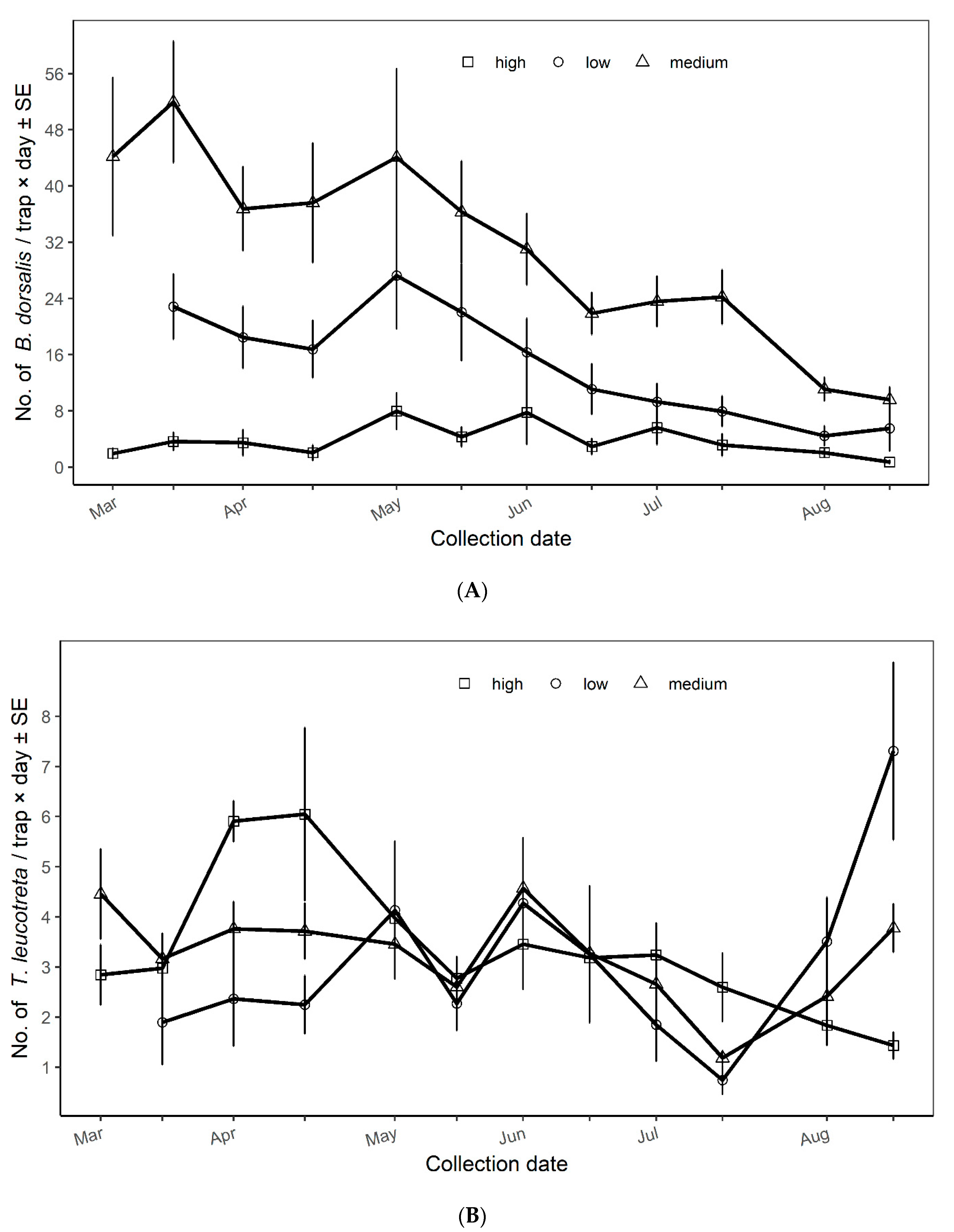
3.2. Population Dynamics of Bactrocera dorsalis and Thaumatotibia leucotreta across Vegetation Productivity Classes
3.3. Distribution of Bactrocera dorsalis and Thaumatotibia leucotreta along the Altitude
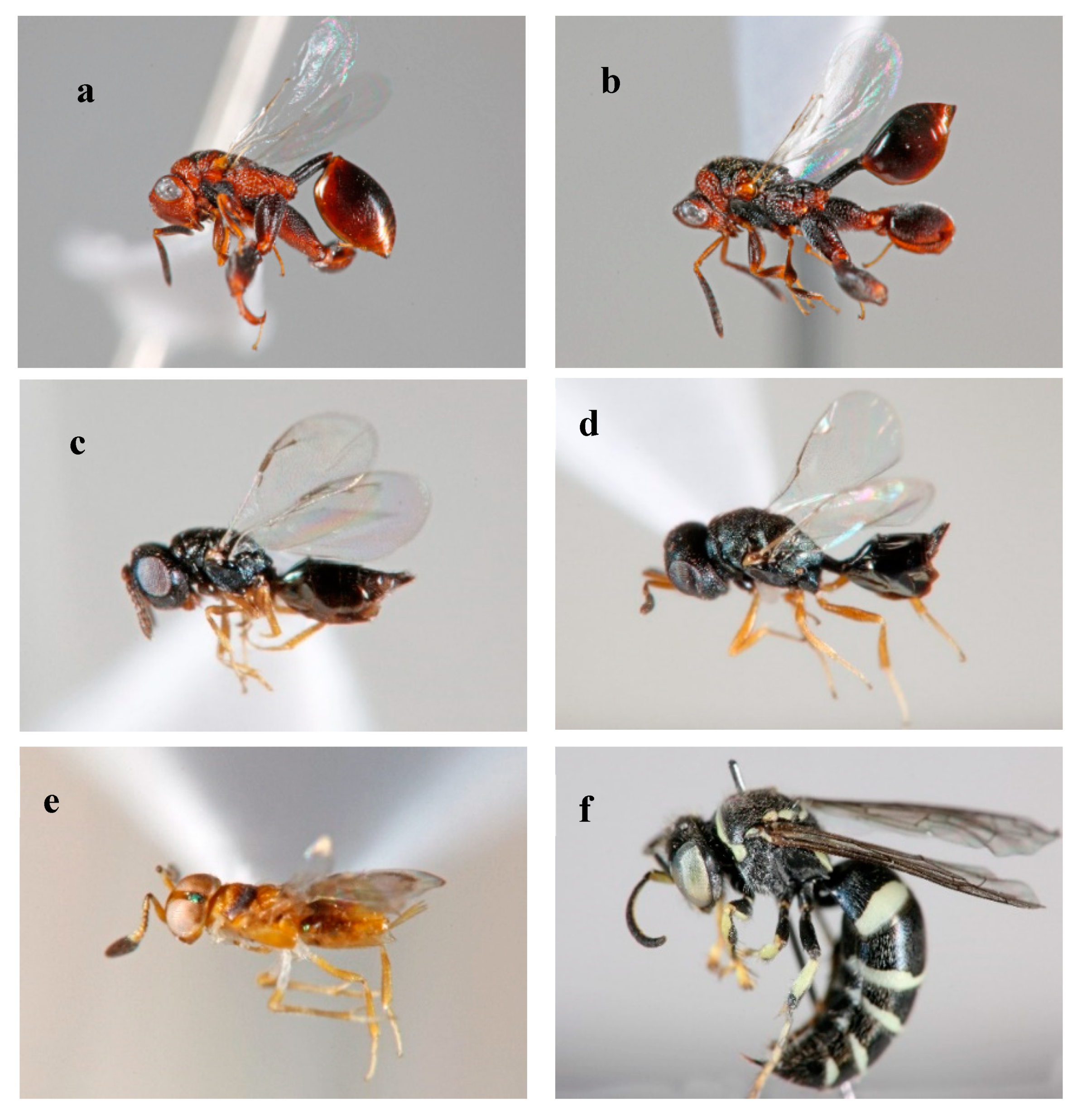
3.4. Emerged Pests and Parasitoids during Fruit Incubation
4. Discussions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity-ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- O’Rourke, M.E.; Jones, L.E. Analysis of landscape-scale insect pest dynamics and pesticide use: An empirical and modelling study. Ecol. Appl. 2011, 21, 3199–3210. [Google Scholar] [CrossRef]
- Veres, A.; Tóth, F.; Kiss, J.; Fetykó, K.; Orosz, S.; Lavigne, C.; Otto, S.; Bohan, D. Spatio-temporal dynamics of Orius spp. (Heteroptera: Anthocoridae) abundance in the agricultural landscape. Agric. Ecosyst. Environ. 2012, 162, 45–51. [Google Scholar] [CrossRef]
- Schellhorn, N.A.; Gagic, V.; Bommarco, R. Time will tell: Resource continuity bolsters ecosystem services. Trends Ecol. Evol. 2015, 30, 524–530. [Google Scholar] [CrossRef]
- Paredes, D.; Cayuela, L.; Gurr, G.M.; Campos, M. Is ground cover vegetation an effective biological control enhancement strategy against olive pests? PLoS ONE 2015, 10, e0117265. [Google Scholar] [CrossRef]
- Orr, D.B.; Landis, D.A.; Mutch, D.R.; Manley, G.V.; Stuby, S.A.; King, R.L. Ground cover influence on microclimate and Trichogramma (Hymenoptera: Trichogrammatidae) augmentation in seed corn production. Environ. Entomol. 1997, 26, 433–438. [Google Scholar] [CrossRef]
- Pettorelli, N. The Normalized Difference Vegetation Index; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Wang, J.; Rich, P.M.; Price, K.P. Temporal responses of NDVI to precipitation and temperature in the central Great Plains, USA. Int. J. Remote Sens. 2003, 24, 2345–2364. [Google Scholar] [CrossRef]
- Dubovyk, O.; Landmann, T.; Erasmus, B.F.; Tewes, A.; Schellberg, J. Monitoring vegetation dynamics with medium resolution MODIS-EVI time series at sub-regional scale in southern Africa. Int. J. Appl. Earth Obs. Geoinf. 2015, 38, 175–183. [Google Scholar] [CrossRef]
