Molecular Cloning and Characterization of Five Glutathione S-Transferase Genes and Promoters from Micromelalopha troglodyta (Graeser) (Lepidoptera: Notodontidae) and Their Response to Tannic Acid Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect and Cell Cultures
2.2. Cloning the cDNA Sequences of the Glutathione S-Transferases (GSTs)
2.3. Sequence Identity and Phylogenetic Analysis
2.4. Induction of GST Transcription in Micromelalopha troglodyta by Tannic Acid
2.5. Cloning the Sequences of the Five GST Promoter Genes
2.6. Analysing the Sequences of the Five GST Promoter Genes
2.7. Construction of the MtGSTd2 Promoter-PGL 4.10, MtGST01 Promoter-PGL 4.10, MtGSTs1 Promoter-PGL 4.10, MtGSTt1 Promoter-PGL 4.10 and MtGSTz1 Promoter-PGL 4.10 Constructs
2.8. Transient Transfection and Dual Luciferase Assay
2.9. Data Analysis
3. Results
3.1. Cloning and Identity of the GST cDNA
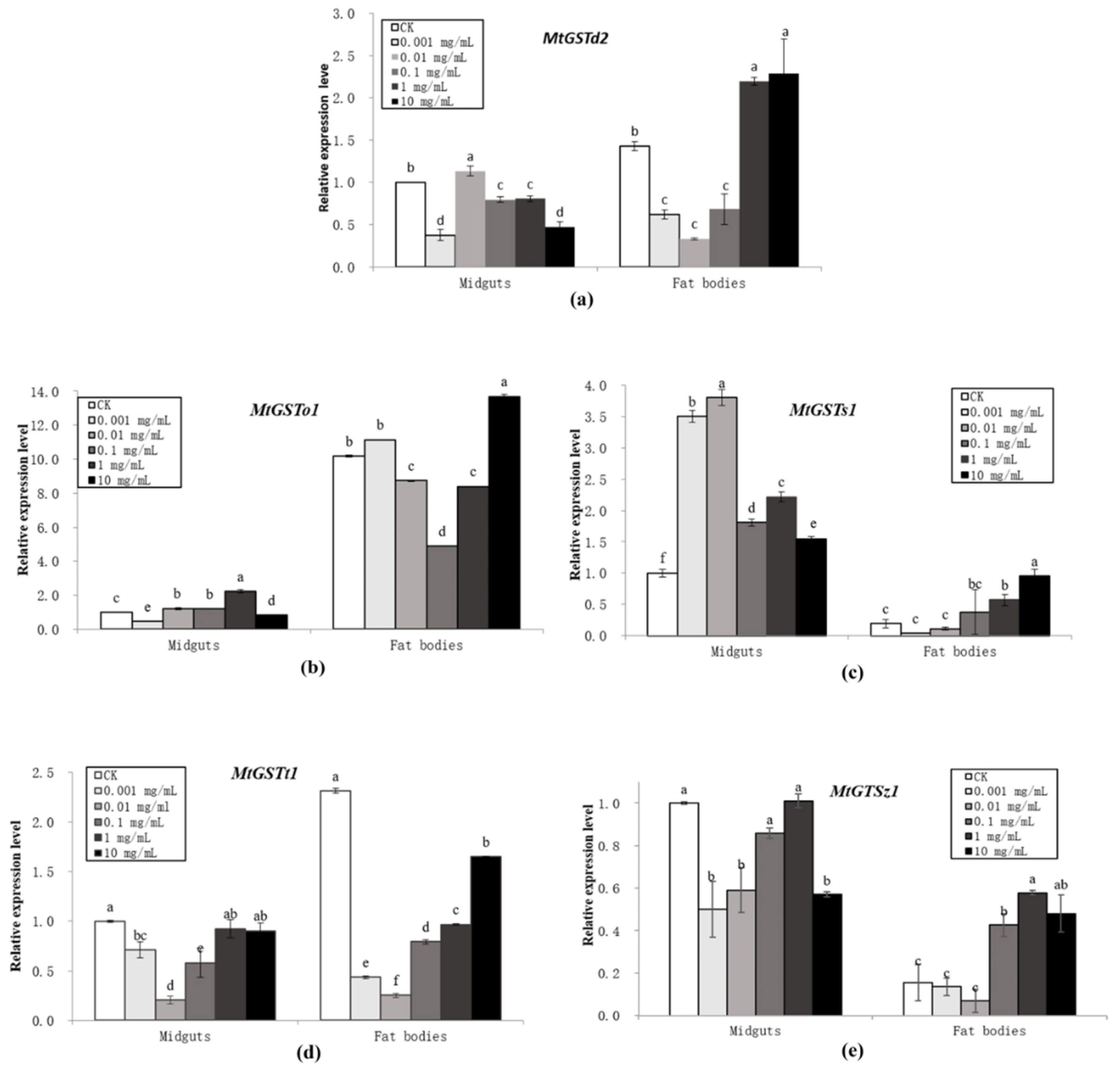
3.2. Induction of GST Gene Expression in M. troglodyta by Tannic Acid
3.3. Characterization of the 5′-Flanking Promoter Sequences of the Five MtGST Genes
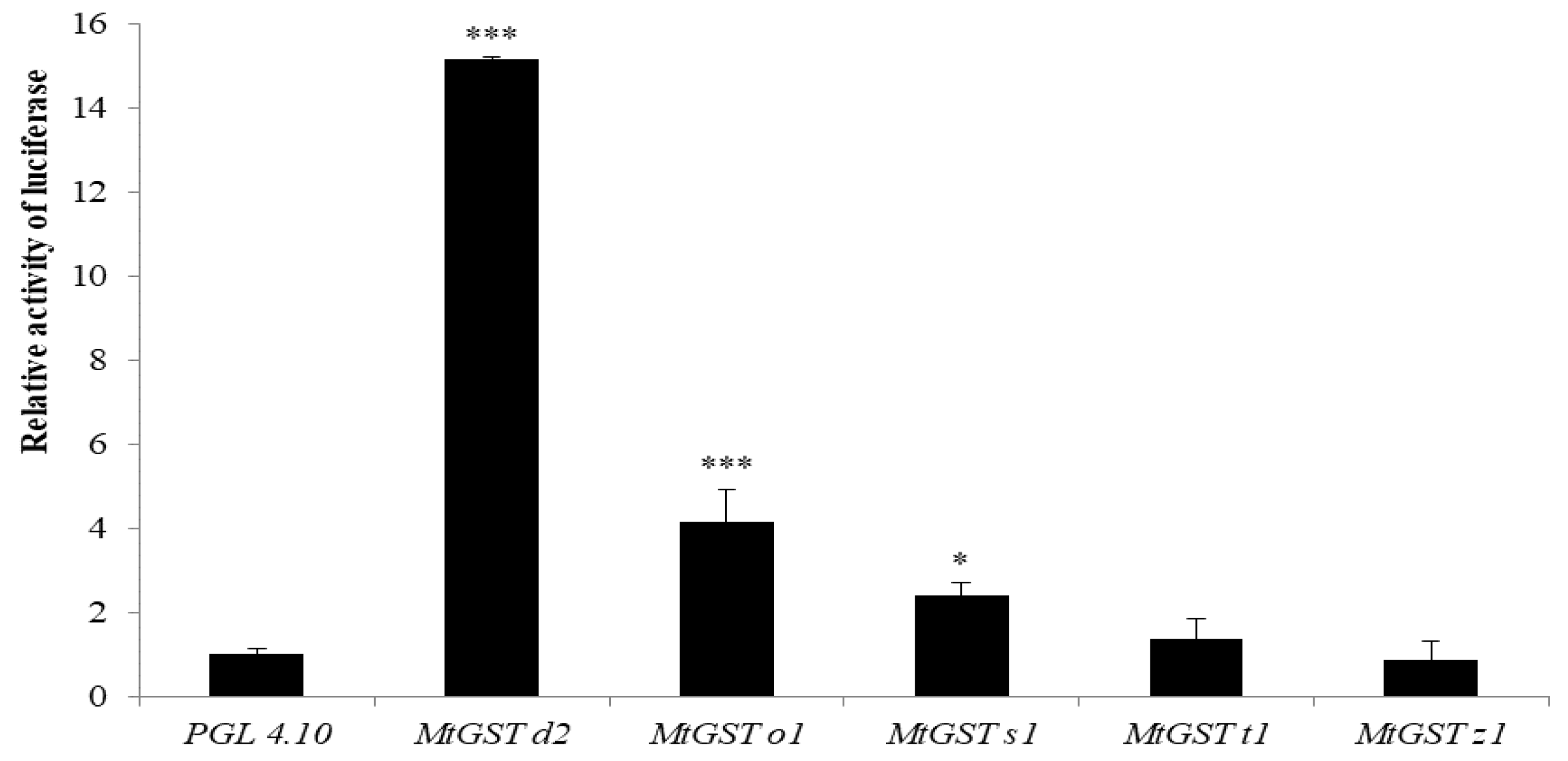
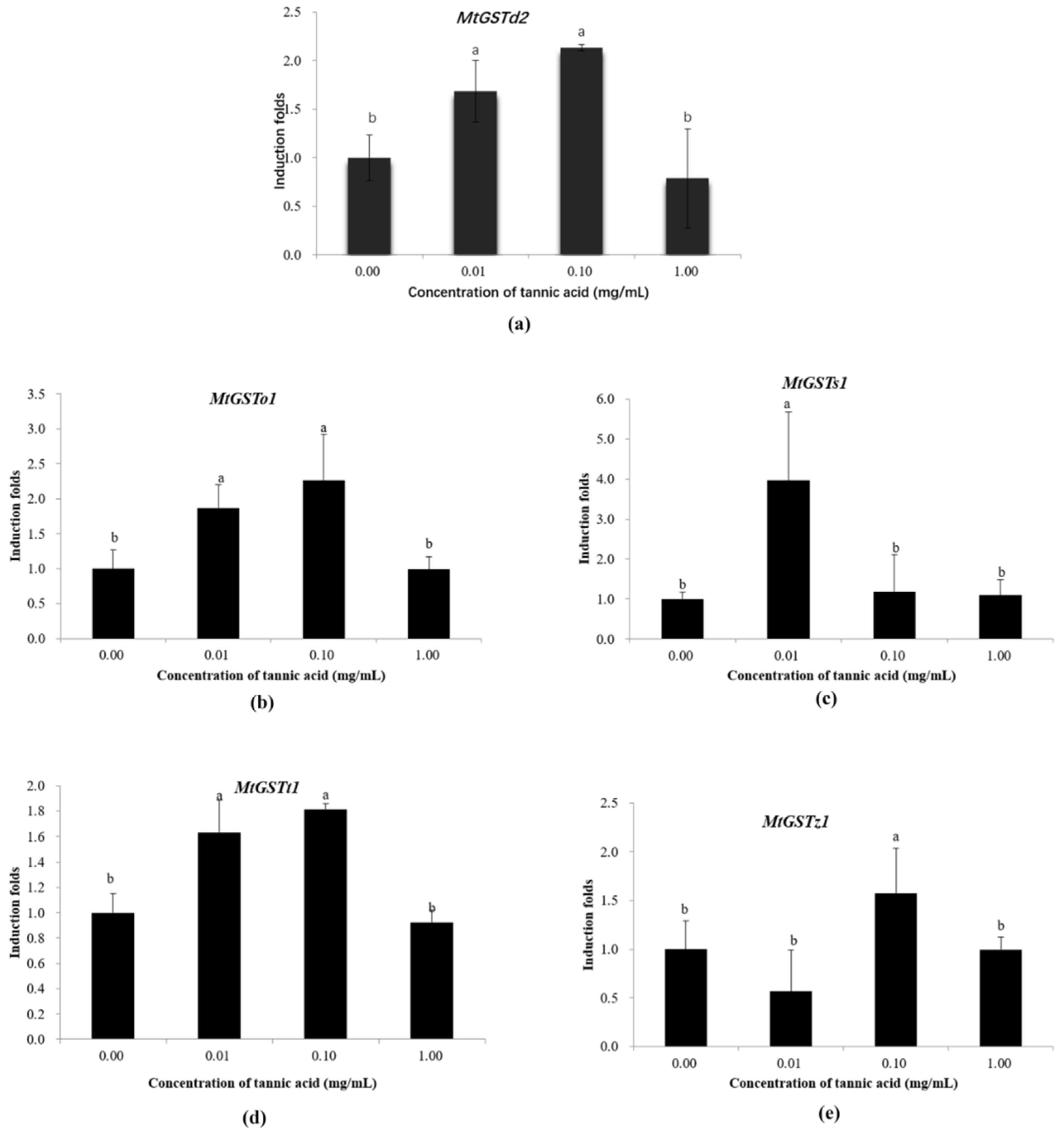
3.4. Functional Analysis of MtGST Promoters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Todaro, L.; Russo, D.; Cetera, P.; Milella, L. Effects of thermo-vacuum treatment on secondary metabolite content and antioxidant activity of poplar (Populus nigra L.) wood extracts. Ind. Crop. Prod. 2017, 109, 384–390. [Google Scholar] [CrossRef]
- Berenbaum, M.R. The chemistry of defense: Theory and practice. Proc. Natl. Acad. Sci. USA 1995, 92, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Boyland, E.; Chasseaud, L.F. The role of glutathione and glutathione S-transferases in mercapturic acid biosynthesis. Adv. Enzymol. Relat. Areas. Mol. Biol. 1969, 32, 173–219. [Google Scholar] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Morel, F.; Rauch, C.; Petit, E.; Piton, A.; Theret, N.; Coles, B.; Guillouzo, A. Gene and protein characterization of the human glutathione S-transferase Kappa and evidence for a peroxisomal localization. J. Biol. Chem. 2004, 279, 16246–16253. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione Transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef]
