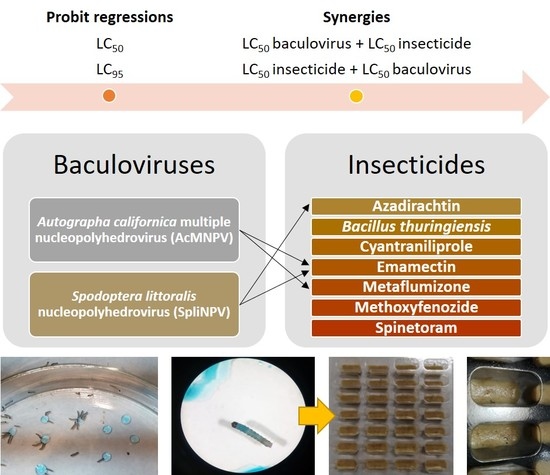
Synergy of Lepidopteran Nucleopolyhedroviruses AcMNPV and SpliNPV with Insecticides
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Species
2.2. Baculovirus Isolates
2.3. Determination of the LC50 and LC95 of NPV and Insecticides at the Second Instar Larval Stage
2.4. Combined Effect of Sequential Feeding of NPV and Insecticides
2.5. Statistical Analysis
3. Results
3.1. Determination of the LC50 and LC95 of NPV and Insecticides at Second Instar Larval Stage
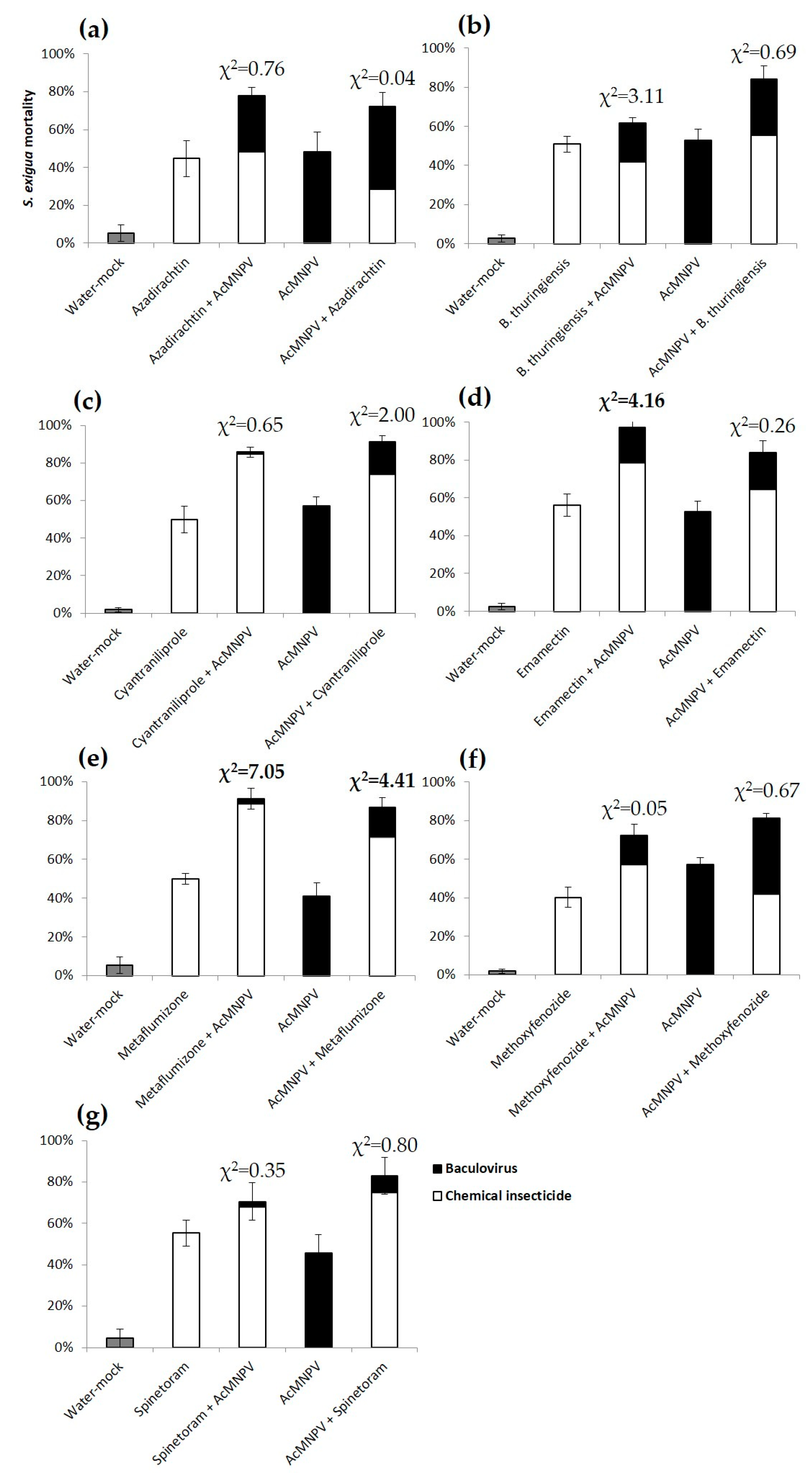
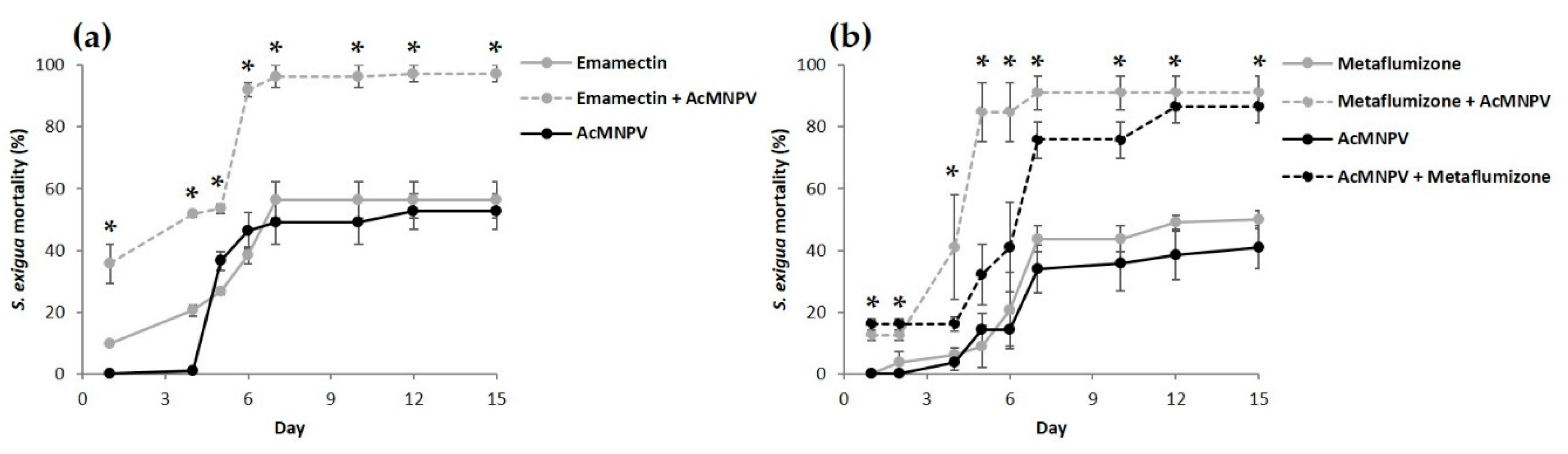
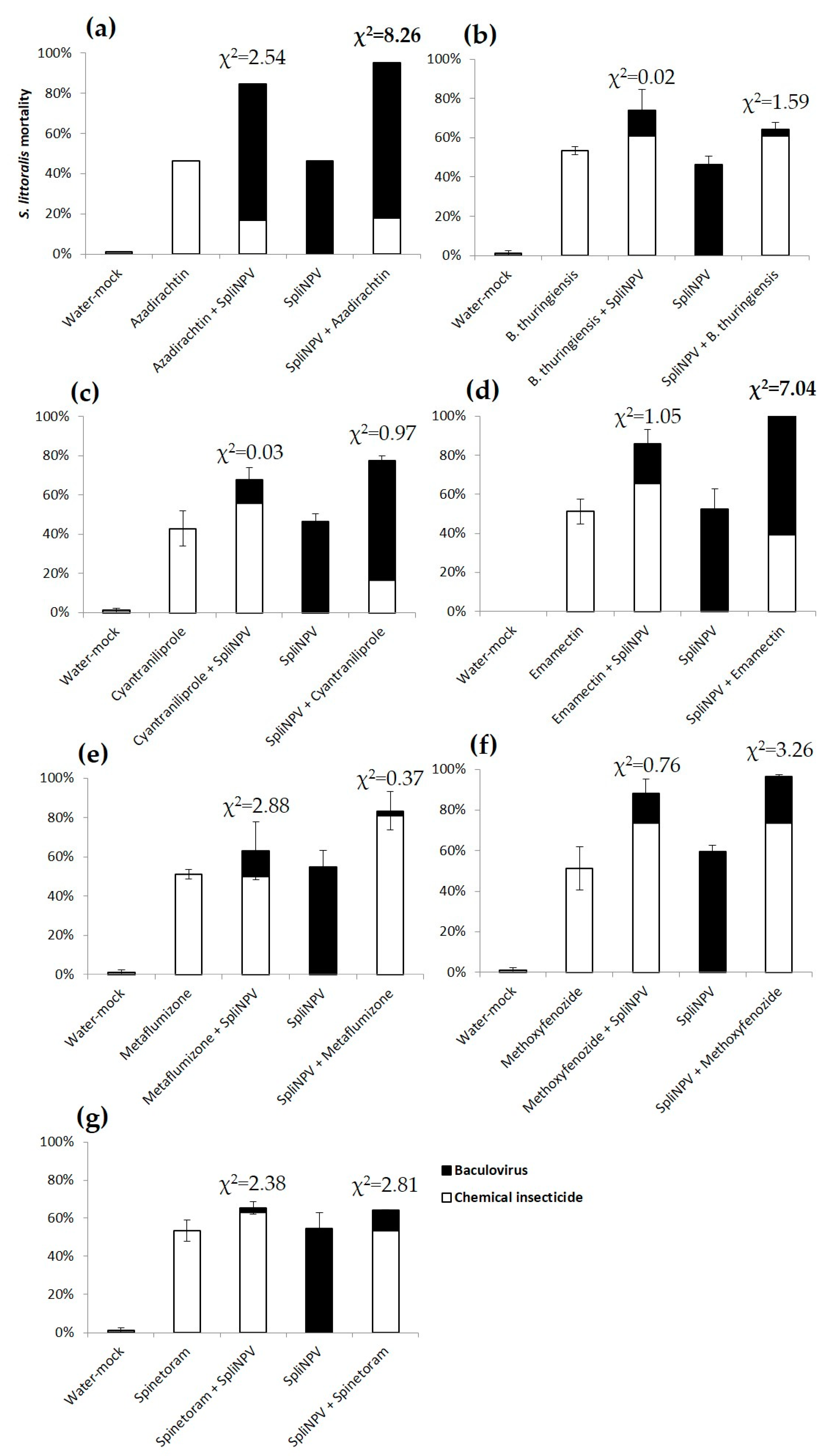
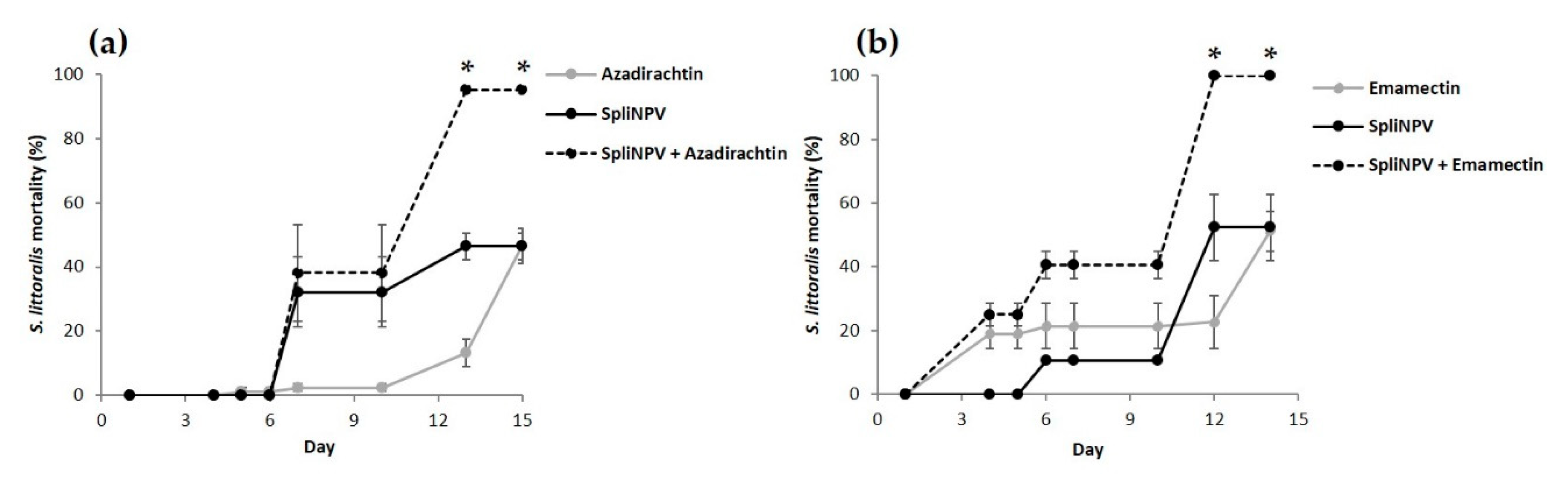
3.2. Combined Effect of the Sequential Feeding of NPV and Insecticides
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Official Journal of the European Union (OJEU). Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009, establishing a framework for Community action to achieve the sustainable use of pesticides. OJEU 2009, 309, 71–86. [Google Scholar]
- Czaja, K.; Góralczyk, K.; Struciński, P.; Hernik, A.; Korcz, W.; Minorczyk, M.; Łyczewska, M.; Ludwicki, J.K. Biopesticides—Towards increased consumer safety in the European Union. Pest Manag. Sci. 