- Traore, S.S.; Forkue, E.K.; Traore, P.C.; Landmann, T. Assessing the inter-relationship between vegetation productivity, rainfall, population and land cover over the Bani River Basin in Mali (West Africa). IOSR J. Eng. 2015, 5, 10–18. [Google Scholar]
- Yengoh, G.T.; Dent, D.; Olsson, L.; Tengberg, A.E.; Tucker III, C.J. Use of the Normalized Difference Vegetation Index (NDVI) to Assess. Land Degradation at Multiple Scales: Current Status, Future Trends, and Practical Considerations; Springer: Berlin, Germany, 2015. [Google Scholar]
- Schultz, P.A.; Halpert, M.S. Global correlation of temperature, NDVI and precipitation. Adv. Space Res. 1993, 13, 277–280. [Google Scholar] [CrossRef]
- Bailey, S.-A.; Horner-Devine, M.C.; Luck, G.; Moore, L.A.; Carney, K.M.; Anderson, S.; Betrus, C.; Fleishman, E. Primary productivity and species richness: Relationships among functional guilds, residency groups and vagility classes at multiple spatial scales. Ecography 2004, 27, 207–217. [Google Scholar] [CrossRef]
- Pettorelli, N.; Ryan, S.; Mueller, T.; Bunnefeld, N.; Jędrzejewska, B.; Lima, M.; Kausrud, K. The Normalised Difference Vegetation Index (NDVI): Unforeseen successes in animal ecology. Clim. Res. 2011, 46, 15–27. [Google Scholar] [CrossRef]
- Macfadyen, S.; Kramer, E.A.; Parry, H.R.; Schellhorn, N.A. Temporal change in vegetation productivity in grain production landscapes: Linking landscape complexity with pest and natural enemy communities: Temporal change in agricultural landscapes. Ecol. Entomol. 2015, 40, 56–69. [Google Scholar] [CrossRef]
- Richard, K.; Abdel-Rahman, E.M.; Mohamed, S.A.; Ekesi, S.; Borgemeister, C.; Landmann, T. Importance of remotely-sensed vegetation variables for predicting the spatial distribution of african citrus Triozid (Trioza erytreae) in Kenya. ISPRS Int. J. Geo Inf. 2018, 7, 429. [Google Scholar] [CrossRef]
- Tratalos, J.A.; Cheke, R.A. Can NDVI GAC imagery be used to monitor desert locust breeding areas? J. Arid Environ. 2006, 64, 342–356. [Google Scholar] [CrossRef]