- Feng, Q.L.; Davey, K.G.; Pang, A.S.D.; Ladd, T.M.; Retnakaran, A.; Tomkins, B.L.; Zheng, S.C.; Palli, S.R. Developmental expression and stress induction of glutathione S-transferase in the spruce budworm, Choristoneura fumiferana. J. Insect Physiol. 2001, 47, 1–10. [Google Scholar] [CrossRef]
- Enayati, A.A.; Ranson, H.; Hemingway, J. Insect glutathione transferases and insecticide resistance. Insect Mol. Biol. 2005, 14, 3–8. [Google Scholar] [CrossRef]
- McLellan, L.I.; Wolf, C.R. Glutathione and glutathione-dependent enzymes in cancer drug resistance. Drug Resist. Update 1999, 2, 153–164. [Google Scholar] [CrossRef]
- Lee, K. Glutathione S-transferase activities in phytophagous insects: Induction and inhibition by plant phototoxins and phenols. Insect Biochem. 1991, 21, 353–361. [Google Scholar] [CrossRef]
- Gao, X.W.; Dong, X.L.; Zheng, B.Z.; Chen, Q. Glutathiones S-transferase (GSTS) of cotton bollworm: Induction of pesticides and plant secondary substances and metabolism of GSTS to pesticides. Acta Entomol. Sin. 1997, 40, 122–127. [Google Scholar]
- Robert, J.A.; Pitt, C.; Bonnett, T.R.; Yuen, M.M.S.; Keeling, C.I.; Bohlmann, J. Disentangling detoxifification: Gene expression analysis of feeding Mountain pine beetle illuminates molecular-level host chemical defense detoxifification mechanisms. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Pitt, C.; Robert, J.A.; Bonnett, T.R.; Keeling, C.I.; Bohlmann, J.; Huber, D.P.W. Proteomics indicators of the rapidly shifting physiology from whole mountain pine beetle, Dendroctonus ponderosae (Coleoptera: Curculionidae), adults during early host colonization. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Dai, L.L.; Ma, J.N.; Ma, M.Y.; Zhang, H.Q.; Shi, Q.; Zhang, R.R.; Chen, H. Characterisation of GST genes from the Chinese white pine beetle Dendroctonus armandi (Curculionidae: Scolytinae) and their response to host chemical defence. Pest Manag. Sci. 2016, 72, 816–827. [Google Scholar] [CrossRef]