2015, 71, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Cabello, T. Natural enemies of noctuid pests in alfalfa, corn, cotton and soybean crops in Southern Spain. J. Appl. Entomol. 1989, 108, 80–88. [Google Scholar] [CrossRef]
- Smagghe, G.; Pineda, S.; Carton, B.; Del Estal, P.; Budia, F.; Viñuela, E. Toxicity and kinetics of methoxyfenozide in greenhouse-selected Spodoptera exigua (Lepidoptera: Noctuidae). Pest Manag. Sci. 2003, 59, 1203–1209. [Google Scholar] [CrossRef]
- Lasa, R.; Pagola, I.; Ibañez, I.; Belda, J.E.; Williams, T.; Caballero, P. Efficacy of Spodoptera exigua multiple nucleopolyhedrovirus as a biological insecticide for beet armyworm control in greenhouses of southern Spain. Biocontrol Sci. Technol. 2007, 17, 221–232. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Organization for Economic Cooperation and Development (OECD). Data requirements for registration of biopesticides in OECD member countries: Survey results (OECD/GD(96)13). OECD Environ. Monogr. 1996, 106, 1–122. [Google Scholar]
- Eberle, K.E.; Jehle, J.A.; Huber, J. Microbial control of crop pests using insect viruses. In Integrated Pest Management: Principles and Practice; Abrol, D.P., Shankar, U., Eds.; CAB International: Wallingford, UK, 2012; pp. 281–298. [Google Scholar]