- Despland, E.; Rosenberg, J.; Simpson, S.J. Landscape structure and locust swarming: A satellite’s eye view. Ecography 2004, 27, 381–391. [Google Scholar] [CrossRef]
- Muriithi, B.W.; Matz, J.A. Welfare effects of vegetable commercialization: Evidence from smallholder producers in Kenya. Food Policy 2015, 50, 80–91. [Google Scholar] [CrossRef]
- Gyau, A.; Mbugua, M.; Oduol, J. Determinants of participation and intensity of participation in collective action: Evidence from smallholder avocado farmers in Kenya. J. Chain Netw. Sci. 2016, 16, 147–156. [Google Scholar] [CrossRef]
- Grové, T.; De Beer, M.S.; Joubert, P.H. Developing a systems approach for Thaumatotibia leucotreta (Lepidoptera: Tortricidae) on ‘Hass’ avocado in South Africa. J. Econ. Entomol. 2010, 103, 1112–1128. [Google Scholar] [CrossRef]
- Mwatawala, M.W.; De Meyer, M.; Makundi, R.H.; Maerere, A.P. Seasonality and host utilization of the invasive fruit fly, Bactrocera invadens (Dipt., Tephritidae) in central Tanzania. J. Appl. Entomol. 2006, 130, 530–537. [Google Scholar] [CrossRef]
- De Graaf, J. Host status of avocado (‘Hass’) to Ceratitis capitata, Ceratitis rosa, and Ceratitis cosyra (Diptera: Tephritidae) in South Africa. J. Econ. Entomol. 2009, 102, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Ware, A.B.; du Toit, C.L.N.; du Toit, E.; Collins, R.; Clowes, R.; Ekesi, S.; Mohamed, S.A. Host suitability of three avocado cultivars (Persea americana Miller: Lauraceae) to oriental fruit fly (Bactrocera (invadens) dorsalis (Hendel) (Diptera: Tephritidae)). Crop. Prot. 2016, 90, 84–89. [Google Scholar] [CrossRef]
- Odanga, J.J.; Mohamed, S.; Mwalusepo, S.; Olubayo, F.; Nyankanga, R.; Khamis, F.; Rwomushana, I.; Johansson, T.; Ekesi, S. Spatial distribution of Bactrocera dorsalis and Thaumatotibia leucotreta in smallholder avocado orchards along an altitudinal gradient of Taita Hills and Mount Kilimanjaro. Insects 2018, 9, 71. [Google Scholar] [CrossRef]
- Centre for Agriculture and Bioscience International. CABI Hosts/Species affected. In Bactrocera Dorsalis (Oriental Fruit Fly); Compendium Record: Wallingford, UK, 2020. [Google Scholar]