- Huang, Y.F.; Xu, Z.B.; Lin, X.Y.; Feng, Q.L.; Zheng, S.C. Structure and expression of glutathione S-transferase genes from the midgut of the common cutworm, Spodoptera litura (Noctuidae) and their response to xenobiotic compounds and bacteria. J. Insect Physiol. 2011, 57, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Xie, J.H.; Ma, X.Q.; Li, D. Molecular cloning of Phosphoethanolamine N-methyltransferase (PEAMT) gene and its promoter from the halophyte Suaeda liaotungensis and their response to salt stress. Acta Physiol. Plant 2016, 38. [Google Scholar] [CrossRef]
- Chen, S.; Lu, M.; Zhang, N.; Zou, X.; Mo, M.; Zheng, S. Nuclear factor erythroid-derived 2-related factor 2 activates glutathione S-transferase expression in the midgut of Spodoptera litura (Lepidoptera: Noctuidae) in response to phytochemicals and insecticides. Insect Mol. Biol. 2018, 27, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, H.; Wei, Q.; Ren, M.M.; Mburu, D.K.; Tian, X.R.; Su, J.Y. Transcription factors CncC/Maf and AhR/ARNT coordinately regulate the expression of multiple GSTs conferring resistance to chlorpyrifos and cypermethrin in Spodoptera exigua. Pest Manag. Sci. 2019, 75, 2009–2019. [Google Scholar] [CrossRef]
- Cheng, H.P.; Tang, F.; Li, W.; Xu, M. Tannic acid induction of a glutathione S-transferase in Micromelalopha troglodyta (Lepidoptera: Notodontidae) larvae. J. Entomol. Sci. 2015, 50, 350–362. [Google Scholar] [CrossRef]
- Zhang, K.; Das, N.P. Inhibitory effects of plant polyphenols on rat liver glutathione S-transferases. Biochem. Pharmacol. 1994, 47, 2063–2068. [Google Scholar] [CrossRef]
- Tang, F.; Zhang, X.B.; Liu, Y.S.; Gao, X.W.; Liu, N.N. In vitro inhibition of glutathione S-transferases by several insecticides and allelochemicals in two moth species. Int. J. Pest Manag. 2014, 60, 33–38. [Google Scholar] [CrossRef]
- Yamamoto, K.; Zhang, P.B.; Miake, F.; Kashige, N.; Aso, Y.; Banno, Y.; Fujii, H. Cloning, expression and characterization of theta-class glutathione S-transferase from the silkworm, Bombyx mori. Comp. Biochem. Physiol. 2005, 141, 340–346. [Google Scholar] [CrossRef]
