Floral Resources for Trissolcus japonicus, a Parasitoid of Halyomorpha halys
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Colonies
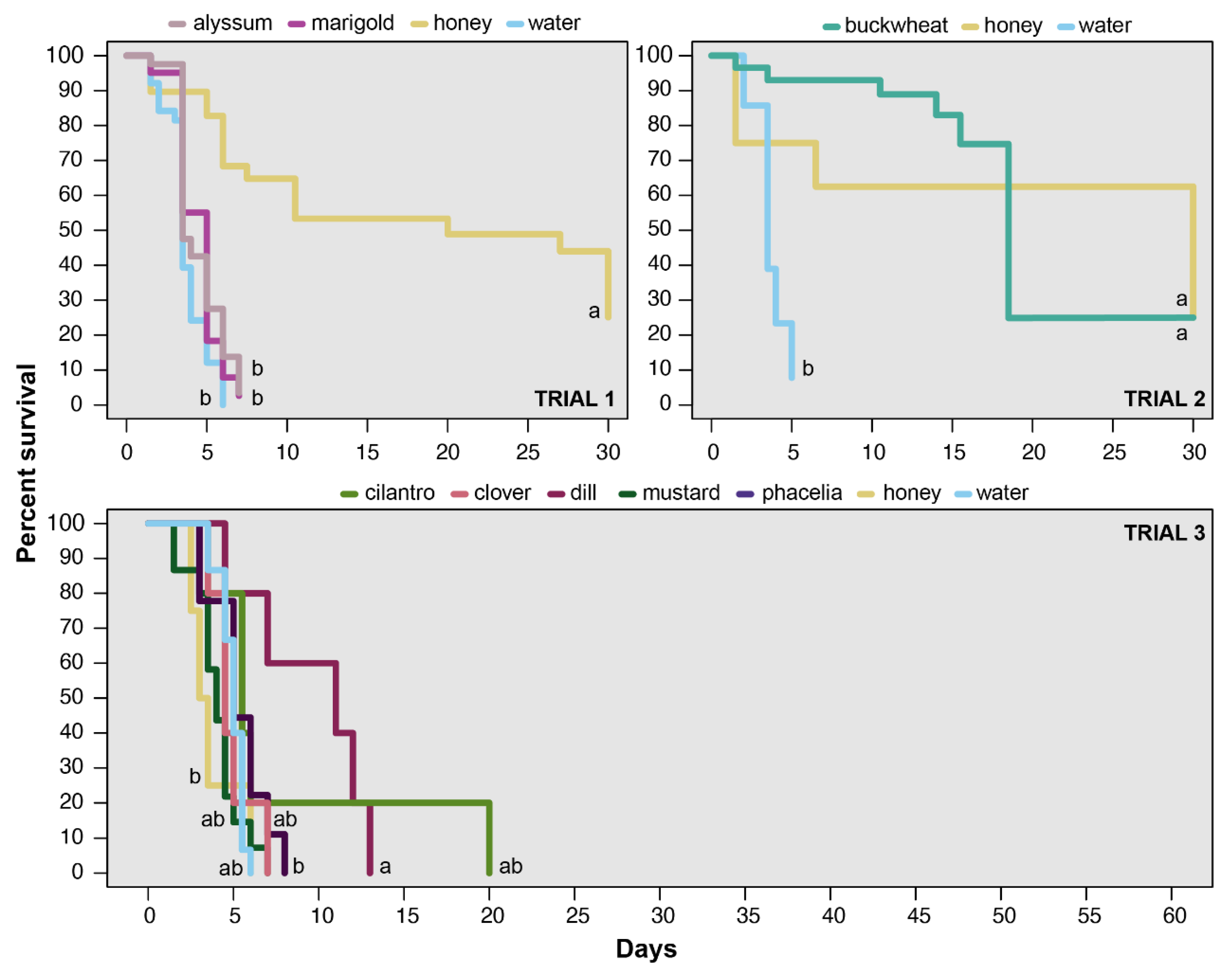
2.2. Survival/Longevity
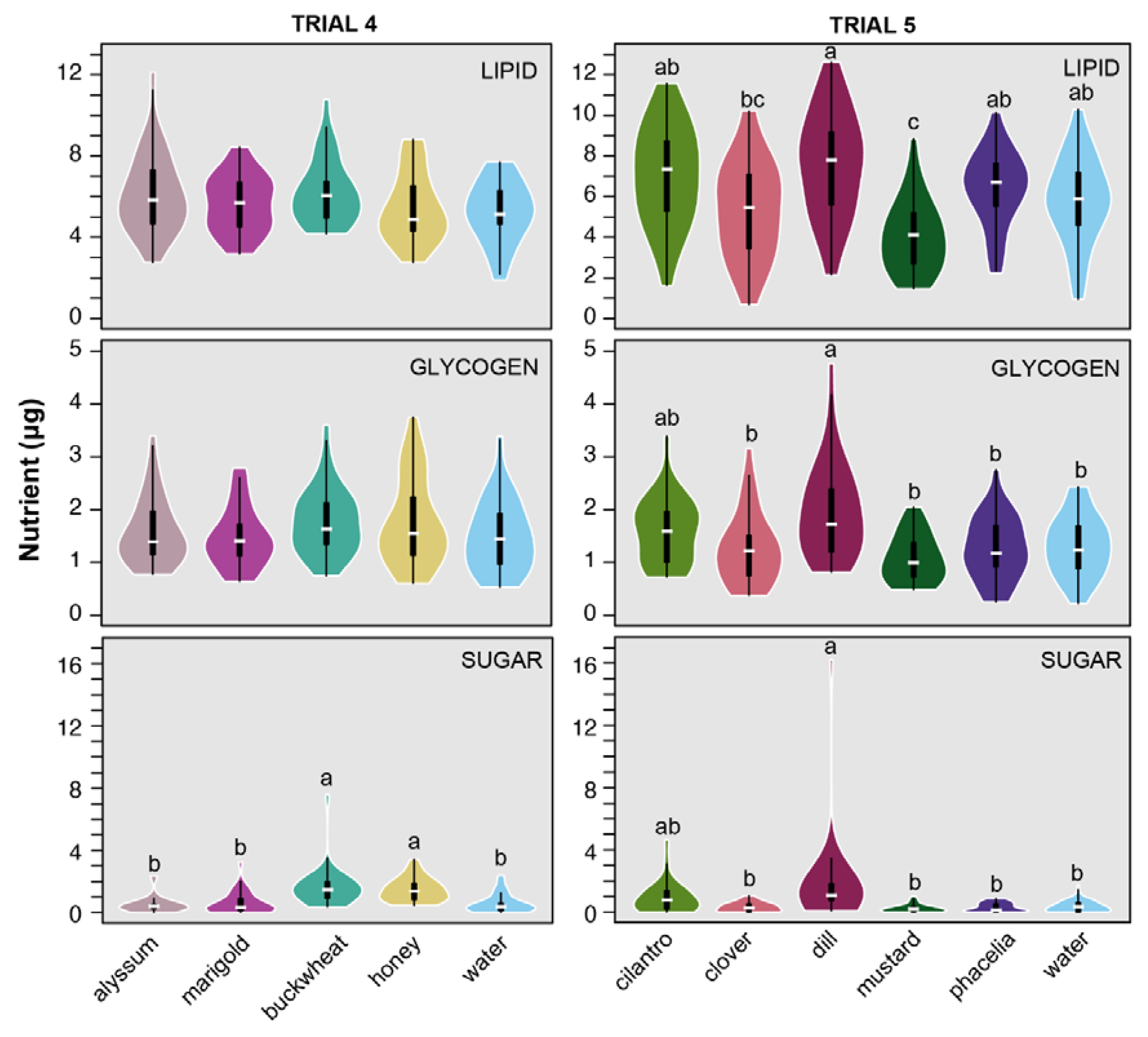
2.3. Nutrient Levels
2.4. Data Analysis
3. Results
3.1. Survival/Longevity
3.2. Nutrient Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Trial | χ2 | df | p < χ2 |
|---|---|---|---|
| 1 | 52.06 | 3 | <0.001 |
| 2 | 28.6 | 2 | <0.001 |
| 3 | 18.35 | 6 | 0.0054 |
References
- Hoebeke, E.R.; Carter, M.E. Halyomorpha halys (Stål) (Heteroptera: Pentatomidae): A polyphagous plant pest from Asia newly detected in North America. Proc. Entomol. Soc. Washingt. 2003, 105, 225–237. [Google Scholar]
- Fogain, R.; Graff, S. First Records of the Invasive Pest, Halyomorpha halys (Hemiptera: Pentatomidae), in Ontario and Quebec. J. Entomol. Soc. Ontario 2011, 142, 45–48. [Google Scholar]
- Wermelinger, B.; Wyniger, D.; Forster, B. First records of an invasive bug in Europe: Halyomorpha halys Stål (Heteroptera: Pentatomidae), a new pest on woody ornamentals and fruit trees? Bull. la Société Entomol. Suisse 2008, 81, 1–8. [Google Scholar]
- Faúndez, E.I.; Rider, D. The brown marmorated stink bug Halyomorpha halys (Stål, 1855) (Heteroptera: Pentatomidae) in Chile. Arq. Entomolóxicos 2017, 17, 305–307. [Google Scholar] [CrossRef]
- Leskey, T.C.; Nielsen, A.L. Impact of the Invasive Brown Marmorated Stink Bug in North America and Europe: History, Biology, Ecology, and Management. Annu. Rev. Entomol. 2018, 63, 599–618. [Google Scholar] [CrossRef]
- Rice, K.B.; Bergh, C.J.; Bergmann, E.J.; Biddinger, D.J.; Dieckhoff, C.; Dively, G.; Fraser, H.; Gariepy, T.; Hamilton, G.; Haye, T.; et al. Biology, Ecology, and Management of Brown Marmorated Stink Bug (Hemiptera: Pentatomidae). J. Integr. Pest. Manag. 2014, 5, 1–13. [Google Scholar] [CrossRef]