- Rodgers, P.B. Potential of biopesticides in agriculture. Pestic. Sci. 1993, 39, 117–129. [Google Scholar] [CrossRef]
- Armenta, R.; Martínez, A.M.; Chapman, J.W.; Magallanes, R.; Goulson, D.; Caballero, P.; Cave, R.D.; Cisneros, J.; Valle, J.; Castillejos, V.; et al. Impact of a nucleopolyhedrovirus bioinsecticide and selected synthetic insecticides on the abundance of insect natural enemies on maize in Southern Mexico. J. Econ. Entomol. 2003, 96, 649–661. [Google Scholar] [CrossRef]
- Nathan, S.S.; Kalaivani, K. Efficacy of nucleopolyhedrovirus and azadirachtin on Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Biol. Control 2005, 34, 93–98. [Google Scholar] [CrossRef]
- Nathan, S.S.; Kalaivani, K. Combined effects of azadirachtin and nucleopolyhedrovirus (SpltNPV) on Spodoptera litura Fabricius (Lepidoptera: Noctuidae) larvae. Biol. Control 2006, 39, 96–104. [Google Scholar] [CrossRef]
- Kumar, N.S.; Murugan, K.; Zhang, W. Additive interaction of Helicoverpa armigera nucleopolyhedrovirus and azadirachtin. Biol. Control 2008, 53, 869–880. [Google Scholar]
