Bioactive Excreted/Secreted Products of Entomopathogenic Nematode Heterorhabditis bacteriophora Inhibit the Phenoloxidase Activity during the Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect and Nematodes
2.2. Nematode Virulence Assay
2.3. Isolation of ESPs
2.4. Proteolytic Activity of ESPs
2.5. Purification of ESPs
2.6. Phenoloxidase Assay
2.7. Mass Spectrometry
2.8. Statistical Analysis
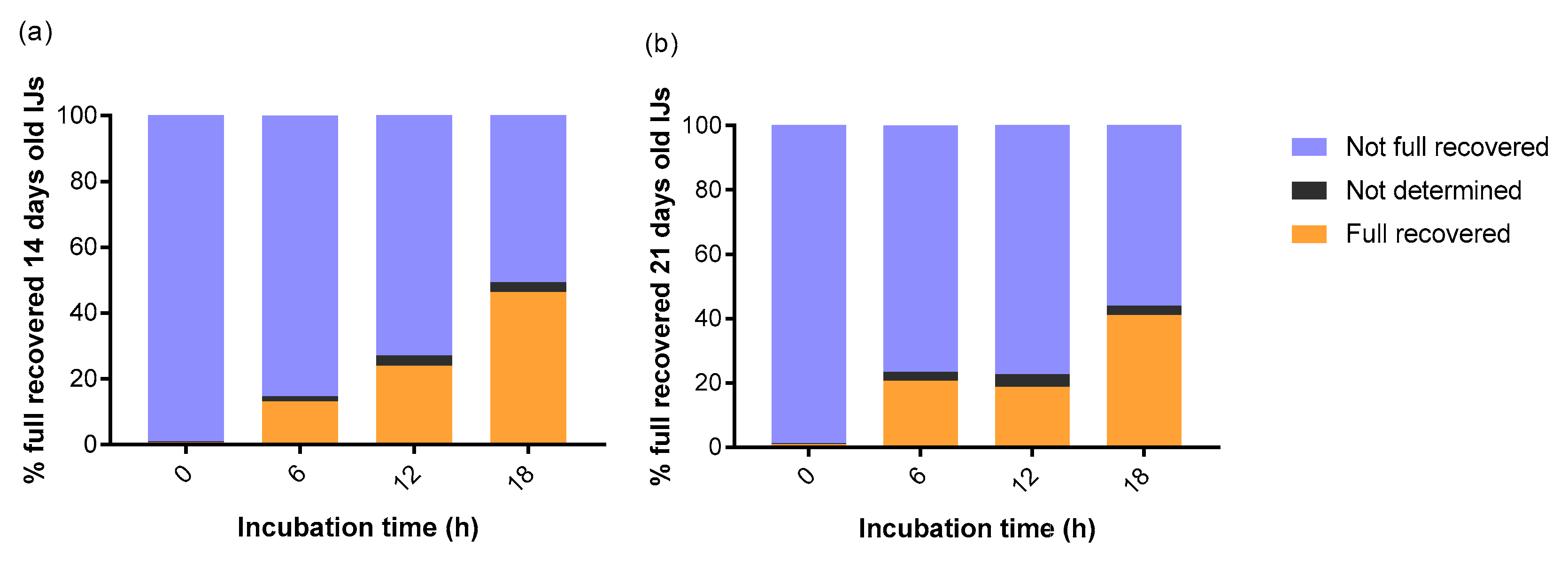
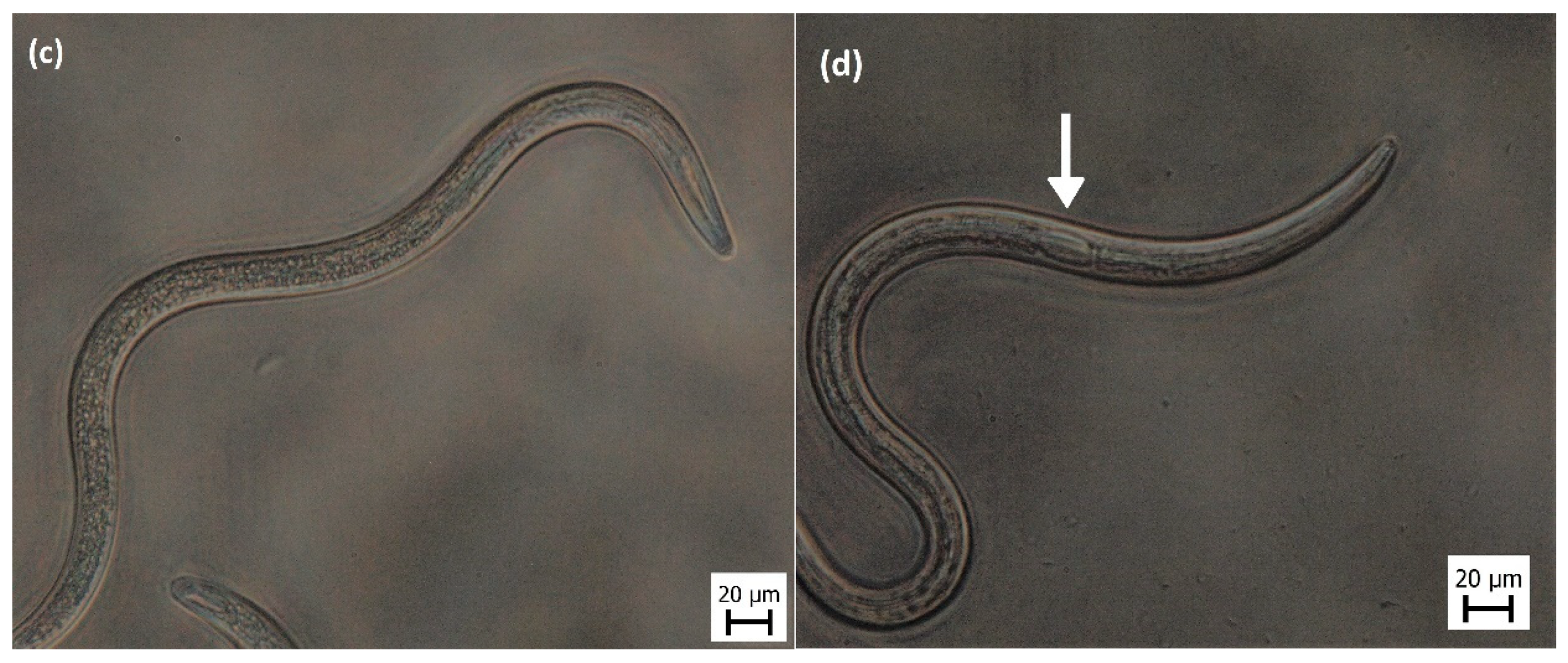
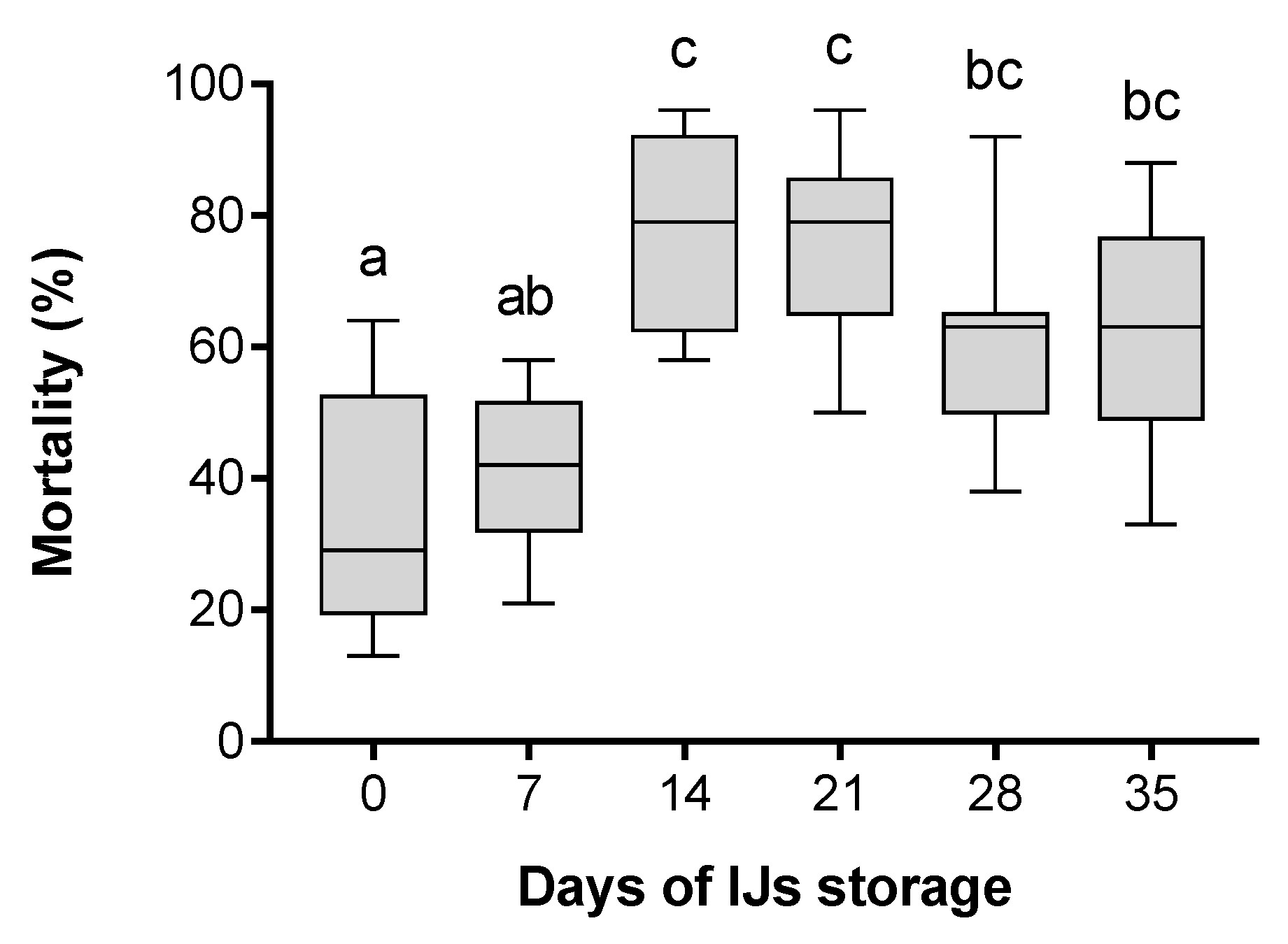
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| N | Total 1 | %Cov (95%) 2 | % Conf. Score 3 | Accessions | Modifications 4 | Cleavages 5 | dMass 6 | Observed MW 7 | Observed m/z 8 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 5.703 | 99.00 | GG5294|c2_g1_i8 | Carbamidomethyl(C)@12 | −0.0939 | 1733.8923 | 867.9534 | |
| 2 | 2 | 6.227 | 99.00 | GG7279|c2_g1_i6 | missed K-N@13; missed K-E@15 | −0.0881 | 1909.9857 | 956.0001 | |
| 3 | 2 | 12.750 | 99.00 | GG2408|c0_g1_i2 | missed K-Q@1 | −0.0379 | 2261.0654 | 1131.5400 | |
| 4 | 2 | 8.531 | 99.00 | GG16163|c66_g1_i9 | Carbamidomethyl(C)@18 | missed K-E@2; missed R-V@16 | 0.0996 | 2030.1835 | 1016.0990 |
| 5 | 2 | 6.522 | 99.00 | GG15187|c0_g1_i1 | missed K-E@4; missed R-Q@8 | −0.0823 | 1909.9438 | 955.9792 | |
| 6 | 2 | 5.015 | 99.00 | GG10285|c3_g1_i4 | Carbamidomethyl(C)@17 | missed K-L@12; missed R-Y@14 | 0.0959 | 1947.0917 | 974.5531 |
| N | Theoretical MW 9 | Theoretical m/z 10 | Theoretical z 11 | Score 12 | Spectrum 13 | Time 14 | Elution Peak Width (Peptide) 15 | Acquisition Time 16 | Intensity (Peptide) 17 | Precursor Intensity Acquisition 18 | MS2Counts 19 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1733.9862 | 868.0004 | 2 | 3 | 1.1.1.710.22 | 11.19 | 1.11 | 11.2966 | 271.28 | 237.27 | 39.1209 |
| 2 | 1910.0737 | 956.0442 | 2 | 3 | 1.1.1.715.28 | 11.63 | 0.27 | 11.5227 | 197.27 | 171.01 | 21.9854 |
| 3 | 2261.1035 | 1131.5590 | 2 | 3 | 1.1.1.762.37 | 13.71 | 0.71 | 13.6088 | 208.23 | 191.34 | 8.9114 |
| 4 | 2030.0830 | 1016.0490 | 2 | 3 | 1.1.1.724.32 | 11.90 | 0.26 | 11.9234 | 239.11 | 215.69 | 20.2243 |
| 5 | 1910.0261 | 956.0204 | 2 | 3 | 1.1.1.723.19 | 11.63 | 0.84 | 11.8707 | 253.12 | 182.25 | 20.9448 |
| 6 | 1946.9959 | 974.5052 | 2 | 3 | 1.1.1.762.25 | 13.62 | 1.63 | 13.5994 | 171.85 | 161.82 | 17.1024 |
References
- Georgis, R.; Koppenhöfer, A.M.; Lacey, L.A.; Bélair, G.; Duncan, L.W.; Grewal, P.S.; Samish, M.; Tan, L.; Torr, P.; van Tol, R.W.H.M. Successes and failures in the use of parasitic nematodes for pest control. Biol. Control 2006, 38, 103–123. [Google Scholar] [CrossRef]
- Ehlers, R.-U. Mass production of entomopathogenic nematodes for plant protection. Appl. Microbiol. Biotechnol. 2001, 56, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Lacey, L.A.; Georgis, R. Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J. Nematol. 2012, 44, 218–225. [Google Scholar] [PubMed]
- Dillman, A.R.; Sternberg, P.W. Entomopathogenic nematodes. Curr. Biol. 2012, 22, R430–R431. [Google Scholar] [CrossRef]