- Kuhar, T.P.; Kamminga, K. Review of the chemical control research on Halyomorpha halys in the USA. J. Pest. Sci. 2017, 90, 1021–1031. [Google Scholar] [CrossRef]
- Lee, D.-H.; Short, B.D.; Nielsen, A.L.; Leskey, T.C. Impact of Organic Insecticides on the Survivorship and Mobility of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) in the Laboratory. Fla. Entomol. 2014, 97, 414–421. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Y.; Qiu, L.; Li, Z.-X. A New Species of Trissolcus (Hymenoptera: Scelionidae) Parasitizing Eggs of Halyomorpha halys (Heteroptera: Pentatomidae) in China with Comments on Its Biology. Ann. Entomol. Soc. Am. 2009, 102, 39–47. [Google Scholar] [CrossRef]
- Talamas, E.J.; Herlihy, M.V.; Dieckhoff, C.; Hoelmer, K.A.; Buffington, M.L.; Bon, M.C.; Weber, D.C. Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae) emerges in North America. J. Hymenopt. Res. 2015, 43, 119–128. [Google Scholar] [CrossRef]
- Milnes, J.M.; Wiman, N.G.; Talamas, E.J.; Brunner, J.F.; Hoelmer, K.A.; Buffington, M.L.; Beers, E.H. Discovery of an Exotic Egg Parasitoid of the Brown Marmorated Stink Bug, Halyomorpha halys (Stål) in the Pacific Northwest. Proc. Entomol. Soc. Washingt. 2016, 118, 466–470. [Google Scholar] [CrossRef]
- Hedstrom, C.; Lowenstein, D.; Andrews, H.; Bai, B.; Wiman, N. Pentatomid host suitability and the discovery of introduced populations of Trissolcus japonicus in Oregon. J. Pest. Sci. 2017, 90, 1169–1179. [Google Scholar] [CrossRef]
- Jarrett, B.J.; Pote, J.; Talamas, E.; Gut, L.; Szucs, M. The Discovery of Trissolcus japonicus (Hymenoptera: Scelionidae) in Michigan. Gt. Lakes Entomol. 2019, 52, 5. [Google Scholar]
- Kaser, J.M.; Akotsen-Mensah, C.; Talamas, E.J.; Nielsen, A.L. First Report of Trissolcus japonicus Parasitizing Halyomorpha halys in North American Agriculture. Fla. Entomol. 2019, 101, 680. [Google Scholar] [CrossRef]