- Zamora-Avilés, N.; Alonso-Vargas, J.; Pineda, S.; Isaac-Figueroa, J.; Lobit, P.; Martínez-Castillo, A.M. Effects of a nucleopolyhedrovirus in mixtures with azadirachtin on Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) larvae and viral occlusion body production. Biocontrol Sci. Technol. 2013, 23, 521–534. [Google Scholar] [CrossRef]
- Subramanian, S.; Rabindra, R.J.; Palaniswamy, S.; Sathiah, N.; Rajasekaran, B. Impact of granulovirus infection on susceptibility of Spodoptera litura to insecticides. Biol. Control 2005, 33, 165–172. [Google Scholar] [CrossRef]
- Méndez, W.A.; Valle, J.; Ibarra, J.E.; Cisneros, J.; Penagos, D.I.; Williams, T. Spinosad and nucleopolyhedrovirus mixtures for control of Spodoptera frugiperda (Lepidoptera: Noctuidae). Biol. Control 2002, 25, 195–206. [Google Scholar] [CrossRef]
- El-Helaly, A.A.; El-bendary, H.M. Field study to evaluate the joint action of certain insecticides, IGR’s and baculoviruses against Spodoptera littoralis (Bosid.). J. Entomol. Zool. Stud. 2015, 3, 289–293. [Google Scholar]
- Espinel, C.; Gómez, J.; Villamizar, L.; Cotes, A.M.; Léry, X.; López-Ferber, M. Biological activity and compatibility with chemical pesticides of a Colombian granulovirus isolated from Tecia solanivora. IOBC/WPRS Bull. 2009, 45, 145–148. [Google Scholar]
