Nest Entrances, Spatial Fidelity, and Foraging Patterns in the Red Ant Myrmica rubra: A Field and Theoretical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Collective Exploitation of Food Sources
2.2. Ant Fidelity to Nest Entrances and to Food Sources
2.3. Model
3. Results
3.1. Field Experiments
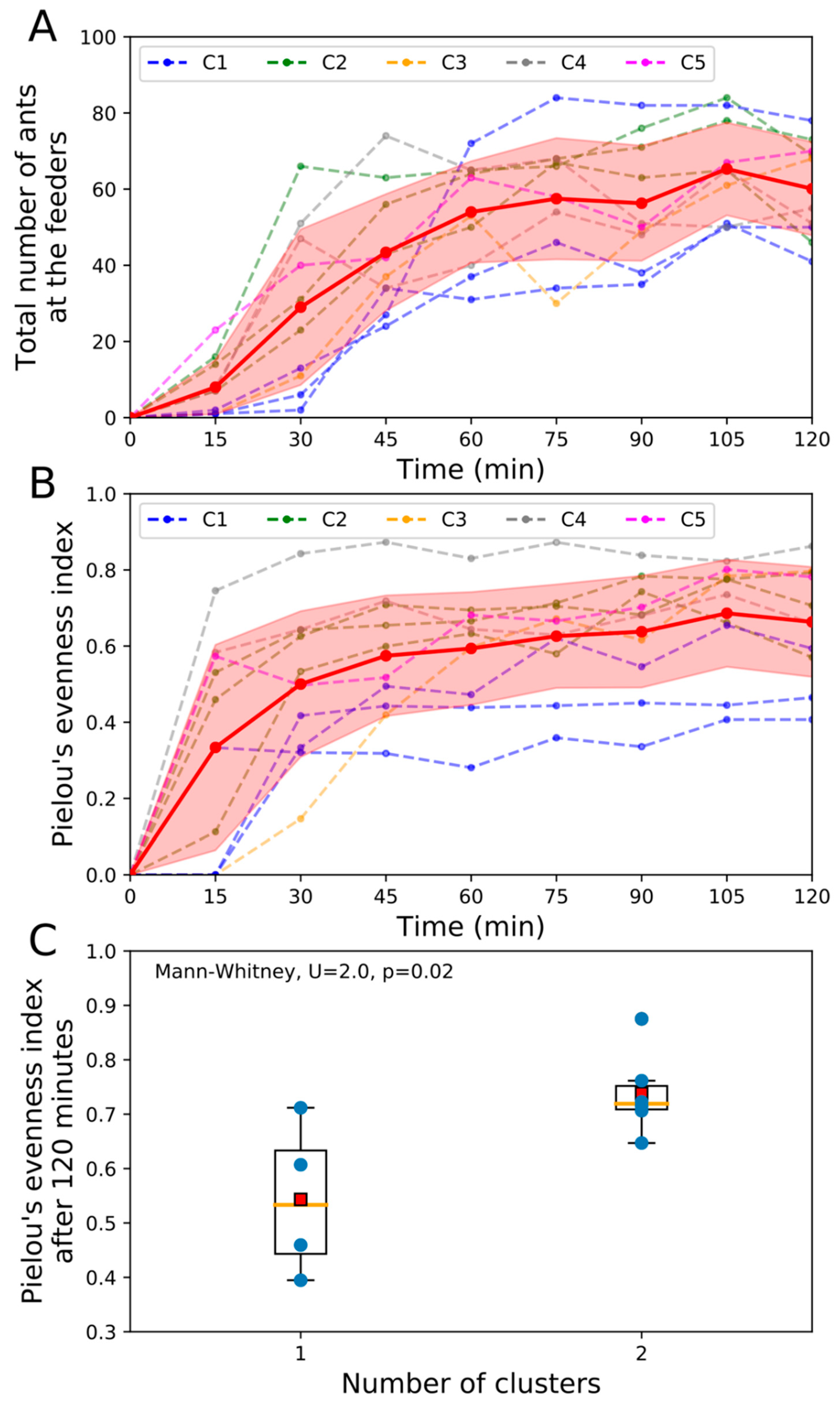
3.1.1. Collective Exploitation of Food Sources
3.1.2. Ant Fidelity to Nest Entrances and Food Sources
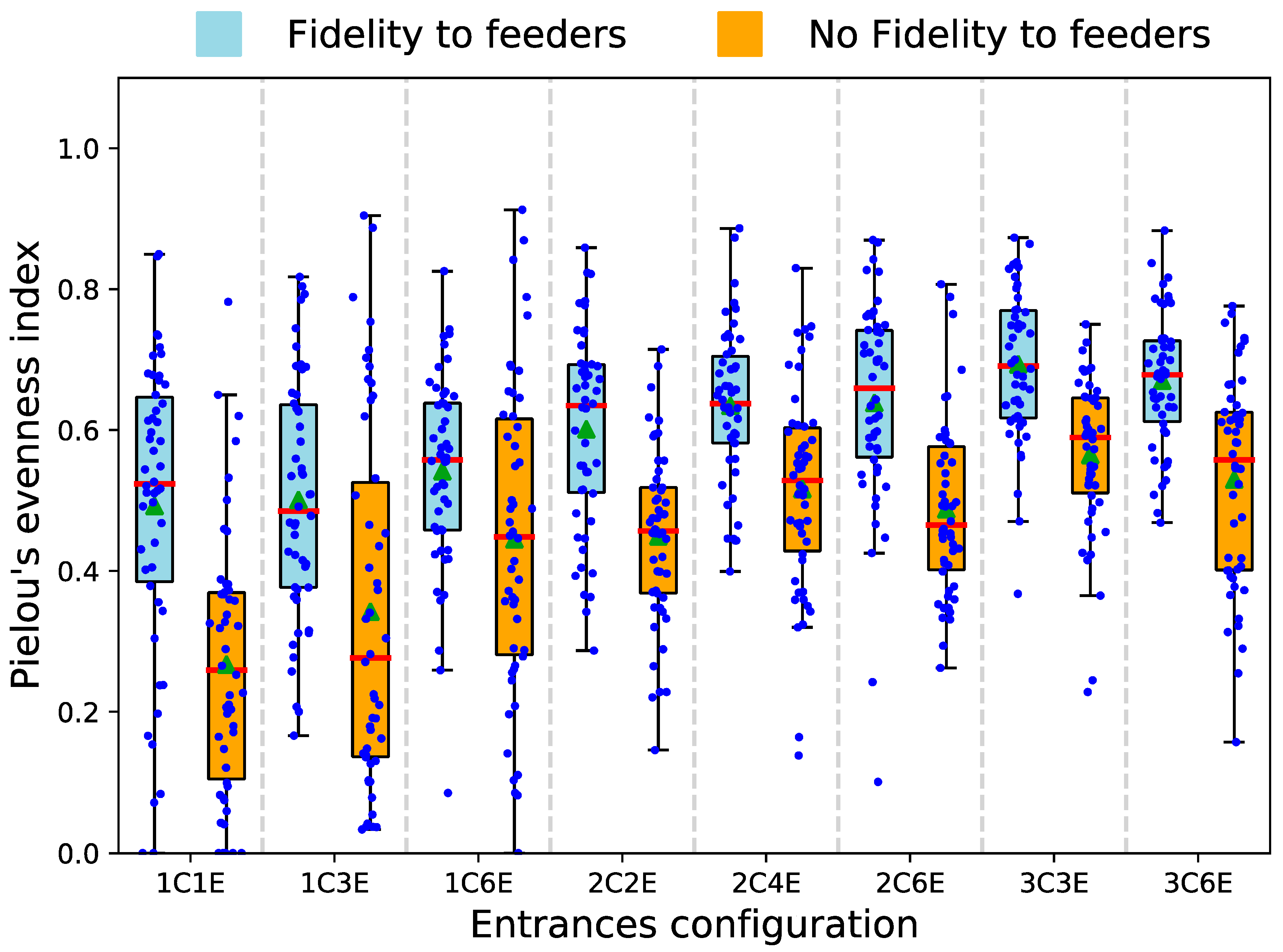
3.2. Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giraldeau, L.-A.; Caraco, T. Social Foraging Theory; Princeton University Press: Princeton, NJ, USA, 2000. [Google Scholar]
- Buckley, N.J. Food Finding and the Influence of Information, Local Enhancement, and Communal Roosting on Foraging Success of North American Vultures. Auk 2012, 113, 473–488. [Google Scholar] [CrossRef]
- Schmidt, V.M.; Dirk, D.L.; Hrncir, M.; Zucchi, R.; Barth, F.G. Collective foraging in a stingless bee: Dependence on food profitability and sequence of discovery. Anim. Behav. 2006, 72, 1309–1317. [Google Scholar] [CrossRef]
- Fitzgerald, T.D.; Peterson, S.C. Elective recruitment by the eastern tent caterpillar (Malacosoma americanum). Anim. Behav. 1983, 31, 417–423. [Google Scholar] [CrossRef]
- Stevens, J.R.; Gilby, I.C. A conceptual framework for nonkin food sharing: Timing and currency of benefits. Anim. Behav. 2004, 67, 603–614. [Google Scholar] [CrossRef]
- Giraldeau, L.-A.; Beauchamp, G. Food exploitation: Searching for the optimal joining policy. Trends Ecol. Evol. 1999, 14, 102–106. [Google Scholar] [CrossRef]
- Götmark, F.; Winkler, D.W.; Andersson, M. Flock-feeding on fish schools increases individual success in gulls. Nature 1986, 319, 589–591. [Google Scholar] [CrossRef]
- Rodman, P.S. Inclusive Fitness and Group Size with a Reconsideration of Group Sizes in Lions and Wolves. Am. Nat. 2002, 118, 275–283. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The Ants; Press of Harvard University: Cambridge, MA, USA, 1990. [Google Scholar]
- Camazine, S.; Deneubourg, J.L.; Franks, N.R.; Sneyd, J.; Theraulaz, G.; Bonabeau, E. Self-Organization in Biological Systems; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Detrain, C.; Deneubourg, J.L.; Pasteels, J.M. (Eds.) Information Processing in Social Insects; Birkhauser Verlag: Basel, Switzerland, 1999. [Google Scholar]
- Seeley, T.D. The Wisdom of the Hive: The Social Physiology of Honey Bee Colonies; Harvard University Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Cassill, D. Rules of supply and demand regulate recruitment to food in an ant society. Behav. Ecol. Sociobiol. 2003, 54, 441–450. [Google Scholar] [CrossRef]