- Ibrahim, E.; Dobeš, P.; Kunc, M.; Hyršl, P.; Kodrík, D. Adipokinetic hormone and adenosine interfere with nematobacterial infection and locomotion in Drosophila melanogaster. J. Insect Physiol. 2018, 107, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Arefin, B.; Kucerova, L.; Dobes, P.; Markus, R.; Strnad, H.; Wang, Z.; Hyrsl, P.; Zurovec, M.; Theopold, U. Genome-wide transcriptional analysis of Drosophila larvae infected by entomopathogenic nematodes shows involvement of complement, recognition and extracellular matrix proteins. J. Innate Immun. 2014, 6, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Kunc, M.; Arefin, B.; Hyrsl, P.; Theopold, U. Monitoring the effect of pathogenic nematodes on locomotion of Drosophila larvae. Fly 2017, 11, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.E.; Gaugler, R.; Harrison, R. Entomopathogenic nematode host finding: Response to host contact cues by cruise and ambush foragers. Parasitology 1992, 105, 309–315. [Google Scholar] [CrossRef]
- Lewis, E.E.; Selvan, S.; Campbell, J.F.; Gaugler, R. Changes in foraging behaviour during the infective stage of entomopathogenic nematodes. Parasitology 1995, 110, 583–590. [Google Scholar] [CrossRef]
- Lewis, E.E.; Campbell, J.; Griffin, C.; Kaya, H.; Peters, A. Behavioral ecology of entomopathogenic nematodes. Biol. Control 2006, 38, 66–79. [Google Scholar] [CrossRef]
- Griffin, C.T. Perspectives on the behavior of entomopathogenic nematodes from dispersal to reproduction: Traits contributing to nematode fitness and biocontrol efficacy. J. Nematol. 2012, 44, 177–184. [Google Scholar] [PubMed]
- Campbell, J.F.; Lewis, E.E.; Stock, S.P.; Nadler, S.; Kaya, H.K. Evolution of host search strategies in entomopathogenic nematodes. J. Nematol. 2003, 35, 142–145. [Google Scholar] [PubMed]
- Shapiro-Ilan, D.I.; Han, R.; Dolinksi, C. Entomopathogenic nematode production and application technology. J. Nematol. 2012, 44, 206–217. [Google Scholar] [PubMed]
- Koppenhöfer, A.M.; Grewal, P.S.; Fuzy, E.M. Differences in penetration routes and establishment rates of four entomopathogenic nematode species into four white grub species. J. Invertebr. Pathol. 2007, 94, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Gao, A.; Li, B.; Wang, M.; Shan, L. Two symbiotic bacteria of the entomopathogenic nematode Heterorhabditis spp. against Galleria mellonella. Toxicon 2017, 127, 85–89. [Google Scholar] [CrossRef]
- Bode, H.B. Entomopathogenic bacteria as a source of secondary metabolites. Curr. Opin. Chem. Biol. 2009, 13, 224–230. [Google Scholar] [CrossRef]
- Rodou, A.; Ankrah, D.O.; Stathopoulos, C. Toxins and secretion systems of photorhabdus luminescens. Toxins 2010, 2, 1250–1264. [Google Scholar] [CrossRef]
- Han, R.; Ehlers, R. Pathogenicity, development, and reproduction of heterorhabditis bacteriophora and Steinernema carpocapsae under axenic in vivo conditions. J. Invertebr. Pathol. 2000, 75, 55–58. [Google Scholar] [CrossRef]
- Sicard, M.; Le Brun, N.; Pages, S.; Godelle, B.; Boemare, N.; Moulia, C. Effect of native xenorhabdus on the fitness of their steinernema hosts: Contrasting types of interaction. Parasitol. Res. 2003, 91, 520–524. [Google Scholar] [CrossRef]
- Ehlers, R.; Wulff, A.; Peters, A. Pathogenicity of axenic Steinernema feltiae, Xenorhabdus bovienii, and the bacto–helminthic complex to larvae of tipula oleracea (Diptera) and galleria mellonella (Lepidoptera). J. Invertebr. Pathol. 1997, 69, 212–217. [Google Scholar] [CrossRef]
- Grewal, P.S.; Peters, A. Formulation and quality. In Nematodes as Biocontrol Agents; CABI: Wallingford, UK, 2005; pp. 79–90. ISBN 0851990177. [Google Scholar]
- Cerenius, L.; Lee, B.L.; Söderhäll, K. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. 2008, 29, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Söderhäll, K.; Cerenius, L. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol. 1998, 10, 23–28. [Google Scholar] [CrossRef]