- Chen, F.J.; Gao, X.W.; Lei, M.Q.; Zheng, B.Z. Effects of tannic acid on glutathione S-transferases in Helicoverpa armigera (Hübner). Acta Entomol. Sin. 2003, 46, 684–690. [Google Scholar]
- Tang, F.; Zhang, X.B.; Liu, Y.S.; Gao, X.W. Tissue distribution and properties of glutathione S-transferases in Micromelalopha troglodyta (Lepidoptera: Notodontidae). J. Entomol. Sci. 2008, 43, 268–278. [Google Scholar] [CrossRef]
- Zhang, X.B.; Tang, F.; Liu, Y.S.; Gao, X.W. Induction of glutathione S-transferases by tannic acid in Micromelalopha troglodyta. Chin. Bull. Entomol. 2009, 46, 579–584. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Giulietti, A.; Oververgh, L.; Valckx, D.; Decallonne, B.; Bouillon, R.; Mathieu, C. An overview of real-time quantitative PCR: Applications to quantify cytokine gene expression. Methods 2001, 25, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Pan, Y.; Gao, X.; Xi, J.; Zhang, L.; Yang, C.; Bi, R.; Yang, S.; Xin, X.; Shang, Q. Cytochrome P450 CYP6DA2 regulated by cap ‘n’collar isoform C (CncC) is associated with gossypol tolerance in Aphis gossypii Glover. Insect Mol. Biol. 2016, 25, 450–459. [Google Scholar] [CrossRef]
- Agianian, B.; Tucker, P.A.; Schouten, A.; Leonard, K.; Bullard, B.; Gros, P. Structure of a drosophila sigma class glutathione S-transferase reveales a novel active site topography suited for lipid peroxidation products. J. Mol. Biol. 2003, 326, 151–165. [Google Scholar] [CrossRef]
- Pemble, S.E.; Taylor, J.B. An evolutionary perspective on glutathione transferases inferred from class-theta glutathione transferase cDNA sequences. Biochem. J. 1992, 287, 957–963. [Google Scholar] [CrossRef]
- Ranson, H.; Cornel, A.J.; Fournier, D.; Vaughan, A.; Collins, F.H.; Hemingway, J. Cloning and localization of a glutathione S-transferase classⅠgene from Anopheles gambiae. J. Biol. Chem. 1997, 272, 5464–5468. [Google Scholar] [CrossRef] [PubMed]
