Suitability and Profitability of a Cereal Aphid for the Parasitoid Aphidius platensis in the Context of Conservation Biological Control of Myzus persicae in Orchards
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.1.1. Parasitoids
2.1.2. Aphid Hosts
2.1.3. Insect Material for the Experiments
2.2. Host Preference Assay: Choice Experiment
2.3. Profitability Assay: Non-choice Experiment
2.4. Statistical Analysis
2.4.1. Choice Experiment
2.4.2. Profitability Assay
3. Results
3.1. Preliminary Experiment
3.2. Choice Experiment
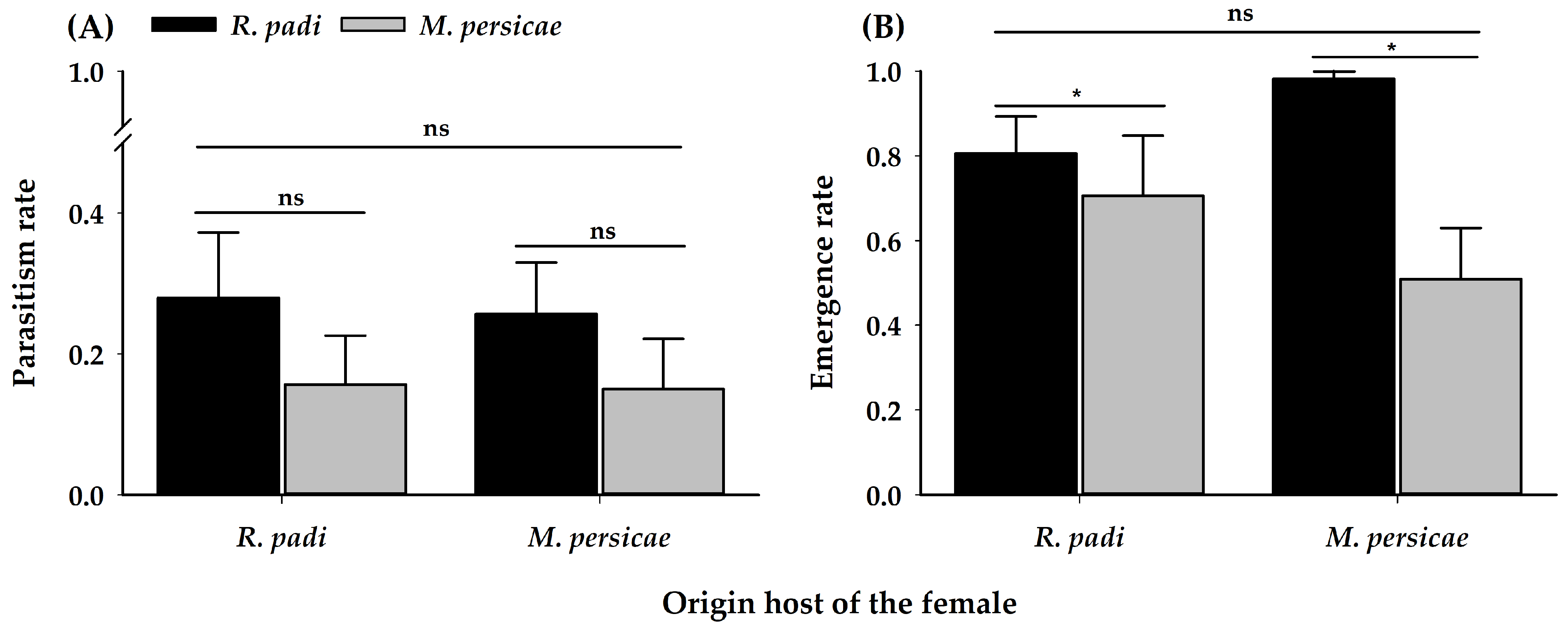
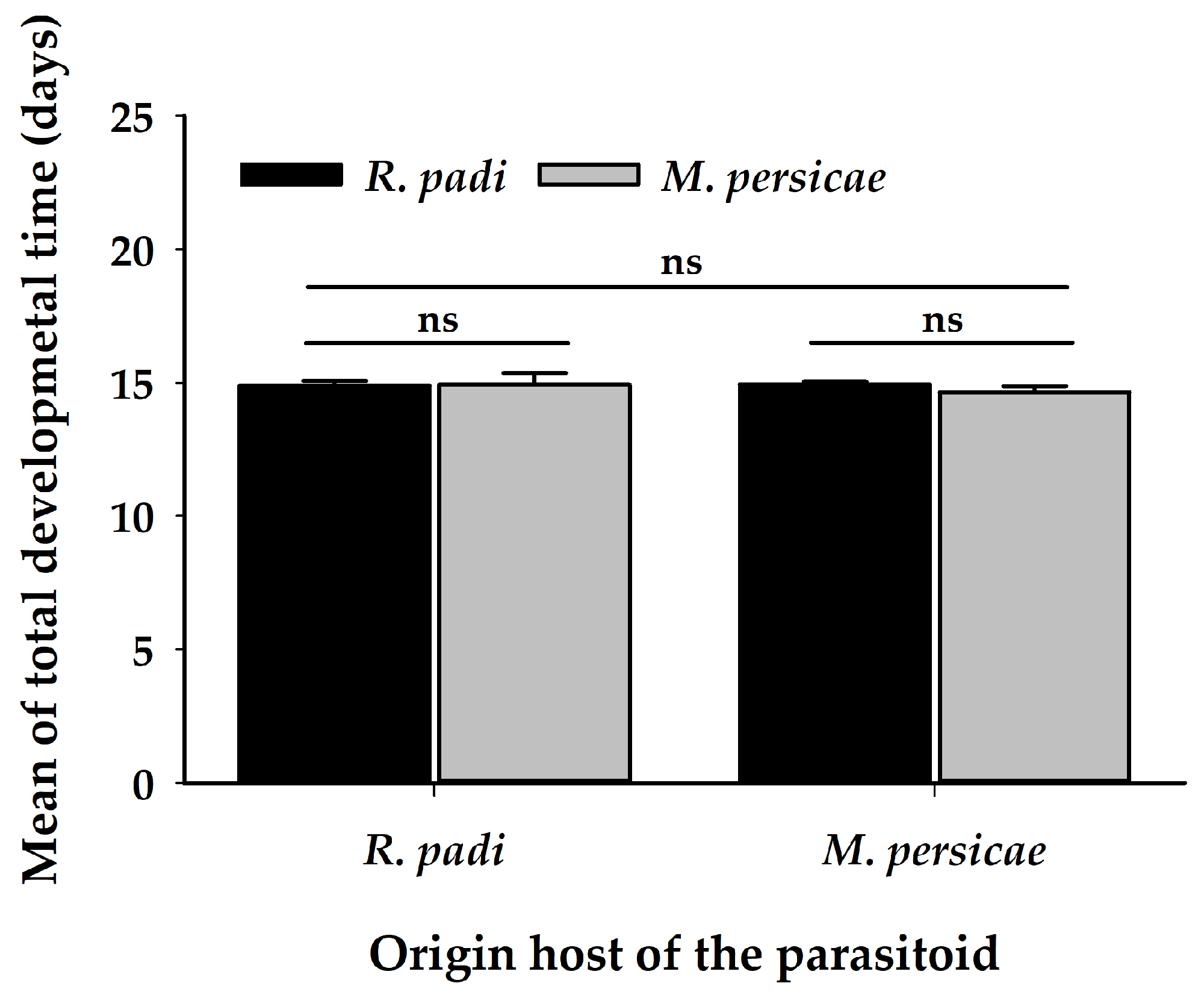
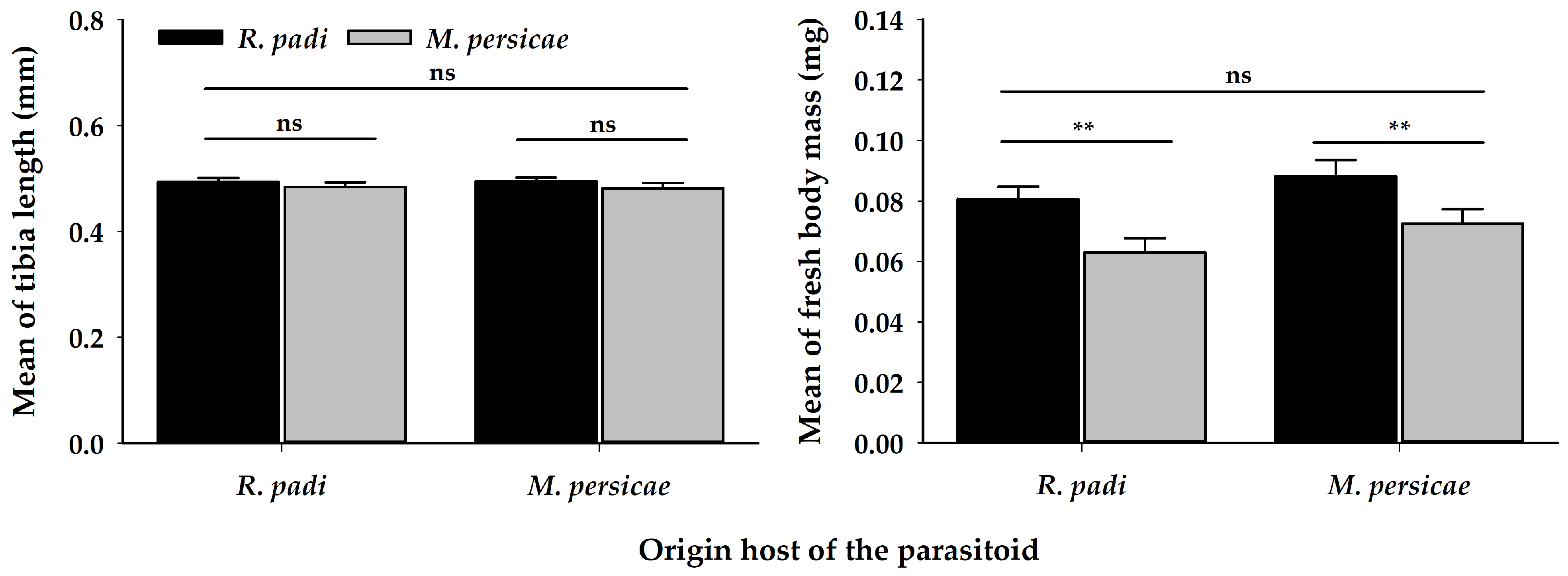
3.3. Profitability Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cardinale, B.J.; Srivastava, D.S.; Duffy, J.E.; Wright, J.P.; Downing, A.L.; Sankaran, M.; Jouseau, C.; Cadotte, M.W.; Carroll, I.T.; Weis, J.J.; et al. Effects of biodiversity on the functioning of ecosystems: A summary of 164 experimental manipulations of species richness. Ecology 2009, 90, 854. [Google Scholar] [CrossRef]
- Pickett, C.H.; Roltsch, W.; Corbett, A. The role of rubidium marked natural enemy refuge in the establishment and movement of Bemisia parasitoids. Int. J. Pest Manag. 2004, 50, 183–191. [Google Scholar] [CrossRef]
- Quijas, S.; Schmid, B.; Balvanera, P. Plant diversity enhances provision of ecosystem services: A new synthesis. Basic Appl. Ecol. 2010, 11, 582–593. [Google Scholar] [CrossRef]
- Fiedler, A.K.; Landis, D.A. Attractiveness of michigan native plants to arthropod natural enemies and herbivores. Environ. Entomol. 2015, 36, 751–765. [Google Scholar] [CrossRef]
- Gurr, G.; Wratten, S. Biological Control: Measures of Success, 1st ed.; Gurr, G., Wratten, S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; ISBN 978-1-4020-1052-1. [Google Scholar]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]
- Holland, J.M.; Bianchi, F.J.; Entling, M.H.; Moonen, A.C.; Smith, B.M.; Jeanneret, P. Structure, function and management of semi-natural habitats for conservation biological control: A review of European studies. Pest Manag. Sci. 2016, 72, 1638–1651. [Google Scholar] [CrossRef]
- Begg, G.S.; Cook, S.M.; Dye, R.; Ferrante, M.; Franck, P.; Lavigne, C.; Lövei, G.L.; Mansion-Vaquie, A.; Pell, J.K.; Petit, S.; et al. A functional overview of conservation biological control. Crop. Prot. 2017, 97, 145–158. [Google Scholar] [CrossRef]
- Fiedler, A.K.; Landis, D.A.; Wratten, S.D. Maximizing ecosystem services from conservation biological control: The role of habitat management. Biol. Control 2008, 45, 254–271. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Ellsworth, P.C. Fifty years of the integrated control concept: Moving the model and implementation forward in Arizona. Pest Manag. Sci. 2009, 65, 1267–1286. [Google Scholar] [CrossRef]
