Host Plant and Antibiotic Effects on Scent Bouquet Composition of Anastrepha ludens and Anastrepha obliqua Calling Males, Two Polyphagous Tephritid Pests
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Host Plant Effects on Male Effluvia Composition
3.2. Antibiotic Treatment Experiment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Müller, M.; Buchbauer, G. Essential oil components as pheromones. A review. Flavour Fragr. J. 2011, 26, 357–377. [Google Scholar] [CrossRef]
- Lancaster, J.; Khrimian, A.; Young, S.; Lehner, B.; Luck, K.; Wallingford, A.; Sparks, M.E. De novo formation of an aggregation pheromone precursor by an isoprenyl diphosphate synthase-related terpene synthase in the harlequin bug. Proc. Natl. Acad. Sci. USA 2018, 115, E8634–E8641. [Google Scholar] [CrossRef]
- Henneken, J.; Goodger, J.Q.D.; Jones, T.M.; Elgar, M.A. Diet-mediated pheromones and signature mixtures can enforce signal reliability. Front. Ecol. Evol. 2017, 4, 145. [Google Scholar] [CrossRef]
- Engl, T.; Kaltenpoth, M. Influence of microbial symbionts on insect pheromones. Nat. Prod. Rep. 2018, 35, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Butenandt, A.; Beckmann, R.; Stamm, D.; Hecker, E. Uber den sexual-lockstoff des seidenspinners Bombyx mori. Reindarstellung und Konstitution. Z. Nat. 1959, 14, 283–284. [Google Scholar]
- Berger, R.S. Isolation, identification and synthesis of sex attractant of the cabbage looper, Trichoplusia ni. Ann. Entomol. Soc. Am. 1966, 59, 767–771. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Rodin, J.; Wood, D.L. 1966. Sex attractants in frass produced by male Ips confusus (LeC.) in Ponderosa pine. Science 1966, 154, 509–510. [Google Scholar]
- Roelofs, W.L.; Brown, R.L. Pheromones and evolutionary relationships of Tortricidae. Annu. Rev. Ecol. Syst. 1982, 3, 395–422. [Google Scholar] [CrossRef]
- Landolt, P.J.; Phillips, T.W. Host plant influences on sex pheromones of phytophagous insects. Annu. Rev. Entomol. 1997, 42, 371–391. [Google Scholar] [CrossRef]
- Tillman, J.A.; Seybold, S.J.; Jurenka, R.A.; Blomquist, G.J. Insect pheromones—An overview of biosynthesis and endocrine regulation. Insect Biochem. Mol. Biol. 1999, 29, 481–514. [Google Scholar] [CrossRef]
- Cardé, R.T.; Minks, A.K. Insect Pheromone Research: New Directions; Springer: New York, NY, USA, 2012; p. 684. [Google Scholar]
- Gregg, P.C.; Del Socorro, A.P.; Landolt, P.J. Advances in attract-and-kill for agricultural pests: Beyond pheromones. Ann. Rev. Entomol. 2018, 63, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Engler-Chaouat, H.S.; Gilbert, L.E. De novo synthesis vs. sequestration: Negatively correlated metabolic traits and the evolution of host plant specialization in cyanogenic butterflies. J. Chem. Ecol. 2007, 33, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Nishida, R. Chemical ecology of insect-plant interactions: Ecological significance of plant secondary metabolites. Biosci. Biotechnol. Biochem. 2014, 78, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Blomquist, G.J.; Tittiger, C.; Jurenka, R. Cuticular Hydrocarbons and pheromones of arthropods. In Hydrocarbons, Oils and Lipids: Diversity, Origin, Chemistry and Fate; Wilkes, H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–31. [Google Scholar]