- McCutchen, B.F.; Hoover, K.; Preisler, H.K.; Betana, M.D.; Herrmann, R.; Robertson, J.L.; Hammock, B.D. Interactions of recombinant and wild-type baculoviruses with classical insecticides and pyrethroid-resistant tobacco budworm (Lepidopteran: Noctuidae). J. Econ. Entomol. 1997, 90, 1170–1180. [Google Scholar] [CrossRef]
- Pingel, R.L.; Lewis, L.C. Effect of Bacillus thuringiensis, Anagrapha falcifera multiple nucleopolyhedrovirus, and their mixture on three lepidopteran corn ear pests. J. Econ. Entomol. 1999, 92, 91–96. [Google Scholar] [CrossRef]
- Elvira, S.; Gorría, N.; Muñoz, D.; Williams, T.; Caballero, P. A simplified low-cost diet for rearing Spodoptera exigua (Lepidoptera: Noctuidae) and its effect on S. exigua nucleopolyhedrovirus production. J. Econ. Entomol. 2010, 103, 17–24. [Google Scholar] [CrossRef]
- Hughes, P.R.; Wood, H.A. In vivo and in vitro bioassay methods for baculoviruses. In The Biology of Baculoviruses; Granados, R.R., Federici, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 1987; Volume II, pp. 1–30. [Google Scholar]
- Insecticide Resistance Action Committee (IRAC). Mode of Action Classification. Available online: http://www.irac-online.org/modes-of-action/ (accessed on 15 January 2020).