- Russell, M. A meta-analysis of physiological and behavioral responses of parasitoid wasps to flowers of individual plant species. Biol. Control 2015, 82, 96–103. [Google Scholar] [CrossRef]
- Lee, J.C.; Heimpel, G.E. Floral resources impact longevity and oviposition rate of a parasitoid in the field. J. Anim. Ecol. 2008, 77, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Segoli, M.; Rosenheim, J.A. Spatial and temporal variation in sugar availability for insect parasitoids in agricultural fields and consequences for reproductive success. Biol. Control 2013, 67, 163–169. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Jervis, M.A. Does floral nectar improve biological control by parasitoids. In Plant-Provided Food for Carnivorous Insects: A Protective Mutualism and Its Applications; Cambridge University Press: Cambridge, MA, USA, 2005; pp. 267–304. ISBN 9780511542220. [Google Scholar]
- Baggen, L.R.; Gurr, G.M.; Meats, A. Flowers in tri-trophic systems: Mechanisms allowing selective exploitation by insect natural enemies for conservation biological control. Entomol. Exp. Appl. 1999, 91, 155–161. [Google Scholar] [CrossRef]
- Arévalo, H.A.; Frank, J.H. Nectar Sources for Larra bicolor (Hymenoptera: Sphecidae), a Parasitoid of Scapteriscus Mole Crickets (Orthoptera: Gryllotalpidae), in Northern Florida. Fla. Entomol. 2005, 88, 146–151. [Google Scholar] [CrossRef]
- Vattala, H.D.; Wratten, S.D.; Phillips, C.B.; Wäckers, F.L. The influence of flower morphology and nectar quality on the longevity of a parasitoid biological control agent. Biol. Control 2006, 39, 179–185. [Google Scholar] [CrossRef]
- Patt, J.M.; Hamilton, G.C.; Lashomb, J.H. Foraging success of parasitoid wasps on flowers: Interplay of insect morphology, floral architecture and searching behavior. Entomol. Exp. Appl. 1997, 83, 21–30. [Google Scholar] [CrossRef]