- Balasubramanian, N.; Hao, Y.-J.; Toubarro, D.; Nascimento, G.; Simões, N. Purification, biochemical and molecular analysis of a chymotrypsin protease with prophenoloxidase suppression activity from the entomopathogenic nematode Steinernema carpocapsae. Int. J. Parasitol. 2009, 39, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.-J.; Montiel, R.; Nascimento, G.; Toubarro, D.; Simoes, N. Identification and expression analysis of the Steinernema carpocapsae elastase-like serine protease gene during the parasitic stage. Exp. Parasitol. 2009, 122, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Toubarro, D.; Lucena-Robles, M.; Nascimento, G.; Costa, G.; Montiel, R.; Coelho, A.V.; Simões, N. An apoptosis-inducing serine protease secreted by the entomopathogenic nematode Steinernema carpocapsae. Int. J. Parasitol. 2009, 39, 1319–1330. [Google Scholar] [CrossRef]
- Balasubramanian, N.; Toubarro, D.; Simões, N. Biochemical study and in vitro insect immune suppression by a trypsin-like secreted protease from the nematode Steinernema carpocapsae. Parasite Immunol. 2010, 32, 165–175. [Google Scholar] [CrossRef]
- Balasubramanian, N.; Nascimento, G.; Ferreira, R.; Martinez, M.; Simões, N. Pepsin-like aspartic protease (Sc-ASP155) cloning, molecular characterization and gene expression analysis in developmental stages of nematode Steinernema carpocapsae. Gene 2012, 500, 164–171. [Google Scholar] [CrossRef]
- Balasubramanian, N.; Toubarro, D.; Nascimento, G.; Ferreira, R.; Simões, N. Purification, molecular characterization and gene expression analysis of an aspartic protease (Sc-ASP113) from the nematode Steinernema carpocapsae during the parasitic stage. Mol. Biochem. Parasitol. 2012, 182, 37–44. [Google Scholar] [CrossRef]
- Toubarro, D.; Avila, M.M.; Hao, Y.; Balasubramanian, N.; Jing, Y.; Montiel, R.; Faria, T.Q.; Brito, R.M.; Simões, N. A serpin released by an entomopathogen impairs clot formation in insect defense system. PLoS ONE 2013, 8, e69161. [Google Scholar] [CrossRef]
- Lu, D.; Macchietto, M.; Chang, D.; Barros, M.M.; Baldwin, J.; Mortazavi, A.; Dillman, A.R. Activated entomopathogenic nematode infective juveniles release lethal venom proteins. PLoS Pathog. 2017, 13, e1006302. [Google Scholar] [CrossRef]
- Toubarro, D.; Avila, M.M.; Montiel, R.; Simões, N. A pathogenic nematode targets recognition proteins to avoid insect defenses. PLoS ONE 2013, 8, e75691. [Google Scholar] [CrossRef] [PubMed]
- Toubarro, D.; Lucena-Robles, M.; Nascimento, G.; Santos, R.; Montiel, R.; Veríssimo, P.; Pires, E.; Faro, C.; Coelho, A.V.; Simões, N. Serine protease-mediated host invasion by the parasitic nematode Steinernema carpocapsae. J. Biol. Chem. 2010, 285, 30666–30675. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Toubarro, D.; Hao, Y.; Simões, N. Cloning, characterisation and heterologous expression of an astacin metalloprotease, Sc-AST, from the entomoparasitic nematode Steinernema carpocapsae. Mol. Biochem. Parasitol. 2010, 174, 101–108. [Google Scholar] [CrossRef]