- Bjostad, L.B.; Wolf, W.A.; Roelofs, W.L. Pheromone biosynthesis in lepidopterans: Desaturation and chain shortening. In Pheromone biochemistry; Academic Press: Cambridge, MA, USA, 1987; pp. 77–120. [Google Scholar]
- Jurenka, R. Regulation of pheromone biosynthesis in moths. Curr. Opin. Insect Sci. 2017, 24, 29–35. [Google Scholar] [CrossRef]
- Lofstedt, C.; Vickers, N.J.; Roelofs, W.L.; Baker, T.C. Diet related courtship success in the Oriental fruit moth, Grapholita molesta (Torticidae). Oikos 1989, 55, 402–408. [Google Scholar] [CrossRef]
- Darragh, K.; Byers, K.J.R.P.; Merrill, R.M.; McMillan, W.O.; Schulz, S.; Jiggins, C.D. Male pheromone composition depends on larval diet but not adult diet in Heliconius melpomene. Ecol. Entomol. 2019, 44, 397–405. [Google Scholar] [CrossRef]
- Boppré, M. Lepidoptera and pyrrolizidine alkaloids exemplification of complexity in chemical ecology. J. Chem. Ecol. 1990, 16, 165–185. [Google Scholar] [CrossRef]
- Nishida, R. Sequestration of defensive substances from plants by Lepidoptera. Annu. Rev. Entomol. 2002, 47, 57–92. [Google Scholar] [CrossRef]
- Foster, S.P.; Anderson, K.G.; Harmon, J.P. Increased allocation of adult-acquired carbohydrate to egg production results in its decreased allocation to sex pheromone production in mated females of the moth Heliothis virescens. J. Exp. Biol. 2014, 217, 499–506. [Google Scholar] [CrossRef]
- Krasnoff, S.B.; Roelofs, W.L. Quantitative and qualitative effects of larval diet on male scent secretions of Estigmene acrea, Phragmatobia foliginosa, and Pyrrharctia isabella (Lepidoptera: Arctiidae). J. Chem. Ecol. 1989, 15, 1077–1093. [Google Scholar] [CrossRef]
- Conner, W.F.; Roach, B.; Benedict, E.; Meinwald, J.; Eisner, T. Courtship pheromone production and body size as correlates of larval diet in males of the arctiid moth, Utetheisa ornatrix. J. Chem. Ecol. 1990, 16, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, R.L.; Mitchell, W.C.; Fukuto, T.R.; Metcalf, E.R. Attraction of the oriental fruit fly, Dacus dorsalis, by methyl eugenol and related olfactory stimulants. Proc. Natl. Acad. Sci. USA 1975, 72, 2501–2505. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.H.; Nishida, R.; Toong, T.C. Floral synomone of a wild orchid, Bulbophyllum cheiri lures Bactrocera fruit flies for pollination. J. Chem. Ecol. 2002, 28, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.H.; Nishida, R. Methyl eugenol: Its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. J. Insect Sci. 2012, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.H.; Nishida, R. Synomone or kairomone?—Bulbophyllum apertum flowers releases raspberry ketone to attract Bactrocera fruit flies. J. Chem. Ecol. 2005, 31, 497–507. [Google Scholar]