- Board, P.G.; Baker, R.T.; Chelvanayagam, G.; Jermiin, L.S. Zeta, a novel class of glutathione transferases in a range of species from plants to humans, Biochem. J. 1997, 328, 929–935. [Google Scholar]
- Toung, Y.P.; Hsieh, T.S.; Tu, C.P. The glutathione S-transferase D genes: A divergently organized, intronless gene family in Drosophila melanogaster. J. Biol. Chem. 1993, 268, 9737–9746. [Google Scholar]
- Yu, Q.Y.; Lu, C.; Li, B.; Fang, S.M.; Zuo, W.D.; Dai, F.Y.; Zhang, Z.; Xiang, Z.H. Identification, genomic organization and expression pattern of glutathione S-transferase in the silkworm, Bombyx mori. Insect Biochem. Mol. 2008, 38, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.C.; Ortelli, F.; Rossiter, L.C.; Hemingway, J.; Ranson, H. The Anopheles gambiae glutathione transferase supergene family: Annotation, phylogeny and expression profiles. BMC Genom. 2003, 35. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Davey, K.G.; Pang, A.S.; Primavera, M.; Ladd, T.R.; Zheng, S.C.; Sohi, S.S.; Retnakaran, A.; Palli, S.R. Glutathione S-transferase from the spruce budworm, Choristoneura fumiferana: Identification, characterization, localization, cDNA cloning, and expression. Insect Biochem. Mol. 1999, 29, 779–793. [Google Scholar] [CrossRef]
- Zheng, S.C.; Deng, H.M.; Ladd, T.; Tomkins, B.L.; Krell, P.J.; Feng, Q.L. Cloning and characterization of two glutathione S-transferase cDNAs in the Spruce Budworm, Choristoneura fumifera. Arch. Insect Biochem. Physiol. 2007, 66, 146–157. [Google Scholar] [CrossRef]
- Tang, A.H.; Tu, C.P. Pentobarbital-induced changes in Drosophila glutathione S-transferase D21 mRNA stability. J. Biol. Chem. 1995, 270, 13819–13825. [Google Scholar] [CrossRef]
- Chrzanowski, G.; Leszczynski, B.; Czerniewicz, P.; Sytykiewicz, H.; Matok, H.; Krzyzanowski, R.; Sempruch, C. Effect of phenolic acids from black currant, sour cherry and walnut on grain aphid (Sitobion avenae F.) development. Crop Prot. 2012, 35, 71–77. [Google Scholar] [CrossRef]