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: London, UK, 1971; p. 151. [Google Scholar]
- Robertson, J.R.; Preisler, H.K.; Russell, R.M. Polo Plus. Probit and Logit Analysis User’s Guide; CRC Press: Boca Raton, FL, USA, 2002; Volume 36. [Google Scholar]
- Preisler, H.K.; Robertson, J.L.; Hoover, K.; McCutchen, B.F. Statistical methods to assessing responses over time in bioassays with mixtures. J. Econ. Entomol. 1999, 92, 598–603. [Google Scholar] [CrossRef]
- Trisyono, A.; Whalon, M. Toxicity of neem applied alone and in combinations with Bacillus thuringiensis to Colorado potato beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1999, 92, 1281–1288. [Google Scholar] [CrossRef]
- Hummelbrunner, L.A.; Isman, M.B. Acute, sublethal, antifeedant, and synergistic effects of monoterpenoid essential oil compounds on tobacco cutworm, Spodoptera litura (Lepidoptera: Noctuidae). J. Agric. Food Chem. 2001, 49, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Koppenhöfer, A.M.; Kaya, H.K. Interactions of a nucleopolyhedrovirus with azadirachtin and imidacloprid. J. Invertebr. Pathol. 2000, 75, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Murillo, R.; Hussey, M.S.; Possee, R.D. Evidence for covert baculovirus infections in a Spodoptera exigua laboratory culture. J. Gen. Virol. 2011, 92, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Virto, C.; Navarro, D.; Téllez, M.M.; Herrero, S.; Williams, T.; Murillo, R.; Caballero, P. Natural populations of Spodoptera exigua are infected by multiple viruses that are transmitted to their offspring. J. Invertebr. Pathol. 2014, 122, 22–27. [Google Scholar] [CrossRef]
- Carballo, A.; Murillo, R.; Jakubowska, A.; Herrero, S.; Williams, T.; Caballero, P. Co-infection with iflaviruses influences the insecticidal properties of Spodoptera exigua multiple nucleopolyhedrovirus occlusion bodies: Implications for the production and biosecurity of baculovirus insecticides. PLoS ONE 2017, 12, e0177301. [Google Scholar] [CrossRef]
- Cook, S.P.; Webb, R.E.; Thorpe, K.W. Potential enhancement of the gypsy moth (Lepidoptera: Lymantriidae) nuclear polyhedrosis virus with the triterpene azadirachtin. Environ. Entomol. 1996, 25, 1209–1214. [Google Scholar] [CrossRef]
- Guido-Cira, N.D.; Tamez-Guerra, P.; Mireles-Martínez, M.; Villegas-Mendoza, J.M.; Rosas-García, N.M. Activity of Bacillus thuringiensis- and baculovirus-based formulations to Spodoptera species. Southwest Entomol. 2017, 42, 191–201. [Google Scholar] [CrossRef]
- Hesketh, H.; Hails, R.S. Bacillus thuringiensis impacts on primary and secondary baculovirus transmission dynamics in Lepidoptera. J. Invertebr. Pathol. 2015, 132, 171–181. [Google Scholar] [CrossRef]