- Koptur, S. Nectar as fuel for plant protectors. In Plant-Provided Food for Carnivorous Insects: A Protective Mutualism and Its Applications; Cambridge University Press: Cambridge, MA, USA, 2005; pp. 75–108. ISBN 9780511542220. [Google Scholar]
- Kaser, J.; Dieckhoff, C.; Hoelmer, K. Reproduction and sperm limitation of the egg parasitoid Trissolcus japonicus. Biol. Control. In preparation.
- Rahat, S.; Gurr, G.M.; Wratten, S.D.; Mo, J.; Neeson, R. Effect of plant nectars on adult longevity of the stinkbug parasitoid, Trissolcus basalis. Int. J. Pest. Manag. 2005, 51, 321–324. [Google Scholar] [CrossRef]
- Steiner, G. Nematodes parasitic on and associated with roots of marigolds (Tagetes hybrids). Proc. Biol. Soc. Washingt. 1941, 54, 31–34. [Google Scholar]
- Souza, I.L.; Marucci, R.C.; Silveira, L.C.P.; de Paulo, N.C.P.; Lee, J.C. Effects of marigold on the behavior, survival and nutrient reserves of Aphidius platensis. BioControl 2018, 63, 543–553. [Google Scholar] [CrossRef]
- Lee, J.C. What we can learn from the energetic levels of insects: A guide and review. Ann. Entomol. Soc. Am. 2019, 112, 220–226. [Google Scholar] [CrossRef]
- McIntosh, H.; Lowenstein, D.M.; Wiman, N.G.; Wong, J.S.; Lee, J.C. Parasitism of frozen Halyomorpha halys eggs by Trissolcus japonicus: Applications for rearing and experimentation. Biocontrol Sci. Technol. 2019, 29, 478–493. [Google Scholar] [CrossRef]
- Van Handel, E. Rapid determination of total lipids in mosquitoes. J. Am. Mosq. Control. Assoc. 1985, 1, 302–304. [Google Scholar]
- Van Handel, E. Rapid determination of glycogen and sugars in mosquitoes. J. Am. Mosq. Control. Assoc. 1985, 1, 299–301. [Google Scholar]
- SAS Version 9.3; SAS Institute Inc.: Cary, NC, USA, 2016.