- Adesanya, A.W.; Held, D.W.; Liu, N.N. Geranium intoxication induces detoxification enzymes in the Japanese beetle, Popillia japonica Newman. Pestic. Biochem. Phys. 2017, 143, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.E.; Ma, H.J.; Feng, D.D.; Lai, X.F.; Chen, Z.M.; Xu, M.Y.; Yu, Q.Y.; Zhang, Z. Induction of detoxification enzymes by quercetin in the silkworm. J. Econ. Entomol. 2012, 105, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Zhu, T.; Gao, X.W.; Yan, A.J. Inhibition of glutathione S-transferases activity from Odontotermes formosanus Shiraki and Reticulitermes chinensis Snyder by seven inhibitors. Acta Entomol. Sin. 2007, 50, 1225–1231. [Google Scholar]
- Chen, F.J.; Zhang, C.Z.; Gao, X.W. In vitro inhibition of glutathione S-transferases by several insecticides and allelochemicals in cotton bollworm, Helicoverpa armigera Hübner. J. Entomol. Sci. 2007, 42, 296–305. [Google Scholar] [CrossRef]
- Yu, S.J.; Abo-Elghar, G.E. Allelochemicals as inhibitors of glutathione S-transferases in the fall armyworm. Pestic. Biochem. Phys. 2000, 68, 173–183. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, F.T.; Jia, D.M.; Li, J.T.; Zhang, Y.M.; Jia, C.G.; Wang, D.P.; Pan, H.Y. Cloning and functional analysis of the promoter of a stress-inducible gene (ZmRXO1) in Maize. Plant Mol. Biol. Rep. 2015, 33, 200–208. [Google Scholar] [CrossRef]
- Fujita, R.; Ono, C.; Ono, I.; Asano, S.; Bando, H. Analysis of the Bombyx mori nucleopolyhedrovirus ie-1 promoter in insect, mammalian, plant, and bacterial cells. Biochem. Biophys. Res. Commun. 2015, 464, 1297–1301. [Google Scholar] [CrossRef]
- Rao, X.J.; Xu, X.X.; Yu, X.Q. Mandnca sexta moricin promoter elements can increase promoter activities of Drosophila melanogaster antimicrobial peptide genes. Insect Biochem. Mol. 2011, 41, 982–992. [Google Scholar] [CrossRef]
- Wang, J.L.; Song, W.H. Regulation of LRRK2 promoter activity and gene expression by Sp1. Mol. Brain 2016, 9. [Google Scholar] [CrossRef]

| GenBank | Gene | Primer | Sequence (5′-3′) | Annealing Temperatures (°C) | Amplicon Size (bp) | Application |
|---|---|---|---|---|---|---|
| KU 963403 | MtGSTd2 | F | TCAGCGTTTGAAGATGTCG | 53 | 678 | ORF |
| R | GCTTCTTACAGCTCGGTTTTAG | ORF | ||||