- Rwomushana, I.; Tanga, C.M. Fruit Fly Species. In Fruit Fly Research and Development in Africa—Towards a Sustainable Management Strategy to Improve Horticulture; Springer: Berlin, Germany, 2016; pp. 71–106. [Google Scholar]
- Zeng, Y.; Reddy, G.V.P.; Li, Z.; Qin, Y.; Wang, Y.; Pan, X.; Jiang, F.; Gao, F.; Zhao, Z.-H. Global distribution and invasion pattern of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). J. Appl. Entomol. 2019, 143, 165–176. [Google Scholar] [CrossRef]
- Nugnes, F.; Russo, E.; Viggiani, G.; Bernardo, U. First record of an invasive fruit fly belonging to Bactrocera dorsalis complex (Diptera: Tephritidae) in Europe. Insects 2018, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Copeland, R.S.; Wharton, R.A.; Luke, Q.; De Meyer, M.; Lux, S.; Zenz, N.; Machera, P.; Okumu, M. Geographic distribution, host fruit, and parasitoids of African fruit fly pests Ceratitis anonae, Ceratitis cosyra, Ceratitis fasciventris, and Ceratitis rosa (Diptera: Tephritidae) in Kenya. Ann. Entomol. Soc. Am. 2006, 99, 261–278. [Google Scholar] [CrossRef]
- Ekesi, S.; Billah, M.K.; Nderitu, P.W.; Lux, S.A.; Rwomushana, I., IV. Evidence for competitive displacement of Ceratitis cosyra by the invasive fruit fly Bactrocera invadens (Diptera: Tephritidae) on mango and mechanisms contributing to the displacement. J. Econ. Entomol. 2009, 102, 981–991. [Google Scholar] [CrossRef]
- Gilligan, T.M.; Epstein, M.E.; Hoffman, K.M. Discovery of false codling moth, Thaumatotibia leucotreta (Meyrick), in California (Lepidoptera: Tortricidae). Proc. Entomol. Soc. Wash. 2011, 113, 426–436. [Google Scholar] [CrossRef]
- Copeland, R.S.; Luke, Q.; Wharton, R.A. Insects reared from the wild fruits of Kenya. J. East Afr. Nat. Hist. 2009, 98, 11–66. [Google Scholar] [CrossRef]
- Grové, T.; Steyn, W.P.; De Beer, M.S. The false codling moth, Cryptophlebia leucotreta (Meyrick) (Lepidoptera: Tortricidae) on avocado: A literature review. South Afr. Avocado Grow. Assoc. Yearb. 1999, 22, 31–33. [Google Scholar]
- Food and Agriculture Organization. FAOSTAT Database; FAO: Rome, Italy; Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 10 February 2020).