- Bottrell, D.G.; Schoenly, K.G. Resurrecting the ghost of green revolutions past: The brown planthopper as a recurring threat to high-yielding rice production in tropical Asia. J. Asia Pac. Entomol. 2012, 15, 122–140. [Google Scholar] [CrossRef]
- Byrne, M.L.; Christie, M.E.; Luke, T.W. Insect pest management in Tropical asian irrigated rice. Annu. Rev. Entomol. 2011, 45, 549–574. [Google Scholar]
- Wyckhuys, K.A.G.; Lu, Y.; Morales, H.; Vazquez, L.L.; Legaspi, J.C.; Eliopoulos, P.A.; Hernandez, L.M. Current status and potential of conservation biological control for agriculture in the developing world. Biol. Control 2013, 65, 152–167. [Google Scholar] [CrossRef]
- Onzo, A.; Hanna, R.; Negloh, K.; Toko, M.; Sabelis, M.W. Biological control of cassava green mite with exotic and indigenous phytoseiid predators-Effects of intraguild predation and supplementary food. Biol. Control 2005, 33, 143–152. [Google Scholar] [CrossRef]
- Pretty, J.; Bharucha, Z.P. Integrated pest management for sustainable intensification of agriculture in Asia and Africa. Insects 2015, 6, 152–182. [Google Scholar] [CrossRef] [PubMed]
- Peñalver-Cruz, A.; Alvarez-Baca, J.K.; Alfaro-Tapia, A.; Gontijo, L.; Lavandero, B. Manipulation of agricultural habitats to improve conservation biological control in South America. Neotrop. Entomol. 2019, 48, 875–898. [Google Scholar] [CrossRef]
- Irvin, N.A.; Bistline-East, A.; Hoddle, M.S. The effect of an irrigated buckwheat cover crop on grape vine productivity, and beneficial insect and grape pest abundance in southern California. Biol. Control 2016, 93, 72–83. [Google Scholar] [CrossRef]
- Landis, D.A. Designing agricultural landscapes for biodiversity-based ecosystem services. Basic Appl. Ecol. 2017, 18, 1–12. [Google Scholar] [CrossRef]
- Welch, K.D.; Harwood, J.D. Temporal dynamics of natural enemy-pest interactions in a changing environment. Biol. Control 2014, 75, 18–27. [Google Scholar] [CrossRef]
- Rusch, A.; Bommarco, R.; Ekbom, B. Conservation biological control in agricultural landscapes. Adv. Bot. Res. 2016, 81, 1–28. [Google Scholar]
- Lundgren, J.G.; Wyckhuys, K.A.G.; Desneux, N. Population responses by Orius insidiosus to vegetational diversity. BioControl 2009, 54, 135–142. [Google Scholar] [CrossRef]
- Gómez-Marco, F.; Urbaneja, A.; Tena, A. A sown grass cover enriched with wild forb plants improves the biological control of aphids in citrus. Basic Appl. Ecol. 2016, 17, 210–219. [Google Scholar] [CrossRef]
- Irvin, N.A.; Scarratt, S.L.; Wratten, S.D.; Frampton, C.M.; Chapman, R.B.; Tylianakis, J.M. The effects of floral understoreys on parasitism of leafrollers (Lepidoptera: Tortricidae) on apples in New Zealand. Agric. Entomol. 2006, 8, 25–34. [Google Scholar] [CrossRef]
- Frank, S.D. Biological control of arthropod pests using banker plant systems: Past progress and future directions. Biol. Control 2010, 52, 8–16. [Google Scholar] [CrossRef]