- Tan, K.H.; Nishida, R. Mutual reproductive benefits between a wild orchid, Bulbophyllum patens, and Bactrocera fruit flies via a floral synomone. J. Chem. Ecol. 2000, 26, 533–546. [Google Scholar] [CrossRef]
- Nishida, R.; Tan, K.H.; Serit, M.; Lajis, N.H.; Sukari, A.M.; Takahashi, S.; Fukami, H. Accumulation of phenylpropanoids in the rectal glands of males of the Oriental fruit fly Dacus dorsalis. Experientia 1988, 44, 534–536. [Google Scholar] [CrossRef]
- Nishida, R.O.; Iwahashi, O.; Tan, K.H. Accumulation of Dendrobium superbum (orchidacea) fragrance in the rectal glands by males of the melon fly, Dacus cucurbitae. J. Chem. Ecol. 1993, 19, 713–722. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R. Sex pheromone and mating competition after methyl eugenol consumption in the Bactrocera dorsalis complex. In Fruit Fly Pests: A World Assessment of Their Biology and Management; McPheron, B.A., Steck, G.J., Eds.; St. Lucie Press: Delray Beach, FL, USA, 1996; pp. 147–153. [Google Scholar]
- Wee, S.L.; Tan, K.H. Female sexual response to male rectal volatile constituents in the fruit fly, Bactrocera carambolae (Diptera: Tephritidae). Appl. Entomol. Zool. 2005, 40, 365–372. [Google Scholar] [CrossRef]
- Tan, K.H.; Tokushima, I.; Ono, H.; Nishida, R. Comparison of phenylpropanoid volatiles in male rectal pheromone gland after methyl eugenol consumption, and molecular phylogenetic relationship of four global pest fruit fly species: Bactrocera invadens, B. dorsalis, B. correcta and B. zonata. Chemoecology 2011, 21, 25–33. [Google Scholar] [CrossRef]
- Epsky, N.D.; Heath, R.R. Food availability and pheromone production by males of Anastrepha suspensa (Diptera: Tephritidae). Environ. Entomol. 1993, 22, 942–947. [Google Scholar] [CrossRef]
- Sivinski, J.; Epsky, N.; Heath, R.R. Pheromone deposition on leaf territories by male Caribbean fruit flies, Anastrepha suspensa (Low) (Diptera: Tephritidae). J. Insect Behav. 1994, 7, 43–51. [Google Scholar] [CrossRef]
- Lu, F.; Teal, P.E.A. Sex pheromone components in oral secretions and crop of male Caribbean fruit flis, Anastrepha suspensa (Loew). Arch. Insect Biochem. Physiol. 2001, 48, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Aluja, M.; Jácome, I.; Macías-Ordóñez, R. Effect of adult nutrition on male sexual performance in four neotropical fruit fly species of the genus Anastrepha (Diptera: Tephritidae). J. Insect Behav. 2001, 14, 759–775. [Google Scholar] [CrossRef]
- Shelly, T.E.; Edu, J.; Pahio, E.; Nishimoto, J. Scented males and choosy females: Does male odor influence female mate choice in the Mediterranean fruit fly? J. Chem. Ecol. 2007, 33, 2308–2324. [Google Scholar] [CrossRef]