- Kareem, O.I. The European Union sanitary and phytosanitary measures and Africa’s exports. Available online: http://hdl.handle.net/1814/33311 (accessed on 10 February 2020).
- European and Mediterranean Plant Protection Organization. Global Database; EPPO: Paris, France; Available online: https://gd.eppo.int/taxon/ARGPLE (accessed on 17 February 2020).
- Otieno, W. KEPHIS experience with market access and compliance with official standards. Acta Hortic. 2011, 73–76. [Google Scholar] [CrossRef]
- Amare, M.; Mariara, J.; Oostendorp, R.; Pradhan, M. The impact of smallholder farmers’ participation in avocado export markets on the labor market, farm yields, sales prices, and incomes in Kenya. Land Use Policy 2019, 88, 104168. [Google Scholar] [CrossRef]
- Walther, S.; Guanter, L.; Heim, B.; Jung, M.; Duveiller, G.; Wolanin, A.; Sachs, T. Assessing the dynamics of vegetation productivity in circumpolar regions with different satellite indicators of greenness and photosynthesis. Biogeosciences 2018, 15, 6221–6256. [Google Scholar] [CrossRef]
- Thavorntam, W.; Tantemsapya, N. Vegetation greenness modelling in response to climate change for Northeast Thailand. J. Geogr. Sci. 2013, 23, 1052–1068. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. NASA Spec. Publ. 1974, 351, 309. [Google Scholar]
- Likas, A.; Vlassis, N.; Verbeek, J.J. The global k-means clustering algorithm. Pattern Recognit. 2003, 36, 451–461. [Google Scholar] [CrossRef]
- QGIS. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2020. Available online: http://qgis.org (accessed on 4 September 2018).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontología Electrónica 2001, 4, 9. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- NASA/METI/AIST/Japan Spacesystems; U.S./Japan ASTER Science Team. ASTER Global Digital Elevation Model V003 [Data Set]; NASA EOSDIS Land Processes DAAC: Sioux Falls, SD, USA, 2019. [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitao, P.J. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Ekesi, S.; Nderitu, P.W.; Rwomushana, I. Field infestation, life history and demographic parameters of the fruit fly Bactrocera invadens (Diptera: Tephritidae) in Africa. Bull. Entomol. Res. 2006, 96, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Mkiga, A.M.; Mohamed, S.A.; du Plessis, H.; Khamis, F.M.; Ekesi, S. Field and laboratory performance of False Codling Moth, Thaumatotibia Leucotreta (Lepidoptera: Tortricidae) on orange and selected vegetables. Insects 2019, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, M. Revision of the subgenus Ceratitis (Ceratalaspis) Hancock (Diptera: Tephritidae). Bull. Entomol. Res. 1998, 88, 257–290. [Google Scholar] [CrossRef]
- Drew, R.A.I.; Romig, M.C. Keys to the Tropical Fruit Flies (Tephritidae: Dacinae) of South-East Asia: Indomalaya to North-West Australasia; CABI: Wallingford, UK, 2016; ISBN 978-1-78064-419-6. [Google Scholar]
- Gilligan, T.M.; Epstein, M.E. Tortricids of Agricultural Importance. Available online: https://idtools.org/id/leps/tortai/Thaumatotibia_leucotreta.htm (accessed on 20 November 2019).