- MacArthur, R.; Pianka, E. On optimal use of a patchy environment. Am. Nat. 1966, 100, 603–609. [Google Scholar] [CrossRef]
- Jaenike, J. On optimal oviposition behavior in phytophagous insects. Theor. Popul. Biol. 1978, 14, 350–356. [Google Scholar] [CrossRef]
- Chesnais, Q.; Ameline, A.; Doury, G.; Le Roux, V.; Couty, A. Aphid parasitoid mothers don’t always know best through the whole host selection process. PLoS ONE 2015, 10, 1–16. [Google Scholar] [CrossRef]
- Gripenberg, S.; Mayhew, P.J.; Parnell, M.; Roslin, T. A meta-analysis of preference-performance relationships in phytophagous insects. Ecol. Lett. 2010, 13, 383–393. [Google Scholar] [CrossRef]
- Henry, L.M.; Gillespie, D.R.; Roitberg, B.D. Does mother really know best? Oviposition preference reduces reproductive performance in the generalist parasitoid Aphidius ervi. Entomol. Exp. Appl. 2005, 116, 167–174. [Google Scholar] [CrossRef]
- Chau, A.; Mackauer, M. Preference of the aphid parasitoid Monoctonus paulensis (Hymenoptera: Braconidae, Aphidiinae) for different aphid species: Female choice and offspring survival. Biol. Control 2001, 20, 30–38. [Google Scholar] [CrossRef]
- Mackauer, M.; Michaud, J.P.; Völkl, W. Host choice by aphidiid parasitoids (Hymenoptera: Aphidiidae): Host recognition, host quality, and host value. Can. Entomol. 1996, 128, 959–980. [Google Scholar] [CrossRef]
- Eoche-Bosy, D.; Outreman, Y.; Oliveira Andrade, T.; Krespi, L.; van Baaren, J. Seasonal variations of host resources influence foraging strategy in parasitoids. Entomol. Exp. Appl. 2016, 161, 11–19. [Google Scholar] [CrossRef]
- Godfray, H.C.J. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994; ISBN 9780691000473. [Google Scholar]
- Straub, C.S.; Ives, A.R.; Gratton, C. Evidence for a trade-off between host-range breadth and host-use efficiency in aphid parasitoids. Am. Nat. 2011, 177, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Drès, M.; Mallet, J. Host races in plant-feeding insects and their importance in sympatric speciation. Philos. Trans. R. Soc. B Biol. Sci. 2002, 357, 471–492. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.F. Differentiation in the Control of Pest Species. In Insect Outbreak Revisited; Barbosa, P., Letourneau, D., Agrawal, A., Eds.; Blackwell Publishing LTD: Hoboken, NJ, USA, 2012; pp. 291–310. [Google Scholar]
- Forbes, A.A.; Powell, T.H.Q.; Stelinski, L.L.; Smith, J.J.; Feder, J.L. Sequential sympatric speciation across trophic levels. Science 2009, 323, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Stamps, J.A. The effect of natal experience on habitat preferences. Trends Ecol. Evol. 2004, 19, 411–416. [Google Scholar] [CrossRef]