- Kenney, E.; Hawdon, J.M.; O’Halloran, D.; Eleftherianos, I. Heterorhabditis bacteriophora excreted-secreted products enable infection by photorhabdus luminescens through suppression of the imd pathway. Front. Immunol. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Harnett, M.M.; Kean, D.E.; Boitelle, A.; McGuiness, S.; Thalhamer, T.; Steiger, C.N.; Egan, C.; Al-Riyami, L.; Alcocer, M.J.; Houston, K.M.; et al. The phosphorycholine moiety of the filarial nematode immunomodulator ES-62 is responsible for its anti-inflammatory action in arthritis. Ann. Rheum. Dis. 2008, 67, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Ditgen, D.; Anandarajah, E.M.; Meissner, K.A.; Brattig, N.; Wrenger, C.; Liebau, E. Harnessing the helminth secretome for therapeutic immunomodulators. Biomed. Res. Int. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, C.; Navarro, S.; Wangchuk, P.; Wilson, D.; Daly, N.L.; Loukas, A. Identifying the immunomodulatory components of helminths. Parasite Immunol. 2015, 37, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Jančaříková, G.; Houser, J.; Dobeš, P.; Demo, G.; Hyršl, P.; Wimmerová, M. Characterization of novel bangle lectin from Photorhabdus asymbiotica with dual sugar-binding specificity and its effect on host immunity. PLoS Pathog. 2017, 13, e1006564. [Google Scholar] [CrossRef]
- Wagenaar, M.M.; Gibson, D.M.; Clardy, J. Akanthomycin, a new antibiotic pyridone from the entomopathogenic fungus akanthomyces gracilis. Org. Lett. 2002, 4, 671–673. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Nakajima, I.; Ihara, F.; Kinoshita, H.; Nihira, T. Cultivation of entomopathogenic fungi for the search of antibacterial compounds. Mycopathologia 2005, 160, 321–325. [Google Scholar] [CrossRef]
- Haydak, M.H. A food for rearing laboratory animals. J. Econ. Entomol. 1936, 29, 1026. [Google Scholar]
- Rosa, J.S.; Bonifassi, E.; Amaral, J.; Lacey, L.A.; Simões, N.; Laumond, C. Natural occurrence of entomopathogenic nematodes (rhabditida: Steinernema, heterorhabditis) in the azores. J. Nematol. 2000, 32, 215–222. [Google Scholar] [PubMed]
- Chang, D.Z.; Serra, L.; Lu, D.; Mortazavi, A.; Dillman, A.R. A core set of venom proteins is released by entomopathogenic nematodes in the genus Steinernema. PLoS Pathog. 2019, 15, e1007626. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Adams, B.J.; Ciche, T.A.; Clifton, S.; Gaugler, R.; Kim, K.; Spieth, J.; Sternberg, P.W.; Wilson, R.K.; Grewal, P.S. A lover and a fighter: The genome sequence of an entomopathogenic nematode heterorhabditis bacteriophora. PLoS ONE 2013, 8, e69618. [Google Scholar] [CrossRef] [PubMed]
- Shapiro-llan, D.I. Virulence of entomopathogenic nematodes to pecan weevil (Coleoptera: Curculionidae) adults. J. Entomol. Sci. 2001, 36, 325–328. [Google Scholar] [CrossRef]
- De Batista, E.S.P.; Auad, A.M.; Andaló, V.; de Monteiro, C.M.O. Virulence of entomopathogenic nematodes (Rhabditida: Steinernematidae, Heterorhabditidae) to spittlebug Mahanarva spectabilis (Hemiptera: Cercopidae). Arq. Inst. Biol. 2014, 81, 145–149. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Grewal, P.S.; Fuzy, E.M. Virulence of the entomopathogenic nematodes Heterorhabditis bacteriophora, Heterorhabditis zealandica, and Steinernema scarabaei against five white grub species (Coleoptera: Scarabaeidae) of economic importance in turfgrass in North America. Biol. Control 2006, 38, 397–404. [Google Scholar] [CrossRef]
- Del Valle, E.E.; Frizzo, L.S.; Lax, P.; Bonora, J.S.; Palma, L.; Bernardi Desch, N.P.; Pietrobón, M.; Doucet, M.E. Biological control of Diloboderus abderus (Coleoptera: Scarabaeidae) larvae using Steinernema rarum CUL (Nematoda: Steinernematidae) and Heterorhabditis bacteriophora SMC (Nematoda: Heterorhabditidae). Crop. Prot. 2017, 98, 184–190. [Google Scholar] [CrossRef]