- Buffin, A.; Denis, D.; Van Simaeys, G.; Goldman, S.; Deneubourg, J.L. Feeding and stocking up: Radio-labelled food reveals exchange patterns in ants. PLoS ONE 2009, 4, e5919. [Google Scholar] [CrossRef]
- Detrain, C.; Deneubourg, J.-L. Collective Decision-Making and Foraging Patterns in Ants and Honeybees. Adv. Insect Physiol. 2008, 35, 123–173. [Google Scholar] [CrossRef]
- Detrain, C.; Deneubourg, J.L.; Goss, S.; Quinet, Y. Dynamics of Collective Exploration in the Ant Pheidole pallidula. Psyche 1991, 98, 21–31. [Google Scholar] [CrossRef]
- Jaffe, K.; Deneubourg, J.L. On foraging, recruitment systems and optimum number of scouts in eusocial colonies. Insectes Soc. 1992, 39, 201–213. [Google Scholar] [CrossRef]
- Dechaume-Moncharmont, F.X.; Dornhaus, A.; Houston, A.I.; McNamara, J.M.; Collins, E.J.; Franks, N.R. The hidden cost of information in collective foraging. Proc. R. Soc. B Biol. Sci. 2005, 272, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.M. The expandable network of ant exploration. Anim. Behav. 1995, 50, 995–1007. [Google Scholar] [CrossRef]
- Cook, Z.; Franks, D.W.; Robinson, E.J.H. Exploration versus exploitation in polydomous ant colonies. J. Theor. Biol. 2013, 323, 49–56. [Google Scholar] [CrossRef]
- Stroeymeyt, N.; Joye, P.; Keller, L. Polydomy enhances foraging performance in ant colonies. Proc. R. Soc. B Biol. Sci. 2017, 284. [Google Scholar] [CrossRef]
- De Biseau, J.; Pasteels, J.M. Response thresholds to recruitment signals and the regulation of foraging intensity in the ant Myrmica sabuleti (Hymenoptera, Formicidae). Behav. Process. 2000, 48, 137–148. [Google Scholar] [CrossRef]
- Collignon, B.; Cervantes Valdivieso, L.E.; Detrain, C. Group recruitment in ants: Who is willing to lead? Behav. Process. 2014, 108, 98–104. [Google Scholar] [CrossRef]
- Bonabeau, E.; Theraulaz, G.; Deneubourg, J.-L.; Aron, S.; Camazine, S. Self-organization in social insects. Trends Ecol. Evol. 1997, 12, 188–193. [Google Scholar] [CrossRef]
- Pagliara, R.; Gordon, D.; Leonard, N. Regulation of harvester ant foraging as a closed-loop excitable system. PLoS Comput. Biol. 2018, 14, e1006200. [Google Scholar] [CrossRef]