- Henry, L.M.; Roitberg, B.D.; Gillespie, D.R. Host-range evolution in Aphidius parasitoids: Fidelity, virulence and fitness trade-offs on an ancestral host. Evolution 2008, 62, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Henry, L.M.; Ma, B.O.; Roitberg, B.D. Size-mediated adaptive foraging: A host-selection strategy for insect parasitoids. Oecologia 2009, 161, 433–445. [Google Scholar] [CrossRef]
- Fuentes-Contreras, E.; Muñoz, R.; Niemeyer, H.M. Diversity of aphids (Hemiptera Aphidoidea) in Chile. Rev. Chil. Hist. Nat. 1997, 70, 531–542. [Google Scholar]
- Nieto Nafría, J.M.; Fuentes-Contreras, E.; Castro Colmenero, M.; Aldea Piera, M.; Ortego, J.; Mier Durante, M.P.; Durante, M.P.M. Catálogo de los áfidos (Hemiptera, Aphididae) de Chile, con plantas hospedadoras y distribuciones regional y provincial. Graellsia 2016, 72, 50. [Google Scholar] [CrossRef][Green Version]
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gutbrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2014, 51, 41–51. [Google Scholar] [CrossRef]
- Fuentes-Contreras, E.; Figueroa, C.; Reyes, M.; Briones, L.; Niemeyer, H. Genetic diversity and insecticide resistance of Myzus persicae (Hemiptera:Aphididae) populations from tobacco in Chile: Evidence for the existence of a single predominant clone. Bull. Entomol. Res. 2004, 94, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Lavandero, B.; Miranda, M.; Ramirez, C.C.; Fuentes-Contreras, E. Landscape composition modulates population genetic structure of Eriosoma lanigerum (Hausmann) on Malus domestica Borkh in central Chile. Bull. Entomol. Res. 2009, 99, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, K.S.; Loxdale, H.D.; Harrington, R.; Brookes, C.P.; Clark, S.J.; Sunnucks, P. Migration and genetic structure of the grain aphid (Sitobion avenae) in Britain related to climate and clonal fluctuation as revealed using microsatellites. Mol. Ecol. 2003, 12, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Blackman, R.; Eastop, V. Aphids on the World’s Crops: An Identification and Information Guide, 2nd ed.; John Wiley & Sons: Chichester, UK, 2000; ISBN 9780471851912. [Google Scholar]
- Ffrench-Constant, R.H. The molecular genetics of insecticide resistance. Genetics 2013, 194, 807–815. [Google Scholar] [CrossRef]
- Starý, P. The Aphidiidae of Chile. Dtsch. Èntomol. Z. 1995, 42, 113–138. [Google Scholar]
- Starý, P. Aphidius platensis Brethes, its distribution and host range (Hym.: Aphidiidae). Orient. Insects 1972, 6, 359–370. [Google Scholar] [CrossRef]
- Zepeda-Paulo, F.A.; Ortiz-Martínez, S.A.; Figueroa, C.C.; Lavandero, B. Adaptive evolution of a generalist parasitoid: Implications for the effectiveness of biological control agents. Evol. Appl. 2013, 6, 983–999. [Google Scholar] [CrossRef]
- Tomanovic, Ž.; Petrovic, A.; Mitrovic, M.; Kavallieratos, N.; Stary, P.; Rakhshani, E.; Rashanipour, M.; Popovic, A.; Shukshuk, A.; Ivanovic, A. Molecular and morphological variability within the Aphidius colemani group with redescription of Aphidius platensis Brethes (Hymenoptera: Braconidae: Aphidiinae). Bull. Entomol. Res. 2014, 104, 552–565. [Google Scholar] [CrossRef]