- Hahn, D.A.; Denlinger, D.L. Meeting the energetic demands of insect diapause: Nutrient storage and utilization. J. Insect Physiol. 2007, 53, 760–773. [Google Scholar] [CrossRef]
- Beenakkers, A.M.T.; Van der Horst, D.J.; Van Marrewijk, W.J.A. Insect flight muscle metabolism. Insect Biochem. 1984, 14, 243–260. [Google Scholar] [CrossRef]
- Idris, A.B.; Grafius, E. Wildflowers as nectar sources for Diadegma insulare (Hymenoptera: Ichneumonidae), a parasitoid of diamondback moth (Lepidoptera: Yponomeutidae). Environ. Entomol. 1995, 24, 1726–1735. [Google Scholar] [CrossRef]
- Orr, D.B.; Pleasants, J.M. The potential of native prairie plant species to enhance the effectiveness of the Ostrinia nubilalis parasitoid Macrocentrus grandii. J. Kans. Entomol. Soc. 1996, 69, 133–143. [Google Scholar] [CrossRef]
- Foti, M.C.; Peri, E.; Wajnberg, E.; Colazza, S.; Rostás, M. Contrasting olfactory responses of two egg parasitoids to buckwheat floral scent are reflected in field parasitism rates. J. Pest. Sci. 2019, 92, 747–756. [Google Scholar] [CrossRef]
- Foti, M.C.; Rostás, M.; Peri, E.; Park, K.C.; Slimani, T.; Wratten, S.D.; Colazza, S. Chemical ecology meets conservation biological control: Identifying plant volatiles as predictors of floral resource suitability for an egg parasitoid of stink bugs. J. Pest. Sci. 2017, 90, 299–310. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat Management to Conserve Natural Enemies of Arthropod Pests in Agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]

| Trial | Measurement | Sex | Treatment (Number of Wasps, Number of Vials) | Dates |
|---|---|---|---|---|
| 1 | survival (30 d) | f | alyssum (51, 15), marigold (58, 16), honey (47, 13), water (50, 14) | May–August 2017 |
| m | alyssum (41, 15), marigold (41, 16), honey (29, 13), water (36, 14) | |||
| 2 | survival (30 d) | f | buckwheat (49, 13), honey (15, 3), water (16, 3) | September 2017–March 2018 |
| m | buckwheat (29, 13), honey (8, 3), water (14, 3) | |||
| 3 | survival (until dead) | f | cilantro (35, 5), crimson clover (35, 5), dill (35, 5), mustard (36, 7), phacelia (35, 5), honey (35, 5), water (99, 15) | June–September 2019 |
| m | cilantro (5, 5), crimson clover (5, 5), dill (5, 5), mustard (11, 7), phacelia (5, 5), honey (15, 5), water (15, 15) | |||
| 4 | nutrient levels (2 d) | f | alyssum (30, 9), marigold (30, 7), buckwheat (30, 7), honey (30, 9), water (30, 9) | 6–26 June 2018 |
| 5 | nutrient levels (2 d) | f | cilantro (30, 4), crimson clover (30, 4), dill (30, 4), mustard (30, 5), phacelia (30, 4), water (50, 9) | June–July 2019 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McIntosh, H.R.; Skillman, V.P.; Galindo, G.; Lee, J.C. Floral Resources for Trissolcus japonicus, a Parasitoid of Halyomorpha halys. Insects 2020, 11, 413. https://doi.org/10.3390/insects11070413
McIntosh HR, Skillman VP, Galindo G, Lee JC. Floral Resources for Trissolcus japonicus, a Parasitoid of Halyomorpha halys. Insects. 2020; 11(7):413. https://doi.org/10.3390/insects11070413
Chicago/Turabian StyleMcIntosh, Hanna R., Victoria P. Skillman, Gracie Galindo, and Jana C. Lee. 2020. "Floral Resources for Trissolcus japonicus, a Parasitoid of Halyomorpha halys" Insects 11, no. 7: 413. https://doi.org/10.3390/insects11070413
APA StyleMcIntosh, H. R., Skillman, V. P., Galindo, G., & Lee, J. C. (2020). Floral Resources for Trissolcus japonicus, a Parasitoid of Halyomorpha halys. Insects, 11(7), 413. https://doi.org/10.3390/insects11070413