- Shelly, T.E.; Edu, J.; Pahio, E. Condition-dependent mating success in male fruit flies: Ingestion of a pheromone precursor compensates for a low-quality diet. J. Insect Behav. 2007, 20, 347–365. [Google Scholar] [CrossRef]
- Liedo, P.; Orozco, D.; Cruz-López, L.; Quintero, J.L.; Becerra-Pérez, C.; Del Refugio-Hernández, M.; Oropeza, A.; Toledo, J. Effect of post-teneral diets on the performance of sterile Anastrepha ludens and Anastrepha obliqua fruit flies. J. Appl. Entomol. 2013, 137, 49–60. [Google Scholar] [CrossRef]
- Merli, D.; Mannucci, B.; Bassetti, F.; Corana, F.; Falchetto, M.; Malacrida, A.R.; Gasperi, G.; Scolari, F. Larval diet affects male pheromone blend in a laboratory strain of the Medfly, Ceratitis capitata (Diptera: Tephritidae). J. Chem. Ecol. 2018, 44, 339–353. [Google Scholar] [CrossRef]
- Shelly, T.E. Larval host plant influences male body size and mating success in a tephritid fruit fly. Entomol. Exp. Appl. 2018, 166, 41–52. [Google Scholar] [CrossRef]
- Birke, A.; Aluja, M. Do mothers really know best? Complexities in testing the preference-performance hypothesis in polyphagous frugivorous fruit flies. Bull. Entomol. Res. 2018, 108, 674–684. [Google Scholar] [CrossRef]
- Thomas, D.B. Mexican fruit fly (Diptera: Tephritidae) and the phenology of its native host plant yellow chapote (Rutaceae) in Mexico. J. Entomol. Sci. 2012, 47, 1–16. [Google Scholar] [CrossRef]
- Birke, A.; Guillén, L.; Midgarden, D.; Aluja, M. Fruit flies, Anastrepha ludens (Loew), A. obliqua (Macquart) and A. grandis (Macquart) (Diptera: Tephritidae): Three pestiferous tropical fruit flies that could potentially expand their range to temperate areas. In Potential Invasive Pests of Agricultural Crops; Peña, J., Ed.; CABI International: Wallingford, UK, 2013; pp. 192–213. [Google Scholar]
- Aluja, M.; Piñero, J.; López, M.; Ruíz, C.; Zúñiga, A.; Piedra, E.; Díaz-Fleischer, F.; Sivinski, J. New host plant and distribution records in Mexico for Anastrepha spp., Toxotrypana curvicauda Gerstacker, Rhagoletis zoqui Bush, Rhagoletis sp., and Hexachaeta sp. (Diptera: Tephritidae). Proc. Entomol. Soc. Wash. 2000, 10, 802–815. [Google Scholar]
- Birke, A.; Aluja, M. Anastrepha ludens and Anastrepha serpentina (Diptera: Tephritidae) do not infest Psidium guajava (Myrtaceae), but Anastrepha obliqua occasionally shares this resource with Anastrepha striata in nature. J. Econ. Entomol. 2011, 104, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Bressan, S.; Da Costa Teles, M. Lista de hospedeiros e índices de infestatação de algumas espécies do gênero Anastrepha Schiner, 1868 (Diptera: Tephritidae) na região de Ribeirão Preto-SP. An. Soc. Entomol. Bras. 1991, 20, 5–15. [Google Scholar]
- Hernández-Ortíz, V.; Aluja, M. Listado de especies del género neotropical Anastrepha (Diptera: Tephritidae) con notas sobre su distribución y plantas hospederas. Folia Entomol. Mex. 1993, 88, 89–105. [Google Scholar]