- Crook, M. The dauer hypothesis and the evolution of parasitism: 20 years on and still going strong. Int. J. Parasitol. 2014, 44, 1–8. [Google Scholar] [CrossRef]
- Yoder, C.A.; Grewal, P.S.; Taylor, R.A.J. Rapid age-related changes in infection behavior of entomopathogenic nematodes. J. Parasitol. 2004, 90, 1229–1234. [Google Scholar] [CrossRef]
- Lee, J.H.; Dillman, A.R.; Hallem, E.A. Temperature-dependent changes in the host-seeking behaviors of parasitic nematodes. BMC Biol. 2016, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.; Fitters, P. Spontaneous and induced activity of Heterorhabditis megidis infective juveniles during storage. Nematology 2004, 6, 911–917. [Google Scholar] [CrossRef]
- Gaugler, R.; Campbell, J.F.; Lewis, E.E. The effects of aging on the foraging behaviour of Steinernema carpocapsae (Rhabdita: Steinernematidae). Nematologica 1997, 43, 355–362. [Google Scholar] [CrossRef]
- Leinwand, S.G.; Yang, C.J.; Bazopoulou, D.; Chronis, N.; Srinivasan, J.; Chalasani, S.H. Circuit mechanisms encoding odors and driving aging-associated behavioral declines in Caenorhabditis elegans. Elife 2015, 4, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K. Effects of storage temperature on survival and infectivity of three indigenous entomopathogenic nematodes strains (Steinernematidae and Heterorhabditidae) from Meghalaya, India. J. Parasit. Dis. 2016, 40, 1150–1154. [Google Scholar]
- Yadav, S.; Eleftherianos, I. Prolonged storage increases virulence of Steinernema entomopathogenic nematodes toward drosophila larvae. J. Parasitol. 2018, 104, 722–725. [Google Scholar] [CrossRef]
- Griffin, C.T. Effects of prior storage conditions on the infectivity of Heterorhabditis sp. (Nematoda: Heterorhabditidae). Fundam. Appl. Nematol. 1996, 19, 95–102. [Google Scholar]
- Fitters, P.F.L.; Dunne, R.; Griffin, C.T. Improved control of otiorhynchus sulcatus at 9 °C by cold-stored heterorhabditis megidis UK211. Biocontrol Sci. Technol. 2001, 11, 483–492. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.I.; Hazir, S.; Lete, L. Viability and virulence of entomopathogenic nematodes exposed to ultraviolet radiation. J. Nematol. 2015, 47, 184–189. [Google Scholar]
- Perez, E.E.; Lewis, E.E.; Shapiro-Ilan, D.I. Impact of the host cadaver on survival and infectivity of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) under desiccating conditions. J. Invertebr. Pathol. 2003, 82, 111–118. [Google Scholar] [CrossRef]
- Hominick, W.M.; Reid, A.P. Perspectives on Entomopathogenic Nematology; CRC Press: Boca Raton, FL, USA, 1990; ISBN 0849345413. [Google Scholar]
- Dempsey, C.M.; Griffin, C.T. Phased activity in Heterorhabditis megidis infective juveniles. Parasitology 2002, 124, 605–613. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Campbell, J.F.; Koppenhöfer, A.M.; Kaya, H.K.; Chinnasri, B. Are there temporarily non-infectious dauer stages in entomopathogenic nematode populations: A test of the phased infectivity hypothesis. Parasitology 1999, 118, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Hall, D.H.; Hedgecock, E.M.; Kao, G.; Karanzta, V.; Vogel, B.E.; Hutter, H.; Chisholm, A.D.; Yurchenco, P.D.; Wadswarth, W.G. Laminin subunits and their role in C. elegans development. Development 2003, 130, 3343–3358. [Google Scholar] [CrossRef] [PubMed]