- Pinter-Wollman, N.; Bala, A.; Merrell, A.; Queirolo, J.; Stumpe, M.C.; Holmes, S.; Gordon, D.M. Harvester ants use interactions to regulate forager activation and availability. Anim. Behav. 2013, 86, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Pless, E.; Queirolo, J.; Pinter-Wollman, N.; Crow, S.; Allen, K.; Mathur, M.B.; Gordon, D.M. Interactions increase forager availability and activity in harvester ants. PLoS ONE 2015, 10, e0141971. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L. The relocation of ant nest entrances: Potential consequences for ant-dispersed seeds. Aust. J. Ecol. 1990, 16, 207–214. [Google Scholar] [CrossRef]
- Robinson, E.J.H. Polydomy: The organisation and adaptive function of complex nest systems in ants. Curr. Opin. Insect Sci. 2014, 5, 37–43. [Google Scholar] [CrossRef]
- McIver, J.D. Dispersed central place foraging in Australian meat ants. Insectes Soc. 1991, 38, 129–137. [Google Scholar] [CrossRef]
- Debout, G.; Schatz, B.; Elias, M.; Mckey, D. Polydomy in ants: What we know, what we think we know, and what remains to be done. Biol. J. Linn. Soc. 2007, 90, 319–348. [Google Scholar] [CrossRef]
- Traniello, J.F.A.; Levings, S.C. Intra- and intercolony patterns of nest dispersion in the ant Lasius neoniger: Correlations with territoriality and foraging ecology. Oecologia 1986, 69, 413–419. [Google Scholar] [CrossRef]
- Holway, D.A.; Case, T.J. Mechanisms of dispersed central-place foraging in polydomous colonies of the Argentine ant. Anim. Behav. 2000, 59, 433–441. [Google Scholar] [CrossRef]
- Holway, D.A.; Case, T.J. Effects of colony-level variation on competitive ability in the invasive Argentine ant. Anim. Behav. 2001, 61, 1181–1192. [Google Scholar] [CrossRef]
- Völkl, W.; Woodring, J.; Fischer, M.; Lorenz, M.; Hoffman, K. Ant-aphid mutualisms: The impact of honeydew production and honeydew sugar composition on ant preferences. Oecologia 1999, 118, 483–491. [Google Scholar] [CrossRef]
- Detrain, C.; Prieur, J. Sensitivity and feeding efficiency of the black garden ant Lasius niger to sugar resources. J. Insect Physiol. 2014, 64, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A. Geschmacksphysiologische Untersuchungen an Ameisen. Z. Vgl. Physiol. 1938, 25, 351–378. [Google Scholar] [CrossRef]
- Ettershank, G. The three-dimensional gallery structure of the nest of the meat ant Iridomyrmex purpureus (SM.) (Hymenoptera: Formicidae). Aust. J. Zool. 1968, 16, 715–723. [Google Scholar] [CrossRef]
- Zee, J.; Holway, D. Nest raiding by the invasive Argentine ant on colonies of the harvester ant, Pogonomyrmex subnitidus. Insectes Soc. 2006, 53, 161–167. [Google Scholar] [CrossRef]
- Kabashima, J.N.; Greenberg, L.; Rust, M.K.; Paine, T.D. Aggressive interactions between Solenopsis invicta and Linepithema humile (Hymenoptera: Formicidae) under laboratory conditions. J. Econ. Entomol. 2007, 100, 148–154. [Google Scholar] [CrossRef]