| Q-F | AAAGCCGATGAAGCCAAGTT | 60 | 105 | qPCR | ||
| Q-R | TGCCAGGGTCAGTTTATCTCC | qPCR | ||||
| Common 1 | GTAATACGACTCACTATAGGGC | 67 | - | Genome Walker | ||
| 1 | ATCGTCTACTATCGTGGGAATGGTGTG | Genome Walker | ||||
| Common 2 | ACTATAGGGCACGCGTGGT | 67 | 975 | Genome Walker | ||
| 2 | GACGAGCTTCAGGTTGAGTTGGATGT | Genome Walker | ||||
| F-1 | AACTCGAGAGATTACTATAGGGCACG | 62 | 920 | Constructs | ||
| R-1 | AAGCTAGCAGGTAGTACAGGTCGATC | Constructs | ||||
| KU 963404 | MtGSTo1 | F | TCGCTGCCATCATGTCTG | 53 | 778 | ORF |
| R | GTTTATTCCTTCTTCTTCCTGG | ORF | ||||
| Q-F | ACTATACAGCTGCCTTCAACGC | 60 | 153 | qPCR | ||
| Q-R | GCCACAATGTGAAGTCAACGAG | qPCR | ||||
| Common 1 | GTAATACGACTCACTATAGGGC | 67 | - | Genome Walker | ||
| 1 | ACTAAAACTGTTCTCTCGGCGTATGGG | Genome Walker | ||||
| Common 2 | ACTATAGGGCACGCGTGGT | 67 | 977 | Genome Walker | ||
| 2 | GGGCAGAATCTCATAGCGAATACACG | Genome Walker | ||||
| F-1 | AACTCGAGATTACTATAGGGCACGC | 62 | 847 | Constructs | ||
| R-1 | AAGCTAGCGGTTTGTAAATGTTTTTC | Constructs | ||||
| KU 963405 | MtGSTs1 | F | GAGTCCTTGACAATGGCTA | 53 | 660 | ORF |
| R | GCTCGCTATTGCACAACC | ORF | ||||
| Q-F | GACTTTTGGGCCAACATCAG | 60 | 112 | qPCR | ||
| Q-R | CAAATCTGGGCAAGAAGAACAC | qPCR | ||||
| Common 1 | GTAATACGACTCACTATAGGGC | 67 | - | Genome Walker | ||
| 1 | AACCTTACATCCTCAAATTCCTGTTTAGTG | Genome Walker | ||||
| Common 2 | ACTATAGGGCACGCGTGGT | 67 | 1012 | Genome Walker | ||
| 2 | GCTAAGGCAGGTGCTTCAAAATAATACAG | Genome Walker | ||||
| F-1 | AACTCGAGCGACGAAGGCTT | 62 | 926 | Constructs | ||
| R-1 | AAGCTAGCATTTGCTGCACTATCA | Constructs | ||||
| KU 963408 | MtGSTt1 | F | GTTCAATACCTTCAAGTTTTTC | 53 | 722 | ORF |
| R | ACACTTTAGACTTAACTTTAGACTGC | ORF | ||||
| Q-F | CCACTGTCGCTGATCTGCTG | 60 | 126 | qPCR | ||
| Q-R | AGGGGCTGAAATGTCGTTG | qPCR | ||||
| Common 1 | GTAATACGACTCACTATAGGGC | 67 | - | Genome Walker | ||
| 1 | CAAAGTACAAGAACATAGCCGAGTGGTG | Genome Walker | ||||
| Common 2 | ACTATAGGGCACGCGTGGT | 67 | 1894 | Genome Walker | ||
| 2 | TCTTCACAGAGTGGTCGTTATTTCAGTTC | Genome Walker | ||||
| F-1 | AACTCGAGTGCCTGCAGGTC | 62 | 1853 | Constructs | ||