- Starý, P. Biology of Aphids Parasites (Hymenoptera: Aphidiidae) with Respect to Integrated Control; Springer: The Hague, The Netherlands, 1970; ISBN 978-90-6193-116-4. [Google Scholar]
- Rakhshani, E.; Barahoei, H.; Ahmad, Z.; Starý, P.; Ghafouri-Moghaddam, M.; Mehrparvar, M.; Kavallieratos, N.G.; Čkrkić, J.; Tomanović, Ž. Review of aphidiinae parasitoids (hymenoptera: Braconidae) of the middle east and north Africa: Key to species and host associations. Eur. J. Taxon. 2019, 2019, 1–132. [Google Scholar] [CrossRef]
- Hwang, H.S.; Jung, D.O.; Kim, J.W.; Lee, K.Y. Molecular identification of Aphidius transcaspicus (Hymenoptera: Braconidae: Aphidiinae), a closely associated species of Aphidius colemani, in Korea. J. Asia Pac. Entomol. 2018, 21, 1246–1252. [Google Scholar] [CrossRef]
- Van Baaren, J.; Héterier, V.; Hance, T.; Krespi, L.; Cortesero, A.M.; Poinsot, D.; Le Ralec, A.; Outreman, Y. Playing the hare or the tortoise in parasitoids: Could different oviposition strategies have an influence in host partitioning in two Aphidius species? Ethol. Ecol. Evol. 2004, 16, 231–242. [Google Scholar] [CrossRef]
- Le Lann, C.; Outreman, Y.; Van Alphen, J.J.M.; Krespi, L.; Pierre, J.S.; Van Baaren, J. Do past experience and competitive ability influence foraging strategies of parasitoids under interspecific competition? Ecol. Entomol. 2008, 33, 691–700. [Google Scholar] [CrossRef]
- Le Lann, C.; Wardziak, T.; van Baaren, J.; van Alphen, J.J.M. Thermal plasticity of metabolic rates linked to life-history traits and foraging behaviour in a parasitic wasp. Funct. Ecol. 2011, 25, 641–651. [Google Scholar] [CrossRef]
- Le Ralec, A.; Anselme, C.; Outreman, Y.; Poirié, M.; Van Baaren, J.; Le Lann, C.C.; Van Alphen, J.J.M. Evolutionary ecology of the interactions between aphids and their parasitoids. C. R. Biol. 2010, 333, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Ottoni, E.B. EthoLog 2.2: A tool for the transcription and timing of behavior observation sessions. Behav. Res. Methods Instrum. Comput. 2000, 32, 446–449. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Henry, L.M.; Roitberg, B.D. Parasitoids. In Encyclopedia of Animal Behavior; Breed, M.D., Moore, J., Eds.; Academic Press: Cambridge, MA, USA, 2010; pp. 651–656. ISBN 978-0-08-045337-8. [Google Scholar]
- Antolin, M.F.; Bjorksten, T.A.; Vaughn, T.T. Host-related fitness trade-offs in a presumed generalist parasitoid, Diaeretiella rapae (Hymenoptera: Aphidiidae). Ecol. Entomol. 2006, 31, 242–254. [Google Scholar] [CrossRef]
- Henry, L.M.; May, N.; Acheampong, S.; Gillespie, D.R.; Roitberg, B.D. Host-adapted parasitoids in biological control: Does source matter? Ecol. Appl. 2010, 20, 242–250. [Google Scholar] [CrossRef]