- Beckers, R.; Deneubourg, J.L.; Goss, S.; Pasteels, J.M. Collective decision making through food recruitment. Insectes Soc. 1990, 37, 258–267. [Google Scholar] [CrossRef]
- De Biseau, J.C.; Deneubourg, J.L.; Pasteels, J.M. Collective flexibility during mass recruitment in the ant Myrmica sabuleti (Hymenoptera: Formicidae). Psyche 1991, 98, 323–336. [Google Scholar] [CrossRef]
- Greaves, T.; Hughes, L. The population biology of the meat ant. J. Aust. Entomol. Soc. 1974, 13, 329–351. [Google Scholar] [CrossRef]
- Latty, T.; Beekman, M. Keeping track of changes: The performance of ant colonies in dynamic environments. Anim. Behav. 2013, 85, 637–643. [Google Scholar] [CrossRef]
- Fresneau, D. Individual foraging and path fidelity in a ponerine ant. Insectes Soc. 1985, 32, 109–116. [Google Scholar] [CrossRef]
- Schultheiss, P.; Cheng, K. Finding food: Outbound searching behavior in the Australian desert ant Melophorus bagoti. Behav. Ecol. 2012, 128–135. [Google Scholar] [CrossRef]
- Azevedo, D.L.O.; Medeiros, J.C.; Araújo, A. Adjustments in the time, distance and direction of foraging in Dinoponera quadriceps workers. J. Insect Behav. 2014, 27, 177–191. [Google Scholar] [CrossRef]
- Czaczkes, T.J.; Salmane, A.K.; Klampfleuthner, F.A.M.; Heinze, J. Private information alone can trigger trapping of ant colonies in local feeding optima. J. Exp. Biol. 2016, 219, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Pasteels, J.M.; Deneubourg, J.-L.; Goss, S. Self-organization mechanisms in ant societies (I): Trail recruitment to newly discovered food sources. In From Individual to Collective Behavior in Social Insects; Pasteels, J.M., Deneubourg, J.L., Eds.; Birkhauser Verlag: Basel, Switzerland, 1987; Volume 54, pp. 155–175. [Google Scholar]
- Detrain, C.; Natan, C.; Deneubourg, J.L. The influence of the physical environment on the self-organised foraging patterns of ants. Naturwissenschaften 2001, 88, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Grüter, C.; Czaczkes, T.J.; Ratnieks, F.L.W. Decision making in ant foragers (Lasius niger) facing conflicting private and social information. Behav. Ecol. Sociobiol. 2011, 65, 141–148. [Google Scholar] [CrossRef]
- Oberhauser, F.B.; Schlemm, A.; Wendt, S.; Czaczkes, T.J. Private information conflict: Lasius niger ants prefer olfactory cues to route memory. Anim. Cogn. 2019, 22, 355–364. [Google Scholar] [CrossRef]