- Hollister, K.A.; Conner, E.S.; Zhang, X.; Spell, M.; Bernard, G.M.; Patel, P.; de Carvalho, A.C.G.V.; Butcher, R.A.; Ragains, J.R. Ascaroside activity in Caenorhabditis elegans is highly dependent on chemical structure. Bioorg. Med. Chem. 2013, 21, 5754–5769. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Perez, D.H.; Jones Lipinski, R.A.; Butcher, R.A. Acyl-CoA oxidases fine-tune the production of ascaroside pheromones with specific side chain lengths. ACS Chem. Biol. 2018, 13, 1048–1056. [Google Scholar] [CrossRef]
- Noguez, J.H.; Conner, E.S.; Zhou, Y.; Ciche, T.A.; Ragains, J.R.; Butcher, R.A. A novel ascaroside controls the parasitic life cycle of the entomopathogenic nematode heterorhabditis bacteriophora. ACS Chem. Biol. 2012, 7, 961–966. [Google Scholar] [CrossRef]
- Butcher, R.A.; Fujita, M.; Schroeder, F.C.; Clardy, J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat. Chem. Biol. 2007, 3, 420–422. [Google Scholar] [CrossRef]
- Srinivasan, J.; von Reuss, S.H.; Bose, N.; Zaslaver, A.; Mahanti, P.; Ho, M.C.; O’Doherty, O.G.; Edison, A.S.; Sternberg, P.W.; Schroeder, F.C. A modular library of small molecule signals regulates social behaviors in caenorhabditis elegans. PLoS Biol. 2012, 10, e1001237. [Google Scholar] [CrossRef] [PubMed]
- Macosko, E.Z.; Pokala, N.; Feinberg, E.H.; Chalasani, S.H.; Butcher, R.A.; Clardy, J.; Bargmann, C.I. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 2009, 458, 1171–1175. [Google Scholar] [CrossRef]
- Butcher, R.A. Decoding chemical communication in nematodes. Nat. Prod. Rep. 2017, 34, 472–477. [Google Scholar] [CrossRef]
- Alonso, V.; Nasrolahi, S.; Dillman, A. Host-specific activation of entomopathogenic nematode infective juveniles. Insects 2018, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Rosa, J.S.; Simões, N. Evaluation of twenty-eight strains of Heterorhabditis bacteriophora isolated in Azores for biocontrol of the armyworm, Pseudaletia unipuncta (Lepidoptera: Noctuidae). Biol. Control 2004, 29, 409–417. [Google Scholar] [CrossRef]
- Vadnal, J.; Ratnappan, R.; Keaney, M.; Kenney, E.; Eleftherianos, I.; O’Halloran, D.; Hawdon, J.M. Identification of candidate infection genes from the model entomopathogenic nematode Heterorhabditis bacteriophora. BMC Genom. 2017, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.-J.; Montiel, R.; Lucena, M.A.; Costa, M.; Simoes, N. Genetic diversity and comparative analysis of gene expression between Heterorhabditis bacteriophora Az29 and Az36 isolates: Uncovering candidate genes involved in insect pathogenicity. Exp. Parasitol. 2012, 130, 116–125. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Revenis, C. Role and importance of phenoloxidase in insect hemostasis. J. Innate Immun. 2011, 3, 28–33. [Google Scholar] [CrossRef]
- Smith, V.J. Immunology of Invertebrates: Cellular. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 1–6. ISBN 9780470015902. [Google Scholar]
- Schmidt, O.; Theopold, U.; Strand, M. Innate immunity and its evasion and suppression by hymenopteran endoparasitoids. BioEssays 2001, 23, 344–351. [Google Scholar] [CrossRef]
- Ligoxygakis, P. A serpin mutant links Toll activation to melanization in the host defence of Drosophila. EMBO J. 2002, 21, 6330–6337. [Google Scholar] [CrossRef]
- Park, J.-W.; Kim, C.-H.; Kim, J.-H.; Je, B.-R.; Roh, K.-B.; Kim, S.-J.; Lee, H.-H.; Ryu, J.-H.; Lim, J.-H.; Oh, B.-H.; et al. Clustering of peptidoglycan recognition protein-SA is required for sensing lysine-type peptidoglycan in insects. Proc. Natl. Acad. Sci. USA 2007, 104, 6602–6607. [Google Scholar] [CrossRef]