- Battiste, M.A.; Strekowski, L.; Vanderbilt, D.P.; Visnick, M.; King, R.W.; Nation, J.L. Anastrephin and epianastrephin, novel lactone components isolated from the sex pheromone blend of male Caribbean and Mexican fruit flies. Tetrahedron Lett. 1983, 24, 2611–2614. [Google Scholar] [CrossRef]
- Stokes, J.B.; Uebel, E.C.; Warthen Jr, J.D.; Jacobson, M.; Flippen-Anderson, J.L.; Gilardi, R.; Spishakoff, L.M.; Wilzer, K.R. Isolation and identification of novel lactones from male Mexican fruit flies. J. Agric. Food. Chem. 1983, 31, 1162–1167. [Google Scholar] [CrossRef]
- Robacker, D.C.; Hart, W.G. (Z)-3 nonenol, (Z-Z)-3,6-nonadienol and (S,S)-(-)epianastrephin: Male-produced pheromones of the Mexican fruit fly. Entomol. Exp. Appl. 1985, 39, 103–108. [Google Scholar] [CrossRef]
- Rocca, J.R.; Nation, J.L.; Strekowski, L.; Battiste, M.A. Comparison of volatiles emitted by male Caribbean and Mexican fruit flies. J. Chem. Ecol. 1992, 18, 223–244. [Google Scholar] [CrossRef]
- Heath, R.R.; Landolt, P.J.; Robacker, D.C.; Dueben, B.D.; Epsky, N.D. Sexual pheromones of Tephritid flies: Clues to unravel phylogeny and behavior. In Fruit Flies (Tephritidae) Phylogeny and evolution of Behavior; Aluja, M., Norrbom, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 793–810. [Google Scholar]
- Quintero-Fong, L.; Toledo, J.; Ruiz, L.; Rendón, P.; Orozco-Dávila, D.; Cruz, L.; Liedo, P. Selection by mating competitiveness improves the performance of Anastrepha ludens males of the genetic sexing strain Tapachula-7. Bull. Entomol. Res. 2016, 106, 624–632. [Google Scholar] [CrossRef]
- Bosa, C.F.; Cruz-López, L.; Zepeda-Cisneros, C.S.; Valle-Mora, J.; Guillén-Navarro, K.; Liedo, P. Sexual behavior and male volatile compounds in wild and mass-reared strains of the Mexican fruit fly Anastrepha ludens (Diptera: Tephritidae) held under different colony management regimes. Insect Sci. 2016, 23, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Birke, A.; Acosta, E.; Aluja, M. Limits to the host range of the highly polyphagous tephritid fruit fly Anastrepha ludens in its natural habitat. Bull. Entomol. Res. 2015, 105, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Aluja, M.; Mangan, R.L. Fruit fly (Diptera: Tephritidae) host status determination: Critical conceptual, methodological, and regulatory considerations. Annu. Rev. Entomol. 2008, 53, 473–502. [Google Scholar] [CrossRef] [PubMed]
- Capuzzo, C.; Firrao, G.; Mazzon, L.; Squartini, A.; Girolami, V. ‘Candidatus Erwinia dacicola’, a coevolved symbiotic bacterium of the olive fly Bactrocera oleae (Gmelin). Int. J. Syst. Evol. Microbiol. 2005, 55, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Gavriel, S.; Jurkevitch, E.; Yuval, B. Bacterially enriched diet improves sexual performance of sterile male Mediterranean fruit flies. J. Appl. Entomol. 2011, 135, 564–576. [Google Scholar] [CrossRef]
- Rashid, M.A.; Andongma, A.A.; Dong, Y.C.; Ren, X.M.; Niu, C.Y. Effect of gut bacteria on fitness of the Chinese citrus fly, Bactrocera minax (Diptera: Tephritidae). Symbiosis 2018, 76, 63–69. [Google Scholar] [CrossRef]