- Czaczkes, T.J.; Grüter, C.; Jones, S.M.; Ratnieks, F.L.W. Synergy between social and private information increases foraging efficiency in ants. Biol. Lett. 2011, 7, 521–524. [Google Scholar] [CrossRef]
- Czaczkes, T.J.; Czaczkes, B.; Iglhaut, C.; Heinze, J. Composite collective decision-making. Proc. R. Soc. B Biol. Sci. 2015, 282. [Google Scholar] [CrossRef]
- Beverly, B.D.; Mclendon, H.; Nacu, S.; Holmes, S.; Gordon, D.M. How site fidelity leads to individual differences in the foraging activity of harvester ants. Behav. Ecol. 2009, 20, 633–638. [Google Scholar] [CrossRef]
- Fourcassié, V.; Traniello, J.F.A. Food searching behaviour in the ant Formica schaufussi (Hymenoptera, Formicidae): Response of naive foragers to protein and carbohydrate food. Anim. Behav. 1994, 48, 69–79. [Google Scholar] [CrossRef][Green Version]
- Le Breton, J.; Fourcassié, V. Information transfer during recruitment in the ant Lasius niger L. (Hymenoptera: Formicidae). Behav. Ecol. Sociobiol. 2004, 55, 242–250. [Google Scholar] [CrossRef]
- Cammaerts, M.C. Étude démographique annuelle des sociétés de Myrmica rubra L. des environs de Bruxelles. Insectes Soc. 1977, 24, 147–161. [Google Scholar] [CrossRef]
- Cammaerts, M.C. Recruitment to food in Myrmica rubra L. Biol. Behav. 1978, 4, 159–172. [Google Scholar]
- Detrain, C.; Deneubourg, J.L. Scavenging by Pheidole pallidula: A key for understanding decision-making systems in ants. Anim. Behav. 1997, 53, 537–547. [Google Scholar] [CrossRef][Green Version]
- Ellis, S.; Robinson, E.J.H. Polydomy in red wood ants. Insectes Soc. 2014, 61, 111–122. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehue, M.; Detrain, C.; Collignon, B. Nest Entrances, Spatial Fidelity, and Foraging Patterns in the Red Ant Myrmica rubra: A Field and Theoretical Study. Insects 2020, 11, 317. https://doi.org/10.3390/insects11050317
Lehue M, Detrain C, Collignon B. Nest Entrances, Spatial Fidelity, and Foraging Patterns in the Red Ant Myrmica rubra: A Field and Theoretical Study. Insects. 2020; 11(5):317. https://doi.org/10.3390/insects11050317
Chicago/Turabian StyleLehue, Marine, Claire Detrain, and Bertrand Collignon. 2020. "Nest Entrances, Spatial Fidelity, and Foraging Patterns in the Red Ant Myrmica rubra: A Field and Theoretical Study" Insects 11, no. 5: 317. https://doi.org/10.3390/insects11050317
APA StyleLehue, M., Detrain, C., & Collignon, B. (2020). Nest Entrances, Spatial Fidelity, and Foraging Patterns in the Red Ant Myrmica rubra: A Field and Theoretical Study. Insects, 11(5), 317. https://doi.org/10.3390/insects11050317