- Liang, K.; Zeger, S. Longitudinal data analysis using generalized linear models. Biometrika 1986, 73, 13–22. [Google Scholar] [CrossRef]
- Hardin, J.W.; Hilbe, J.M. Generalized Estimating Equations, 2nd ed.; Chapman & Hall/CRC: New York, NY, USA, 2012; ISBN 978-1-4398-8113-2. [Google Scholar]
- McCullagh, P.; Nelder, J. Generalized Linear Models, 2nd ed.; Chapman & Hall/CRC: New York, NY, USA, 1989; ISBN 9780412317606. [Google Scholar]
- Harrison, X.A.; Donaldson, L.; Correa-Cano, M.E.; Evans, J.; Fisher, D.N.; Goodwin, C.E.D.; Robinson, B.S.; Hodgson, D.J.; Inger, R. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 2018, 2018, 1–32. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- He, X.Z.; Wang, Q.; Teulon, D.A.J. Host age preference behavior in Aphidius ervi Haliday (Hymenoptera: Aphidiidae). J. Insect Behav. 2011, 24, 447–455. [Google Scholar] [CrossRef]
- Cloutier, C.; Duperron, J.; Tertuliano, M.; McNeil, J.N. Host instar, body size and fitness in the koinobiotic parasitoid Aphidius nigripes. Entomol. Exp. Appl. 2000, 97, 29–40. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; Stone, L.; Desneux, N.; Hoelmer, K.A.; Hopper, K.R.; Heimpel, G.E. Parasitism of the soybean aphid, Aphis glycines by Binodoxys communis: The role of aphid defensive behaviour and parasitoid reproductive performance. Bull. Entomol. Res. 2008, 98, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Messing, R.H.; Rabasse, J.M. Oviposition behaviour of the polyphagous aphid parasitoid Aphidius colemani Viereck (Hymenoptera: Aphidiidae). Agric. Ecosyst. Environ. 1995, 52, 13–17. [Google Scholar] [CrossRef]
- Perdikis, D.C.; Lykouressis, D.P.; Garantonakis, N.G.; Iatrou, S.A. Instar preference and parasitization of Aphis gossypii and Myzus persicae (Hemiptera: Aphididae) by the parasitoid Aphidius colemani (Hymenoptera: Aphidiidae). Eur. J. Entomol. 2004, 101, 333–336. [Google Scholar] [CrossRef]
- Starý, P. The fate of released parasitoids (Hymenoptera, Braconidae, Aphidiinae) for biological-control in Chile. Bull. Entomol. Res. 1993, 83, 633–639. [Google Scholar] [CrossRef]
- Starý, P. Aphidius colemani Viereck: Its taxonomy, distribution and host range (Hymenoptera, Aphidiidae). Acta Entomol. Bohemoslov. 1975, 72, 156–173. [Google Scholar]
- Santos, C.; Sampaio, M.; Lau, D.; Redaelli, L.; Jahnke, S.; Pivato, J.; Carvalho, F. Taxonomic status and population oscillations of Aphidius colemani species group (Hymenoptera: Braconidae) in Southern Brazil. Neotrop. Entomol. 2019, 48, 983–991. [Google Scholar] [CrossRef]
- Gianoli, E. Competition in cereal aphids (Homoptera: Aphididae) on wheat plants. Environ. Entomol. 2000, 29, 213–219. [Google Scholar] [CrossRef]