- Klassen, W. Area-wide integrated pest management and the sterile insect technique. In Sterile Insect Technique; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 39–68. [Google Scholar]
- Aluja, M.; Díaz-Fleischer, F.; Arredondo, J. Non-host status of commercial Persea americana cultivar ‘Hass’ to Anastrepha ludens, Anastrepha obliqua, Anastrepha serpentina, and Anastrepha striata (Diptera: Tephritidae) in Mexico. J. Econ. Entomol. 2004, 97, 293–309. [Google Scholar] [CrossRef]
- Aluja, M.; Birke, A.; Ceymann, M.; Guillén, L.; Arrigoni, E.; Baumgartner, D.; Pascacio-Villafán, C.; Samietz, J. Agroecosystem resilience to an invasive insect species that could expand its geographical range in response to global climate change. Agric. Ecosyst. Environ. 2014, 186, 54–63. [Google Scholar] [CrossRef]
- Pascacio-Villafán, C.; Williams, T.; Birke, A.; Aluja, M. Nutritional and non-nutritional food components modulate phenotypic variation but not physiological trade-offs in an insect. Sci. Rep. 2016, 6, 29413. [Google Scholar] [CrossRef]
- Aluja, M.; Sivinski, J.; Ovruski, S.; Guillen, L.; Lopez, M.; Cancino, J.; Torres-Anaya, A.; Gallegos-Chang, G.; Ruiz, L. Colonization and domestication of seven species of native New World hymenopterous larval-prepupal and pupal fruit fly (Diptera: Tephritidae) parasitoids. Biocontrol Sci. Technol. 2009, 19, 49–79. [Google Scholar] [CrossRef]
- Anderson, M.J.; Whitcomb, P.J. RSM Simplified-Optimizing Processes Using Response Surface Methods for Design of Experiments; Productivity Inc.: New York, NY, USA, 2005. [Google Scholar]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Aluja, M.; Ordano, M.; Teal, P.E.; Sivinski, J.; García-Medel, D.; Anzures-Dadda, A. Larval feeding substrate and species significantly influence the effect of a juvenile hormone analog on sexual development/performance in four tropical tephritid flies. J. Insect Physiol. 2009, 55, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Staples, D.; Aluja, M.; Macías-Ordóñez, R.; Sivinski, J. Reproductive trade-offs from mating with a successful male: The case of the tephritid fly Anastrepha obliqua. Behav. Ecol. Sociobiol. 2008, 62, 1333–1340. [Google Scholar] [CrossRef]
- Ibañez-Lopez, A.; Cruz-Lopez, L. Glándulas salivales de Anastrepha obliqua (Macquart) (Diptera: Tephritidae): Análisis químico y morfológico, y actividad biológica de los componentes volátiles. Folia Entomol. Mex. 2001, 40, 221–231. [Google Scholar]
- Meza-Hernández, J.S.; Hernández, E.; Salvador-Figueroa, M.; Cruz-López, L. Sexual compatibility, mating performance and sex pheromone release of mass-reared and wild Anastrepha obliqua (Diptera: Tephritidae) under field-cage conditions. Proceedings of 6th International Fruit Fly Symposium, Stellenbosch, South Africal, 6–10 May 2002; pp. 99–104. [Google Scholar]