| R-1 | AAGCTAGCGCTTTGATTTGGTC | Constructs | ||||
| KU 963410 | MtGSTz1 | F | CTCAAAATACAACGGGAACC | 53 | 707 | ORF |
| R | CGACCGTGACAAGAGGC | ORF | ||||
| Q-F | AGTCAATCCGATGGAGCAGG | 60 | 180 | qPCR | ||
| Q-R | GGTTGGATGCCTGATGCTATT | qPCR | ||||
| Common 1 | GTAATACGACTCACTATAGGGC | 67 | - | Genome Walker | ||
| 1 | CTTGTCTCTTCCAGGTAGTGCATTATGTTC | Genome Walker | ||||
| Common 2 | ACTATAGGGCACGCGTGGT | 67 | 1590 | Genome Walker | ||
| 2 | ATGGGATCTCCTTCAAGTTGAGTGCG | Genome Walker | ||||
| F-1 | AACTCGAGGGCACGCGTG | 62 | 1177 | Constructs | ||
| R-1 | AAGCTAGCTTGTAAGTCGGTATGTATGTAA | Constructs | ||||
| GU 262991 | Actin | Q-F | CTCTGGTCGACTTGAGGCTGGAC | 60 | 241 | qPCR |
| Q-R | CTCTGGTCGACTTGAGGCTGGAC | qPCR |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, F.; Tu, H.; Shang, Q.; Gao, X.; Liang, P. Molecular Cloning and Characterization of Five Glutathione S-Transferase Genes and Promoters from Micromelalopha troglodyta (Graeser) (Lepidoptera: Notodontidae) and Their Response to Tannic Acid Stress. Insects 2020, 11, 339. https://doi.org/10.3390/insects11060339
Tang F, Tu H, Shang Q, Gao X, Liang P. Molecular Cloning and Characterization of Five Glutathione S-Transferase Genes and Promoters from Micromelalopha troglodyta (Graeser) (Lepidoptera: Notodontidae) and Their Response to Tannic Acid Stress. Insects. 2020; 11(6):339. https://doi.org/10.3390/insects11060339
Chicago/Turabian StyleTang, Fang, Huizhen Tu, Qingli Shang, Xiwu Gao, and Pei Liang. 2020. "Molecular Cloning and Characterization of Five Glutathione S-Transferase Genes and Promoters from Micromelalopha troglodyta (Graeser) (Lepidoptera: Notodontidae) and Their Response to Tannic Acid Stress" Insects 11, no. 6: 339. https://doi.org/10.3390/insects11060339
APA StyleTang, F., Tu, H., Shang, Q., Gao, X., & Liang, P. (2020). Molecular Cloning and Characterization of Five Glutathione S-Transferase Genes and Promoters from Micromelalopha troglodyta (Graeser) (Lepidoptera: Notodontidae) and Their Response to Tannic Acid Stress. Insects, 11(6), 339. https://doi.org/10.3390/insects11060339