- Crispo, E. Modifying effects of phenotypic plasticity on interactions among natural selection, adaptation and gene flow. J. Evol. Biol. 2008, 21, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Martel, V.; Darrouzet, E.; Boivin, G. Phenotypic plasticity in the reproductive traits of a parasitoid. J. Insect Physiol. 2011, 57, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Thibert-Plante, X.; Hendry, A.P. The consequences of phenotypic plasticity for ecological speciation. J. Evol. Biol. 2011, 24, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Calvert, D.J. Experimental host preferences of Monoctonus paulensis (Hymenoptera: Braconidae), including a hypothetical scheme of host selection. Ann. Entomol. Soc. Am. 1973, 66, 28–33. [Google Scholar] [CrossRef]
- Ortiz-Martínez, S.; Pierre, J.S.; van Baaren, J.; Le Lann, C.; Zepeda-Paulo, F.; Lavandero, B. Interspecific competition among aphid parasitoids: Molecular approaches reveal preferential exploitation of parasitized hosts. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Barta, R.J.; Hoelmer, K.A.; Hopper, K.R.; Heimpel, G.E. Multifaceted determinants of host specificity in an aphid parasitoid. Oecologia 2009, 160, 387–398. [Google Scholar] [CrossRef] [PubMed]
- De Farias, A.M.I.; Hopper, K.R. Oviposition behavior of Aphelinus asychis (Hymenoptera. Aphelinidae) and Aphidius matricariae (Hymenoptera: Aphidiidae) and defense behavior of their host Diuraphis noxia (Homoptera: Aphididae). Environ. Entomol. 1999, 28, 858–862. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Rosenheim, J.A.; Mangel, M. Predation on adult Aphytis parasitoids in the field. Oecologia 1997, 110, 346–352. [Google Scholar] [CrossRef]
- Ode, P.J.; Hopper, K.R.; Coll, M. Oviposition vs. offspring fitness in Aphidius colemani parasitizing different aphid species. Entomol. Exp. Appl. 2005, 115, 303–310. [Google Scholar] [CrossRef]
- Kouamé, K.L.; Mackauer, M. Influence of aphid size, age and behaviour on host choice by the parasitoid wasp Ephedrus californicus: A test of host-size models. Oecologia 1991, 88, 197–203. [Google Scholar] [CrossRef]
- Cohen, J.E.; Jonsson, T.; Müller, C.B.; Godfray, H.C.J.; Van Savage, M. Body sizes of hosts and parasitoids in individual feeding relationships. Proc. Natl. Acad. Sci. USA 2005, 102, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Shu-sheng, L. Development, adult size and fecundity of Aphidius sonchi reared in two instars of its aphid host, Hyperomyzus lactucae. Entomol. Exp. Appl. 1985, 37, 41–48. [Google Scholar] [CrossRef]
- Sequeira, R.; Mackauer, M. The nutritional ecology of a parasitoid wasp, Ephedrus californicus Baker (Hymenoptera: Aphidiidae). Can. Entomol. 1993, 125, 423–430. [Google Scholar] [CrossRef]
- Giles, K.L.; Madden, R.D.; Stockland, R.; Payton, M.E.; Dillwith, J.W. Host plants affect predator fitness via the nutritional value of herbivore prey: Investigation of a plant-aphid-ladybeetle system. BioControl 2002, 47, 1–21. [Google Scholar] [CrossRef]