- Bohart, R.M.; Bohart, R.M.; Menke, A.S. Sphecid Wasps of the World: A Generic Revision; University of California Press: Berkely, CA, USA, 1976; p. 695. [Google Scholar]
- FAO/IAEA. Trapping Guidelines for Area-Wide Fruit Fly Programmes; IAEA: Wien, Austria, 2003. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. GLM and GAM for count data. In Mixed Effects Models and Extensions In Ecology With R; Springer: Berlin, Germany, 2009; pp. 209–243. [Google Scholar]
- Cowley, J.M.; Baker, R.T.; Harte, D.S. Definition and determination of host status for multivoltine fruit fly (Diptera: Tephritidae) species. J. Econ. Entomol. 1992, 85, 312–317. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2019. Available online: https://www.r-project.org/ (accessed on 20 March 2019).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Heisterkamp, S.; Van Willigen, B.; Maintainer, R. Package ‘nlme.’ Linear Nonlinear Mixed Effects Models; Version 3; CRAN: Wien, Austria, 2017. [Google Scholar]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, aka Least-Squares Means; R Package Version 1; CRAN: Wien, Austria, 2018. [Google Scholar]
- Graves, S.; Piepho, H.P.; Selzer, L.; Dorai-Raj, S. multcompView: Visualizations of Paired Comparisons; R Package Version 0.1-8; CRAN: Wien, Austria, 2019. [Google Scholar]
- Stotter, R.L.; Terblanche, J.S. Low-temperature tolerance of false codling moth Thaumatotibia leucotreta (Meyrick) (Lepidoptera: Tortricidae) in South Africa. J. Therm. Biol. 2009, 34, 320–325. [Google Scholar] [CrossRef]
- Wang, A.; Messing, R.H. The ectoparasitic pupal parasitoid, Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae), attacks other primary tephritid fruit fly parasitoids: Host expansion and potential non-target impact. Biol. Control. 2004, 31, 227–236. [Google Scholar] [CrossRef]
- Oliveira, L.; Medeiros, A.; Falco, J.V.; Beitia, F.; Verdu, M.J.; Garcia, P. Parasitoids from Azores (Hymenoptera: Encyrtidae, Pteromalidae, Braconidae): Potential use in integrated pest management against Ceratitis capitata (Diptera: Tephritidae). Biocontrol Sci. Technol. 2008, 18, 741–744. [Google Scholar] [CrossRef][Green Version]
- Molnár, A.V.; Mészáros, A.; Csathó, A.I.; Balogh, G.; Cs\Hosz, S. On the presence of the subfamily Epitraninae (Hymenoptera: Chalcidoidea, Chalcididae) in Iran. North West J. Zool. 2018, 14, 267–268. [Google Scholar]
- Geden, C.J.; Moon, R.D. Host ranges of gregarious muscoid fly parasitoids: Muscidifurax raptorellus (Hymenoptera: Pteromalidae), Tachinaephagus zealandicus (Hymenoptera: Encyrtidae), and Trichopria nigra (Hymenoptera: Diapriidae). Environ. Entomol. 2009, 38, 700–707. [Google Scholar] [CrossRef]
| B. dorsalis | T. leucotreta | |||
|---|---|---|---|---|
| Vegetation Productivity Class | N | Mean ± SE | N | Mean ± SE |
| Low | 75 | 14.82 ± 1.53 ab | 74 | 3.11 ± 0.36 |
| Medium | 206 | 29.86 ± 1.96 a | 214 | 3.20 ± 0.15 |
| High | 142 | 3.68 ± 0.58 b | 151 | 3.26 ± 0.44 |
| Mean Number of Adults/kg of Fruits (± SE) | ||
|---|---|---|
| Vegetation Productivity Class | From Ground | From Tree |
| Low | 12.50 ± 3.10 a | 0.14 ± 0.14 |
| Medium | 8.84 ± 1.69 ab | 0.32 ± 0.20 |
| High | 3.94 ± 1.31 b | 0.76 ± 0.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toukem, N.K.; Yusuf, A.A.; Dubois, T.; Abdel-Rahman, E.M.; Adan, M.S.; Mohamed, S.A. Landscape Vegetation Productivity Influences Population Dynamics of Key Pests in Small Avocado Farms in Kenya. Insects 2020, 11, 424. https://doi.org/10.3390/insects11070424
Toukem NK, Yusuf AA, Dubois T, Abdel-Rahman EM, Adan MS, Mohamed SA. Landscape Vegetation Productivity Influences Population Dynamics of Key Pests in Small Avocado Farms in Kenya. Insects. 2020; 11(7):424. https://doi.org/10.3390/insects11070424
Chicago/Turabian StyleToukem, Nadia K., Abdullahi A. Yusuf, Thomas Dubois, Elfatih M. Abdel-Rahman, Marian Salim Adan, and Samira A. Mohamed. 2020. "Landscape Vegetation Productivity Influences Population Dynamics of Key Pests in Small Avocado Farms in Kenya" Insects 11, no. 7: 424. https://doi.org/10.3390/insects11070424
APA StyleToukem, N. K., Yusuf, A. A., Dubois, T., Abdel-Rahman, E. M., Adan, M. S., & Mohamed, S. A. (2020). Landscape Vegetation Productivity Influences Population Dynamics of Key Pests in Small Avocado Farms in Kenya. Insects, 11(7), 424. https://doi.org/10.3390/insects11070424