- López-Guillén, G.; Cruz-López, L.; Malo, E.A.; González-Hernández, H.; Llanderal-Cázares, C.; López-Collado, J.; Toledo, J.; Rojas, J.C. Factors influencing the release of volatiles in Anastrepha obliqua males (Diptera: Tephritidae). Environ. Entomol. 2008, 37, 876–882. [Google Scholar] [CrossRef]
- López-Guillén, G.; López, L.C.; Malo, E.A.; Rojas, J.C. Olfactory responses of Anastrepha obliqua (Diptera: Tephritidae) to volatiles emitted by calling males. Fla. Entomol. 2011, 94, 874–881. [Google Scholar] [CrossRef]
- Gonçalves, G.B.; Silva, C.E.; De Lima Mendonça, A.; Vaníčková, L.; Tomčala, A.; Nascimento, R.R.D. Pheromone communication in Anastrepha obliqua (Diptera: Tephritidae): A comparison of the volatiles and salivary gland extracts of two wild populations. Fla. Entomol. 2013, 96, 1365–1374. [Google Scholar] [CrossRef]
- Muñoz-Barrios, R.; Cruz-López, L.; Rojas, J.C.; Hernández, E.; Liedo, P.; Gómez-Simuta, Y.; Malo, E.A. Influence of methoprene on pheromone emission and sexual maturation of Anastrepha obliqua (Diptera: Tephritidae) males. J. Econ. Entomol. 2016, 109, 637–643. [Google Scholar] [CrossRef]
- Orozco-Dávila, D.; Quintero, L.; Hernández, E.; Solís, E.; Artiaga, T.; Hernández, R.; Montoya, P. Mass rearing and sterile insect releases for the control of Anastrepha spp. pests in Mexico–a review. Entomol. Exp. Appl. 2017, 164, 176–187. [Google Scholar] [CrossRef]
- Aguila, J.R.; Suszko, J.; Gibbs, A.G.; Hoshizaki, D.K. The role of larval fat cells in adult Drosophila melanogaster. J. Exp. Biol. 2007, 210, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Dion, E.; Monteiro, A.; Yew, J.Y. Phenotypic plasticity in sex pheromone production in Bicyclus anynana butterflies. Sci. Rep. 2016, 6, 39002. [Google Scholar] [CrossRef] [PubMed]
- West-Eberhard, M.J. Developmental plasticity and evolution; Oxford University Press: New York, NY, USA, 2003; p. 816. [Google Scholar]
- Forsman, A. Rethinking phenotypic plasticity and its consequences for individuals, populations and species. Heredity 2015, 115, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.; Park, S.J.; Taylor, P.W. Domestication modifies the volatile emissions produced by male Queensland fruit flies during sexual advertisement. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fitt, G.P.; O’Brien, R. Bacteria associated with four species of Dacus (Diptera: Tephritidae) and their role in the nutrition of the larvae. Oecologia 1985, 67, 447–454. [Google Scholar] [CrossRef]
- Howard, D.J.; Bush, G.L.; Breznak, J.A. The evolutionary significance of bacteria associated with Rhagoletis. Evolution 1985, 39, 405–417. [Google Scholar] [CrossRef]
- Drew, R.; Lloyd, A. Relationship of fruit flies (Diptera: Tephritidae) and their bacteria to host plants. Ann. Entomol. Soc. Am. 1987, 80, 629–636. [Google Scholar] [CrossRef]
- Ben-Yosef, M.; Jurkevith, E.; Yuval, B. Effect of bacteria on nutritional status and reproductive success of the Mediterranean fruit fly Ceratitis capitata. Physiol. Entomol. 2008, 33, 145–154. [Google Scholar] [CrossRef]