- Großhans, J.; Schnorrer, F.; Nüsslein-Volhard, C. Oligomerisation of tube and pelle leads to nuclear localisation of dorsal. Mech. Dev. 1999, 81, 127–138. [Google Scholar] [CrossRef]
- Huot, L.; George, S.; Girard, P.-A.; Severac, D.; Nègre, N.; Duvic, B. Spodoptera frugiperda transcriptional response to infestation by Steinernema carpocapsae. Sci. Rep. 2019, 9, 12879. [Google Scholar] [CrossRef]
- Ji, S.; Sun, M.; Zheng, X.; Li, L.; Sun, L.; Chen, D.; Sun, Q. Cell-surface localization of Pellino antagonizes Toll-mediated innate immune signalling by controlling MyD88 turnover in Drosophila. Nat. Commun. 2014, 5, 3458. [Google Scholar] [CrossRef] [PubMed]
- Haghayeghi, A.; Sarac, A.; Czerniecki, S.; Grosshans, J.; Schöck, F. Pellino enhances innate immunity in Drosophila. Mech. Dev. 2010, 127, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Cluxton, C.D.; Caffrey, B.E.; Kinsella, G.K.; Moynagh, P.N.; Fares, M.A.; Fallon, P.G. Functional conservation of an ancestral Pellino protein in helminth species. Sci. Rep. 2015, 5, 11687. [Google Scholar] [CrossRef]
- Davies, B.A.; Topp, J.D.; Sfeir, A.J.; Katzmann, D.J.; Carney, D.S.; Tall, G.G.; Friedberg, A.S.; Deng, L.; Chen, Z.; Horazdovsky, B.F. Vps9p CUE domain ubiquitin binding is required for efficient endocytic protein traffic. J. Biol. Chem. 2003, 278, 19826–19833. [Google Scholar] [CrossRef]
- Zhu, L.-L.; Luo, T.-M.; Xu, X.; Guo, Y.-H.; Zhao, X.-Q.; Wang, T.-T.; Tang, B.; Jiang, Y.-Y.; Xu, J.-F.; Lin, X.; et al. E3 ubiquitin ligase Cbl-b negatively regulates C-type lectin receptor–mediated antifungal innate immunity. J. Exp. Med. 2016, 213, 1555–1570. [Google Scholar] [CrossRef]
- Theopold, U.; Rissler, M.; Fabbri, M.; Schmidt, O.; Natori, S. Insect glycobiology: A lectin multigene family in drosophila melanogaster. Biochem. Biophys. Res. Commun. 1999, 261, 923–927. [Google Scholar] [CrossRef]
- Yu, X.-Q.; Gan, H.R.; Kanost, M. Immulectin, an inducible C-type lectin from an insect, Manduca sexta, stimulates activation of plasma prophenol oxidase. Insect Biochem. Mol. Biol. 1999, 29, 585–597. [Google Scholar] [CrossRef]
- Yu, X.-Q.; Kanost, M.R. Immulectin-2, a lipopolysaccharide-specific lectin from an insect, manduca sexta, is induced in response to gram-negative bacteria. J. Biol. Chem. 2000, 275, 37373–37381. [Google Scholar] [CrossRef]
- Kud, J.; Wang, W.; Gross, R.; Fan, Y.; Huang, L.; Yuan, Y.; Gray, A.; Duarte, A.; Kuhl, J.C.; Caplan, A.; et al. The potato cyst nematode effector RHA1B is a ubiquitin ligase and uses two distinct mechanisms to suppress plant immune signaling. PLoS Pathog. 2019, 15, e1007720. [Google Scholar] [CrossRef]
- Chronis, D.; Chen, S.; Lu, S.; Hewezi, T.; Carpenter, S.C.D.; Loria, R.; Baum, T.J.; Wang, X. A ubiquitin carboxyl extension protein secreted from a plant-parasitic nematode Globodera rostochiensis is cleaved in planta to promote plant parasitism. Plant. J. 2013, 74, 185–196. [Google Scholar] [CrossRef]
- Eves-van den Akker, S.; Laetsch, D.R.; Thorpe, P.; Lilley, C.J.; Danchin, E.G.J.; Da Rocha, M.; Rancurel, C.; Holroyd, N.E.; Cotton, J.A.; Szitenberg, A.; et al. The genome of the yellow potato cyst nematode, Globodera rostochiensis, reveals insights into the basis of parasitism and virulence. Genome Biol. 2016, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cui, L.; Chen, Y.; Zhang, H.; Liu, P.; Wu, P.; Qiu, D.; Zou, J.; Yang, D.; Yang, L.; et al. Transcriptional responses of wheat and the cereal cyst nematode Heterodera avenae during their early contact stage. Sci. Rep. 2017, 7, 14471. [Google Scholar] [CrossRef] [PubMed]