- Douglas, A.E. The Nutritional Physiology of Aphids; Academic Press: Cambridge, MA, USA, 2003; Volume 31, ISBN 0120242311. [Google Scholar]
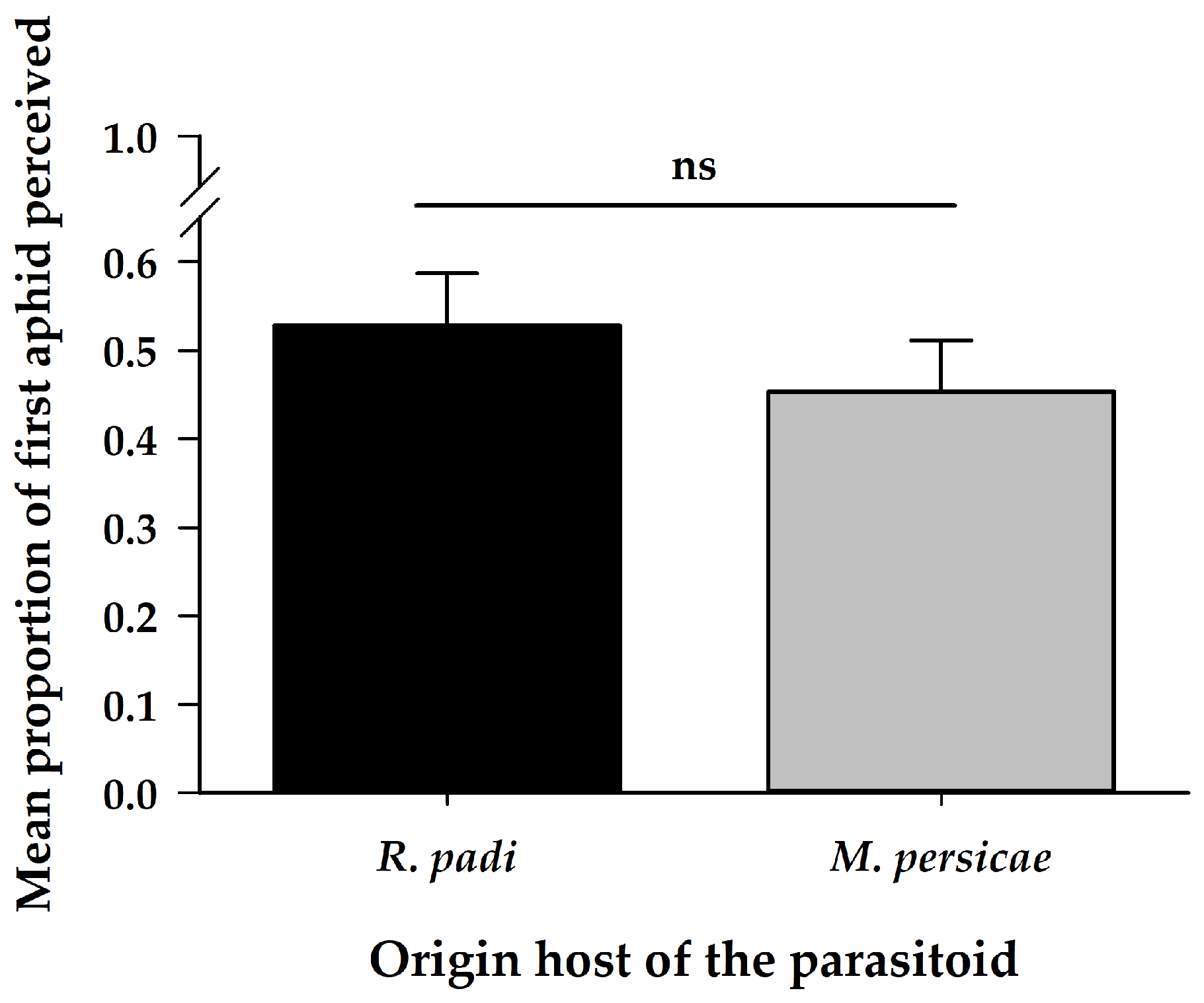
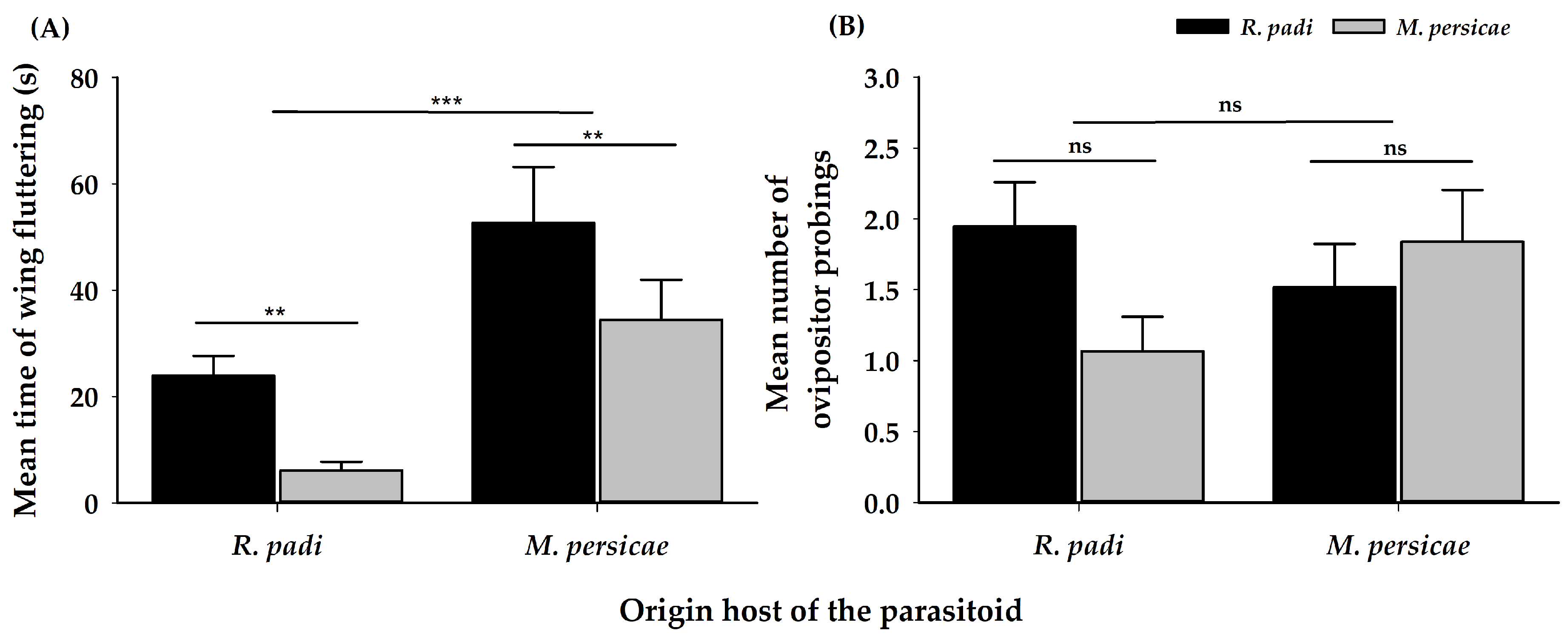
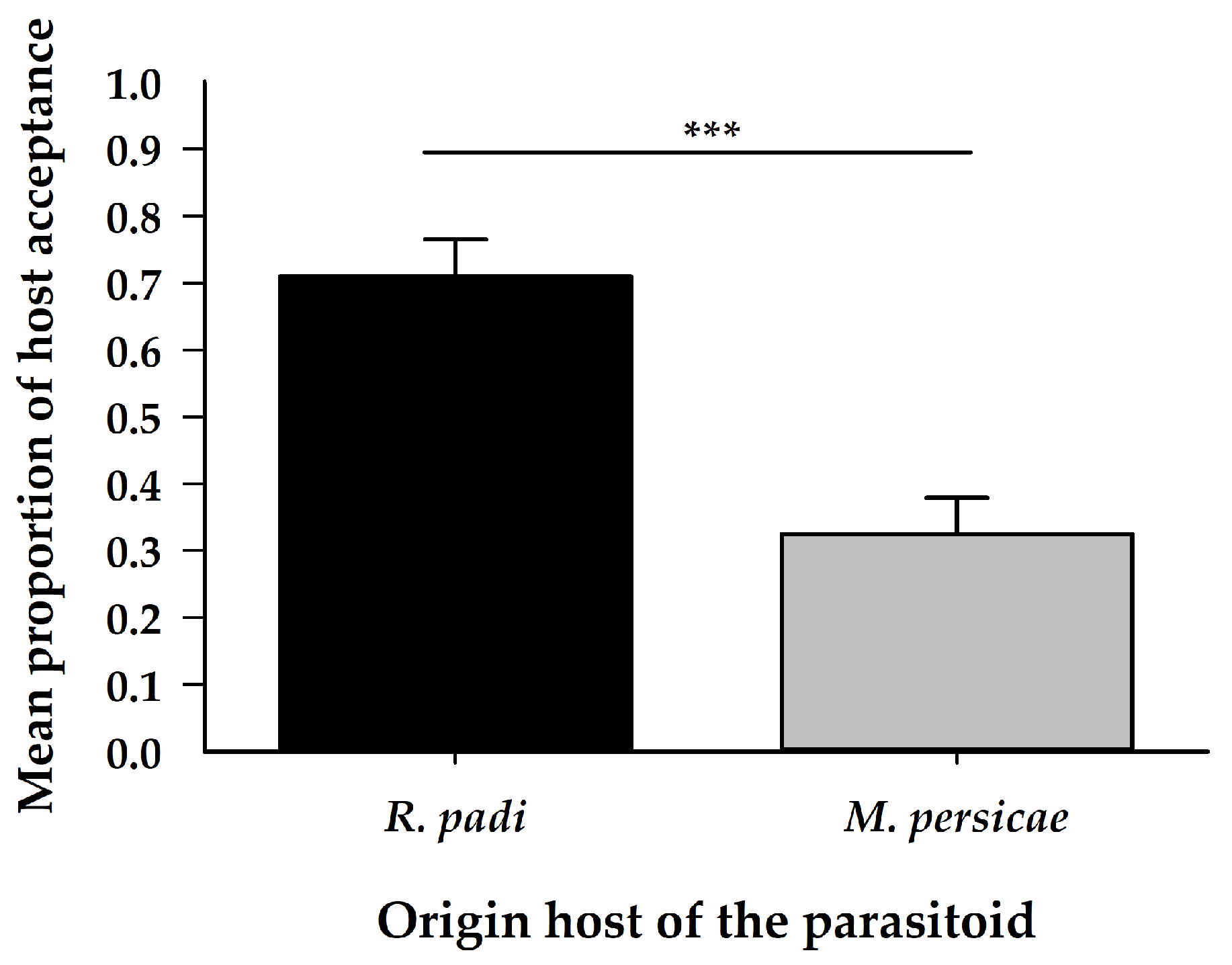

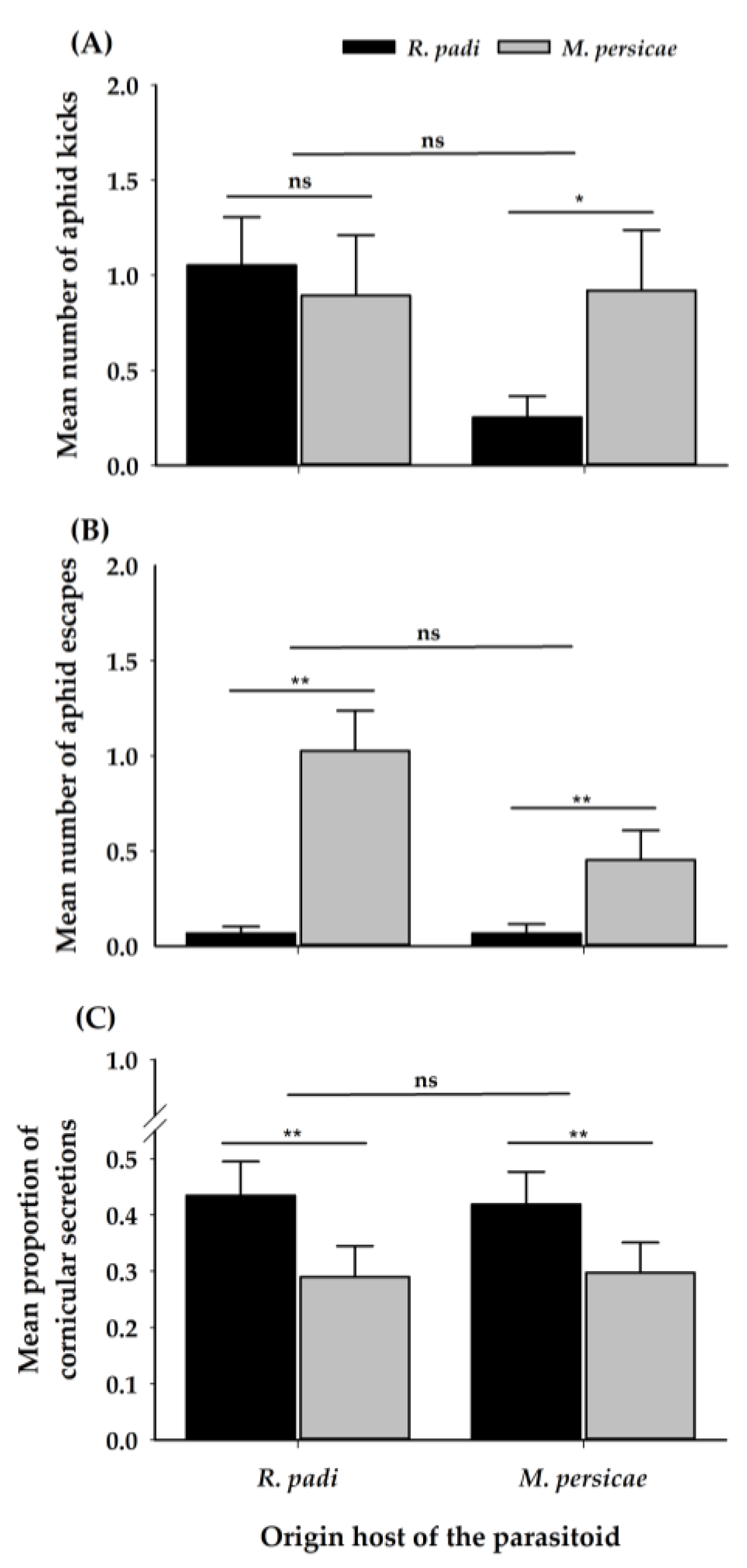
| Variables | Origin | Chosen | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| df | X2 | p-Value | df | X2 | p-value | df | X2 | p-Value | |
| First aphid perceived | 1 | 1.08 | 0.30 | - | - | ||||
| Ovipositor probing | 1 | 0.25 | 0.62 | 1 | 0.65 | 0.42 | 1 | 3.05 | 0.08 |
| Host acceptance | 1 | 14.50 | 1.4 × 10−4 | - | - | ||||
| Handling time for oviposition | 1 | 11.62 | 6.5 × 10−4 | 1 | 1.67 | 0.19 | 1 | 0.06 | 0.81 |
| Wing fluttering | 1 | 13.70 | 2.2 × 10−4 | 1 | 6.00 | 0.01 | 1 | 0.00 | 0.98 |
| Aphid kicking | 1 | 1.79 | 0.18 | 1 | 10.82 | 0.37 | 1 | 3.82 | 0.05 |
| Aphid escaping | 1 | 2.04 | 0.15 | 1 | 20.97 | 4.7 × 10−6 | 1 | 0.61 | 0.44 |
| Cornicular secretions | 1 | 0.00 | 0.97 | 1 | 4.51 | 0.03 | 1 | 0.03 | 0.87 |
| Variables | Origin | Tested | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| df | X2 | p-Value | df | X2 | p-Value | df | X2 | p-Value | |
| Parasitism rate 1 | 1 | 0.04 | 0.84 | 1 | 2.54 | 0.11 | 1 | 0.00 | 0.95 |
| Emergence 2 | 1 | 1.01 | 0.32 | 1 | 4.86 | 0.03 | 1 | 0.51 | 0.47 |
| Total developmental time 2 | 1 | 0.02 | 0.88 | 1 | 0.59 | 0.44 | 1 | 1.13 | 0.29 |
| Tibia length 1 | 1 | 0.00 | 0.99 | 1 | 1.56 | 0.21 | 1 | 0.04 | 0.84 |
| Fresh body mass 1 | 1 | 2.18 | 0.14 | 1 | 7.43 | 0.01 | 1 | 0.08 | 0.78 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Baca, J.K.; Alfaro-Tapia, A.; Lavandero, B.; Le Lann, C.; Van Baaren, J. Suitability and Profitability of a Cereal Aphid for the Parasitoid Aphidius platensis in the Context of Conservation Biological Control of Myzus persicae in Orchards. Insects 2020, 11, 381. https://doi.org/10.3390/insects11060381
Alvarez-Baca JK, Alfaro-Tapia A, Lavandero B, Le Lann C, Van Baaren J. Suitability and Profitability of a Cereal Aphid for the Parasitoid Aphidius platensis in the Context of Conservation Biological Control of Myzus persicae in Orchards. Insects. 2020; 11(6):381. https://doi.org/10.3390/insects11060381
Chicago/Turabian StyleAlvarez-Baca, Jeniffer K., Armando Alfaro-Tapia, Blas Lavandero, Cécile Le Lann, and Joan Van Baaren. 2020. "Suitability and Profitability of a Cereal Aphid for the Parasitoid Aphidius platensis in the Context of Conservation Biological Control of Myzus persicae in Orchards" Insects 11, no. 6: 381. https://doi.org/10.3390/insects11060381
APA StyleAlvarez-Baca, J. K., Alfaro-Tapia, A., Lavandero, B., Le Lann, C., & Van Baaren, J. (2020). Suitability and Profitability of a Cereal Aphid for the Parasitoid Aphidius platensis in the Context of Conservation Biological Control of Myzus persicae in Orchards. Insects, 11(6), 381. https://doi.org/10.3390/insects11060381