- Chang, J.H.; Choi, J.Y.; Jin, B.R.; Roh, J.Y.; Olszewski, J.A.; Seo, S.J.; O’Reilly, D.R.; Je, Y.H. An improved baculovirus insecticide producing occlusion bodies that contain Bacillus thuringiensis insect toxin. J. Invertebr. Pathol. 2003, 84, 30–37. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kanwar, R.K.; Sehgal, A.; Cahill, D.M.; Barrow, C.J.; Sehgal, R.; Kanwar, J.R. Progress on Azadirachta indica based biopesticides in replacing synthetic toxic pesticides. Front. Plant Sci. 2017, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, D.R. Baculovirus-encoded ecdysteroid UDP-glucosyltransferases. Insect Biochem. Mol. Biol. 1995, 25, 541–550. [Google Scholar] [CrossRef]
| Active Ingredient | Commercial Product | Company | Mode of Action IRAC 1 | MFRC 2 |
|---|---|---|---|---|
| Autographa californica multiple nucleopolyhedrovirus (AcMNPV) | - | - | 31 | - |
| Azadirachtin 1% (azadirachtin A) [EC] | NeemAzal T/S® | Agrichem S.A. (Madrid, Spain) | Unknown | 3 mL L−1 |
| Bacillus thuringiensis subspecies aizawai (GC-91) 50% [WP] | Turex® | Mitsui Agriscience S.A. (Brussels, Belgium) | 11A | 2 g L−1 |
| Cyantraniliprole 10% + Acibenzolar-S-methyl 1.25% [SC] | Minecto Alpha® | Syngenta S.A. (Madrid, Spain) | 28 | 1 mL L−1 |
| Emamectin 0.855% [SG] | Affirm® | Syngenta S.A. (Madrid, Spain) | 6 | 1.5 g L−1 |
| Metaflumizone 24% [SC] | Alverde® | BASF S.L. (Barcelona, Spain) | 22B | 1 mL L−1 |
| Methoxyfenozide 24% [SC] | Runner® | Corteva Agroscience S.A. (Sevilla, Spain) | 18 | 0.4 mL L−1 |
| Spinetoram 25% [WP] | Delegate® | Corteva Agroscience S.A. (Sevilla, Spain) | 5 | 0.4 g L−1 |
| Spodoptera littoralis nucleopolyhedrovirus (SpliNPV) | - | - | 31 | - |
| Active Ingredient | Concentrations Tested | LC50 (95% Fiducial Limits) | LC95 (95% Fiducial Limits) | Slope ± SEM | t-Ratio | χ2 | df | Heterogeneity |
|---|---|---|---|---|---|---|---|---|
| AcMNPV | 1.7 × 103, 1.7 × 105, 1.7 × 107, 1.7 × 109 OBs mL−1 | 1.7 × 104 OBs mL−1 (8.1 × 103–3.4 × 104) | 1.6 × 106 OBs mL−1 (4.3 × 105–4.1 × 106) | 0.926 ± 0.122 | 7.572 | 0.292 | 2 | 0.146 |
| Azadirachtin | 0.375, 0.75, 1.5, 3, 6 mL L−1 | 2.108 mL L−1 (1.729–2.437) | 4.635 mL L−1 (3.819–6.520) | 4.807 ± 0.825 | 5.829 | 2.407 | 3 | 0.802 |
| Bacillus thuringiensis | 0.125, 0.25, 0.5, 1, 2, 4 g L−1 | 0.769 g L−1 (0.505–1.034) | 3.616 g L−1 (2.395–8.058) | 2.447 ± 0.320 | 7.644 | 4.307 | 4 | 1.076 |
| Cyantraniliprole | 0.1, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4 mL L−1 | 0.384 mL L−1 (0.154–0.646) | 4.964 mL L−1 (2.441–25.391) | 1.479 ± 0.203 | 7.287 | 8.192 | 5 | 1.638 |
| Emamectin | 0.025, 0.05, 0.1, 0.2, 0.4 g L−1 | 0.082 g L−1 (0.069–0.096) | 0.278 g L−1 (0.220–0.386) | 3.109 ± 0.331 | 9.405 | 2.403 | 3 | 0.801 |
| Metaflumizone | 0.0625, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16, 32 mL L−1 | 4.354 mL L−1 (1.982–6.274) | 15.484 mL L−1 (10.237–46.487) | 2.985 ± 0.538 | 5.551 | 14.08 | 8 | 1.760 |