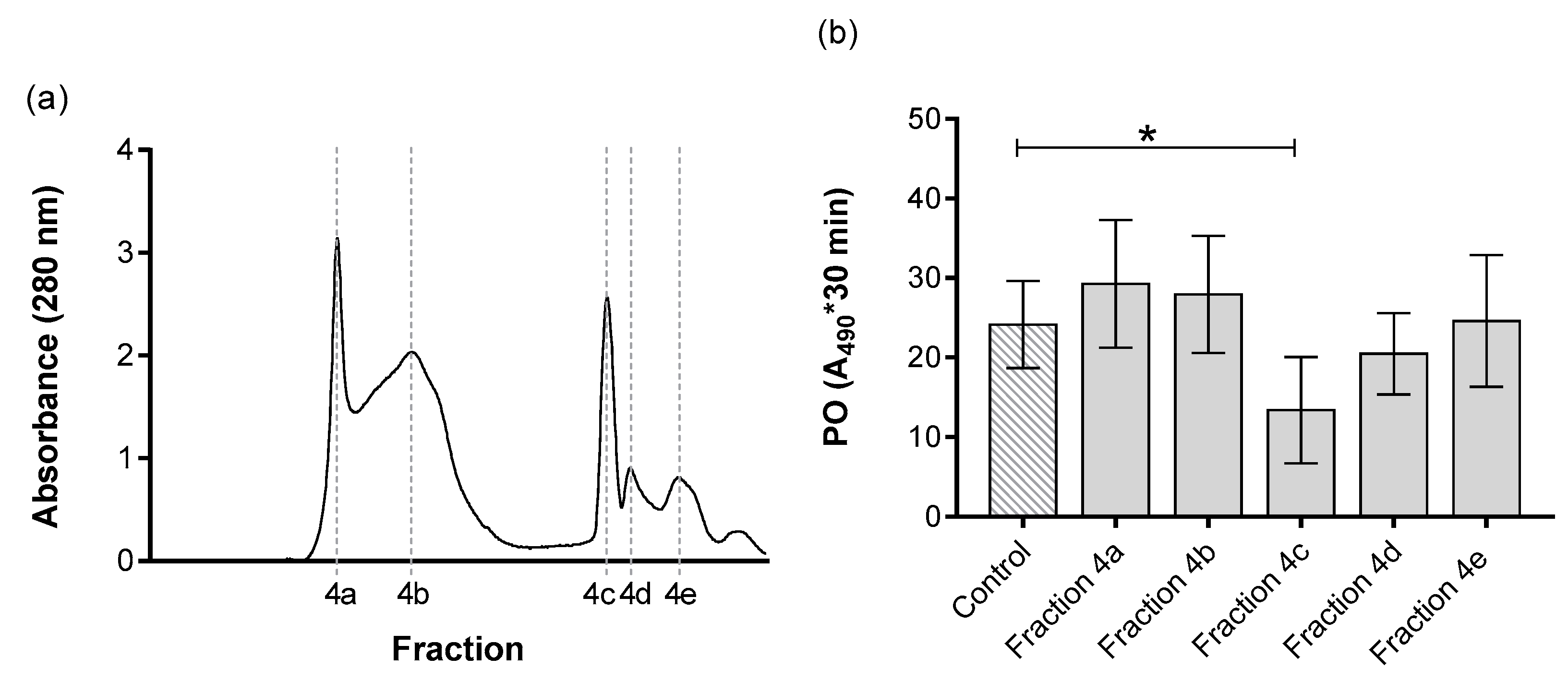

| V (mL) | Protein (mg/mL) | Total Protein (mg) | Proteolytic Activity (U) a | Specific Activity (U/mg) b | Purifications (Folds) c | % Yieldd | |
|---|---|---|---|---|---|---|---|
| Total ESP | 5.00 | 0.67 | 3.37 | 38.01 | 56.48 | ||
| Fraction 1 | 3.96 | 0.02 | 0.10 | 11.91 | 496.25 | 8.79 | 3.57 |
| Fraction 2 | 3.94 | 0.04 | 0.17 | 16.75 | 398.81 | 7.06 | 6.24 |
| Fraction 3 | 3.39 | 0.17 | 0.59 | 14.17 | 81.20 | 1.44 | 25.93 |
| Fraction 4 e | 4.15 | 0.17 | 0.71 | 16.65 | 97.94 | 1.73 | 25.26 |
| Fraction 5 e | 2.93 | 0.03 | 0.10 | 12.71 | 373.82 | 6.62 | 5.05 |
| Fraction 4a | 1.86 | 0.08 | 0.15 | 29.28 | 374.47 | 6.63 | 11.62 |
| Fraction 4b | 3.30 | 0.10 | 0.33 | 27.93 | 281.15 | 4.98 | 14.76 |
| Fraction 4c e | 1.06 | 0.05 | 0.05 | 13.38 | 272.70 | 4.83 | 7.29 |
| Fraction 4d | 0.42 | 0.01 | 0.00322 | 20.48 | 2667.67 | 47.23 | 1.14 |
| Fraction 4e | 1.99 | 0.03 | 0.05 | 24.59 | 952.85 | 16.87 | 3.83 |
| Fraction 5a | 2.12 | 0.02 | 0.04 | 34.66 | 2028.51 | 35.92 | 2.54 |
| Fraction 5b | 1.52 | 0.04 | 0.06 | 14.24 | 354.23 | 6.27 | 5.97 |
| N | de novo Peptide Sequence 1 | Conf Score | Hb Transcript 2 | Identification | E Value | Homology | GO | Pfam |
|---|---|---|---|---|---|---|---|---|
| 1 | KPIILTNLNVPCQPK | 99.0 | Hba_21422 | Pellino | 6 × 10−148 | Ancylostoma ceylanicum | Ubiquitin protein ligase | PF04710 |
| 2 | LLAVHFTVAEGVKNKER | 99.0 | Hba_15515 | Metridin ShK toxin | 5 × 10−148 | Haemonchus contortus | Toxin activity | PF01549 |
| 3 | KQEDNEVESPPQQEVPRPR | 99.0 | Hba_18775 | Ion channel | 9 × 10−127 | Necator americanus | Ion channel activity | PF07885 |
| 4 | QKEILASTSIAQIVDRVC | 99.0 | Hba_18934 | Hypothetical protein | 6 × 10−110 | Ancylostoma ceylanicum | Unnamed protein | NI |
| 5 | YTFKELTRQEEIVVR | 99.0 | Hba_19968 | CUE domain protein | 3 × 10−84 | Haemonchus contortus | CUE domain | PF02845 |
| 6 | MLDEVVPPAGSKLRYIC | 99.0 | Hba_20430 | Unnamed protein | 3 × 10−16 | Haemonchus placei | Unnamed protein | PF02319 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eliáš, S.; Hurychová, J.; Toubarro, D.; Frias, J.; Kunc, M.; Dobeš, P.; Simões, N.; Hyršl, P. Bioactive Excreted/Secreted Products of Entomopathogenic Nematode Heterorhabditis bacteriophora Inhibit the Phenoloxidase Activity during the Infection. Insects 2020, 11, 353. https://doi.org/10.3390/insects11060353
Eliáš S, Hurychová J, Toubarro D, Frias J, Kunc M, Dobeš P, Simões N, Hyršl P. Bioactive Excreted/Secreted Products of Entomopathogenic Nematode Heterorhabditis bacteriophora Inhibit the Phenoloxidase Activity during the Infection. Insects. 2020; 11(6):353. https://doi.org/10.3390/insects11060353
Chicago/Turabian StyleEliáš, Sara, Jana Hurychová, Duarte Toubarro, Jorge Frias, Martin Kunc, Pavel Dobeš, Nelson Simões, and Pavel Hyršl. 2020. "Bioactive Excreted/Secreted Products of Entomopathogenic Nematode Heterorhabditis bacteriophora Inhibit the Phenoloxidase Activity during the Infection" Insects 11, no. 6: 353. https://doi.org/10.3390/insects11060353
APA StyleEliáš, S., Hurychová, J., Toubarro, D., Frias, J., Kunc, M., Dobeš, P., Simões, N., & Hyršl, P. (2020). Bioactive Excreted/Secreted Products of Entomopathogenic Nematode Heterorhabditis bacteriophora Inhibit the Phenoloxidase Activity during the Infection. Insects, 11(6), 353. https://doi.org/10.3390/insects11060353