| Methoxyfenozide | 0.01, 0.02, 0.04, 0.08, 0.16, 0.32, 0.64 mL L−1 | 0.077 mL L−1 (0.052–0.099) | 0.398 mL L−1 (0.262–0.928) | 2.300 ± 0.446 | 5.156 | 3.638 | 5 | 0.728 |
| Spinetoram | 0.005, 0.01, 0.02, 0.04, 0.08, 0.16 g L−1 | 0.008 g L−1 (0.006–0.011) | 0.058 g L−1 (0.041–0.096) | 1.928 ± 0.262 | 7.363 | 3.723 | 4 | 0.931 |
| Active Ingredient | Concentrations Tested | LC50 (95% Fiducial Limits) | LC95 (95% Fiducial Limits) | Slope ± SEM | t-Ratio | χ2 | df | Heterogeneity |
|---|---|---|---|---|---|---|---|---|
| Azadirachtin | 0.75, 1.5, 3, 6, 12, 24, 48, 96 mL L−1 | 3.069 mL L−1 (2.240–4.064) | 53.337 mL L−1 (33.593–101.411) | 1.326 ± 0.129 | 10.31 | 4.010 | 6 | 0.668 |
| Bacillus thuringiensis | 0.125, 0.25, 0.5, 1, 2, 4, 8, 16 g L−1 | 2.604 g L−1 (1.348–3.581) | 9.423 g L−1 (6.310–28.300) | 2.945 ± 0.503 | 5.852 | 9.867 | 6 | 1.645 |
| Cyantraniliprole | 0.1, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4 mL L−1 | 0.546 mL L−1 (0.410–0.699) | 5.227 mL L−1 (3.535–9.199) | 1.677 ± 0.182 | 9.192 | 3.495 | 5 | 0.699 |
| Emamectin | 0.003, 0.006, 0.012, 0.025, 0.05, 0.1, 0.2, 0.4 g L−1 | 0.054 g L−1 (0.038–0.068) | 0.163 g L−1 (0.117–0.346) | 3.430 ± 0.589 | 5.828 | 6.700 | 6 | 1.117 |
| Metaflumizone | 1, 2, 4, 8, 16, 32 mL L−1 | 3.155 mL L−1 (2.401–3.954) | 17.453 mL L−1 (12.556–28.640) | 2.214 ± 0.274 | 8.087 | 1.305 | 4 | 0.326 |
| Methoxyfenozide | 0.02, 0.04, 0.08, 0.16, 0.32, 0.64, 1.28, 2.56, 5.12, 10.24 mL L−1 | 0.858 mL L−1 (0.473–1.256) | 6.199 mL L−1 (3.493–23.395) | 1.915 ± 0.284 | 6.743 | 13.44 | 8 | 1.680 |
| SpliNPV | 1.0 × 102, 1.0 × 103, 1.0 × 104, 1.0 × 105, 1.0 × 106, 1.0 × 107 OBs mL−1 | 1.7 × 105 OBs mL−1 (8.1 × 104–3.2 × 105) | 7.0 × 106 OBs mL−1 (2.8 × 106–3.3 × 107) | 1.019 ± 0.102 | 9.977 | 4.719 | 4 | 1.180 |
| Spinetoram | 0.0025, 0.005, 0.01, 0.02, 0.04, 0.08, 0.16 g L−1 | 0.024 g L−1 (0.017–0.031) | 0.098 g L−1 (0.068–0.184) | 2.676 ± 0.292 | 9.170 | 6.467 | 5 | 1.293 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dáder, B.; Aguirre, E.; Caballero, P.; Medina, P. Synergy of Lepidopteran Nucleopolyhedroviruses AcMNPV and SpliNPV with Insecticides. Insects 2020, 11, 316. https://doi.org/10.3390/insects11050316
Dáder B, Aguirre E, Caballero P, Medina P. Synergy of Lepidopteran Nucleopolyhedroviruses AcMNPV and SpliNPV with Insecticides. Insects. 2020; 11(5):316. https://doi.org/10.3390/insects11050316
Chicago/Turabian StyleDáder, Beatriz, Eduardo Aguirre, Primitivo Caballero, and Pilar Medina. 2020. "Synergy of Lepidopteran Nucleopolyhedroviruses AcMNPV and SpliNPV with Insecticides" Insects 11, no. 5: 316. https://doi.org/10.3390/insects11050316
APA StyleDáder, B., Aguirre, E., Caballero, P., & Medina, P. (2020). Synergy of Lepidopteran Nucleopolyhedroviruses AcMNPV and SpliNPV with Insecticides. Insects, 11(5), 316. https://doi.org/10.3390/insects11050316