- Ben-Yosef, M.; Pasternak, Z.; Jurkevitch, E.; Yuval, B. Symbiotic bacteria enable olive fly larvae to overcome host defences. R. Soc. Open Sci. 2015, 2, 150170. [Google Scholar] [CrossRef]
- Ventura, C.; Briones-Roblero, C.I.; Hernández, E.; Rivera-Orduña, F.N.; Zúñiga, G. Comparative analysis of the gut bacterial community of four Anastrepha fruit flies (Diptera: Tephritidae) based on pyrosequencing. Curr. Microbiol. 2018, 75, 966–976. [Google Scholar] [CrossRef]
- Wybouw, N.; Pauchet, Y.; Heckel, D.G.; Van Leuuwen, T. Horizontal gene transfer contributes to the evolution of arthropod herbivory. Genome Biol. Evol. 2016, 8, 1785–1801. [Google Scholar] [CrossRef] [PubMed]
- Kutchan, T.; Gershenzon, J.; Møller, B.L.; Gang, D. Natural products. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; John Wiley & Sons: Oxford, UK, 2015; pp. 1132–1206. [Google Scholar]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. In Biotechnology of isoprenoids Advances in Biochemical Engineering/Biotechnology; Schrader, J., Bohlmann, J., Eds.; Springer: Cham, Switzerland, 2015; pp. 63–106. [Google Scholar]
- Lichtenthaler, H.K. The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Physiol. 1999, 50, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat. Prod. Rep. 1999, 16, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Sarria, S.; Wong, B.; Martín, H.G.; Keasling, J.D.; Peralta-Yahya, P. Microbial synthesis of pinene. ACS Synth. Biol. 2014, 3, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Kuzuyama, T. Biosynthetic studies on terpenoids produced by Streptomyces. J. Antibiot. 2017, 70, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Beran, F.; Köllner, T.G.; Gershenzon, J.; Tholl, D. Chemical convergence between plants and insects: Biosynthetic origins and functions of common secondary metabolites. New Phytol. 2019, 223, 52–67. [Google Scholar] [CrossRef]
- Bellés, X.; Martín, D.; Piulachs, M.D. The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu. Rev. Entomol. 2005, 50, 181–199. [Google Scholar] [CrossRef]
- Degenhardt, J.; Köllner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef]
- Dickschat, J.S. Bacterial terpene cyclases. Nat. Prod. Rep. 2016, 33, 87–110. [Google Scholar] [CrossRef]
- Aluja, M.; Celedonio-Hurtado, H.; Liedo, P.; Cabrera, M.; Castillo, F.; Guillén, J.; Rios, E. Seasonal population fluctuations and ecological implications for management of Anastrepha fruit flies (Diptera: Tephritidae) in commercial mango orchards in Southern Mexico. J. Econ. Entomol. 1996, 89, 654–667. [Google Scholar] [CrossRef]
- Thomas, D.B. Hot peppers as a host for the Mexican fruit fly Anastrepha ludens (Diptera: Tephritidae). Fla. Entomol. 2004, 87, 603–608. [Google Scholar] [CrossRef]
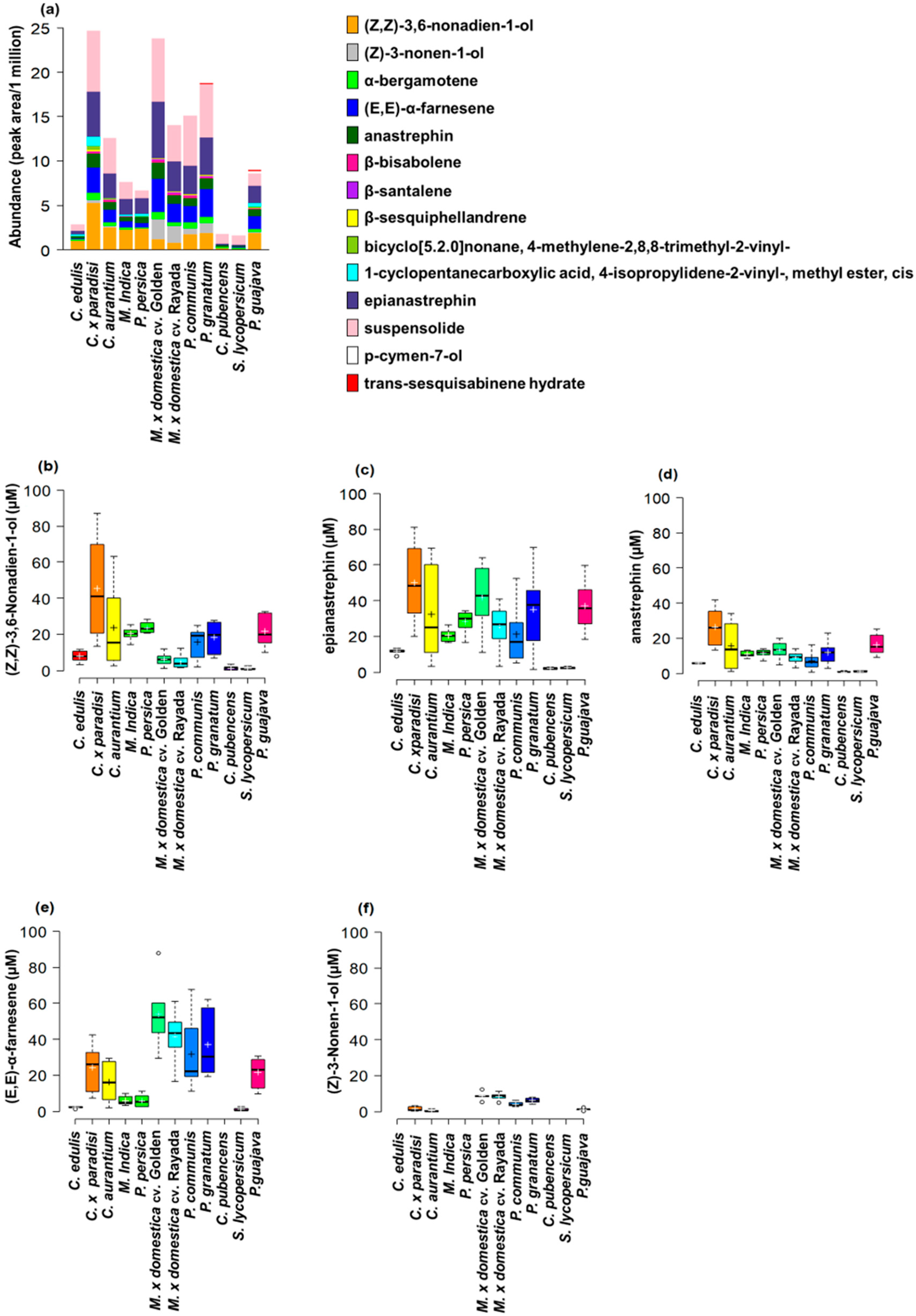
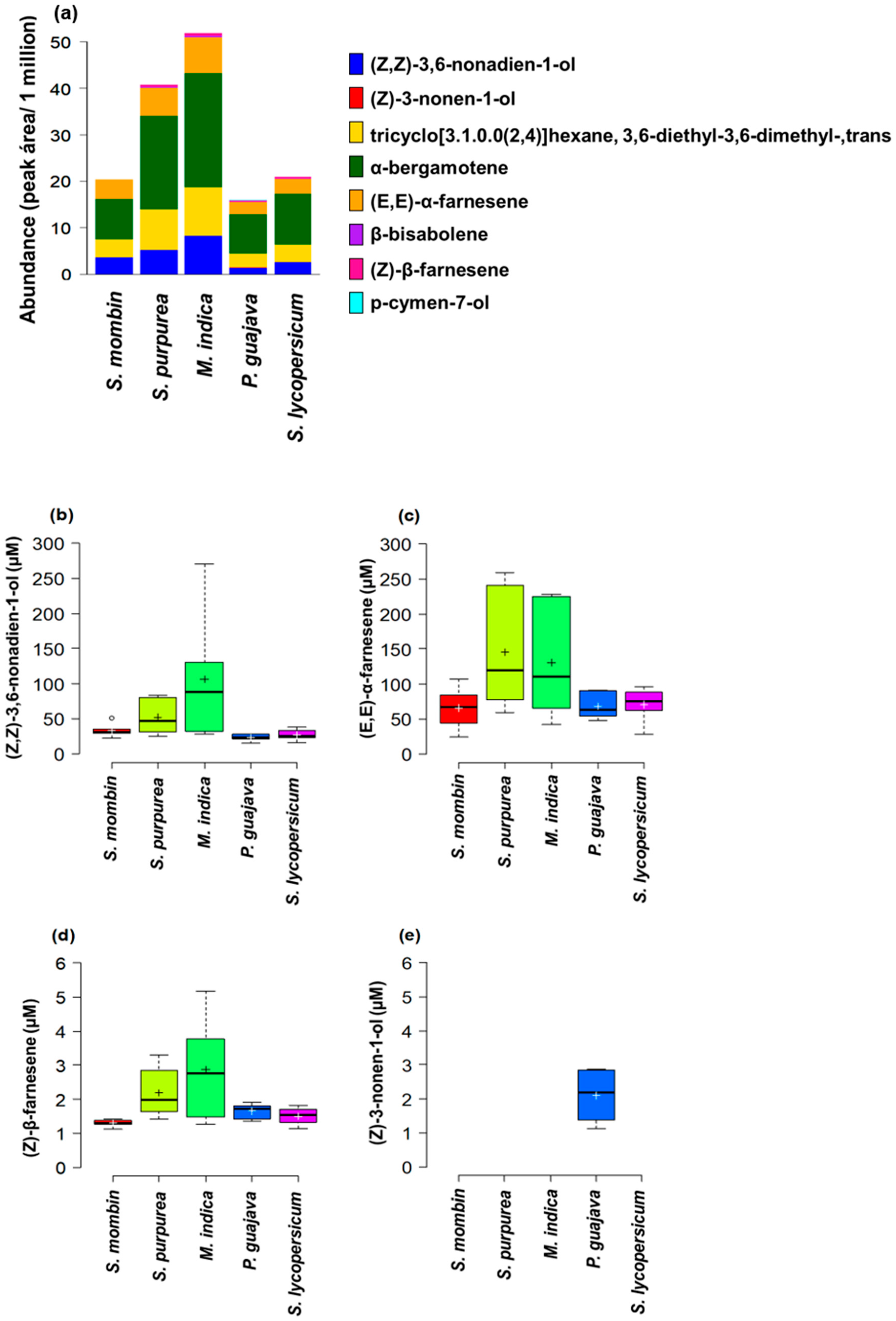
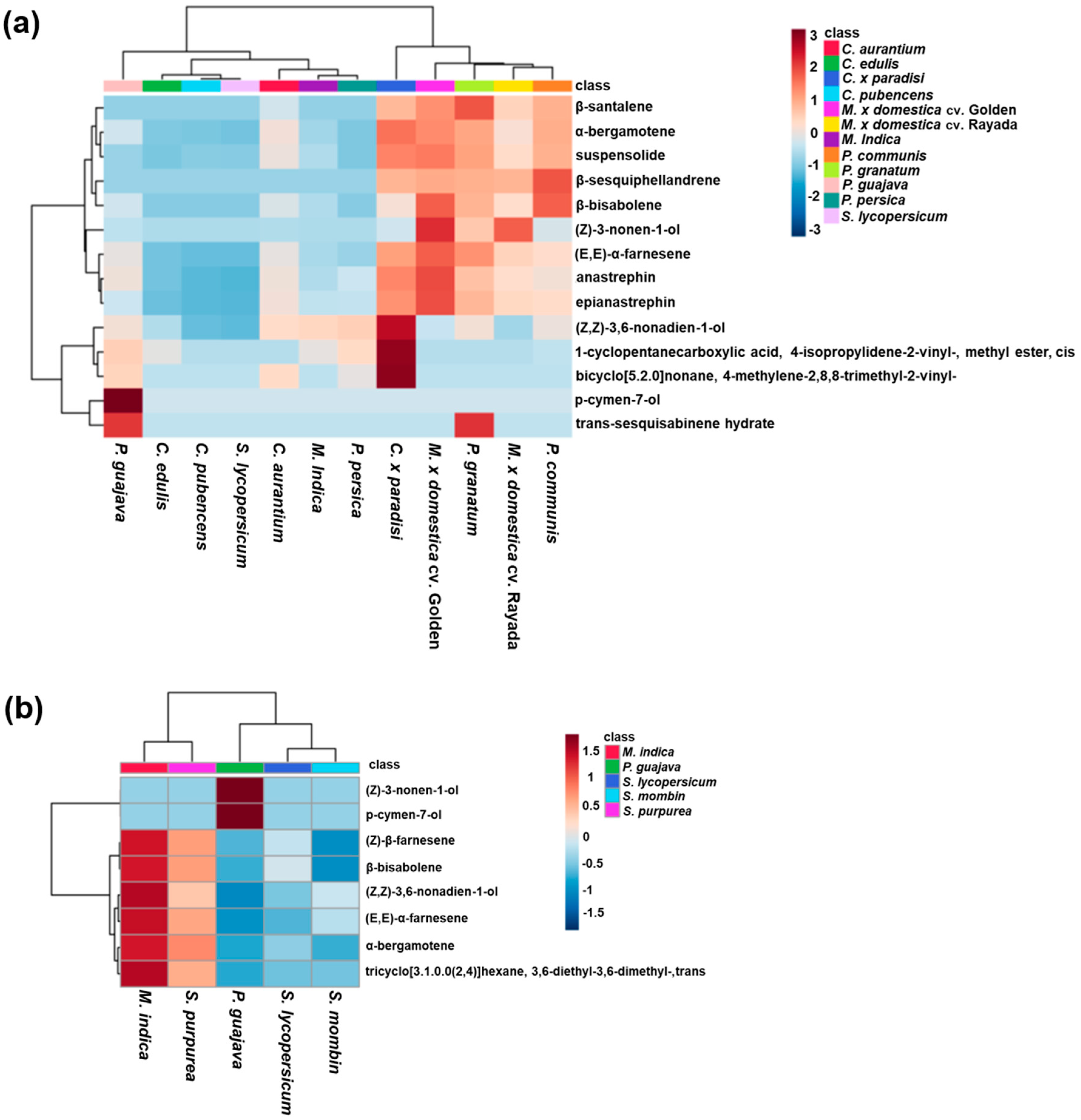
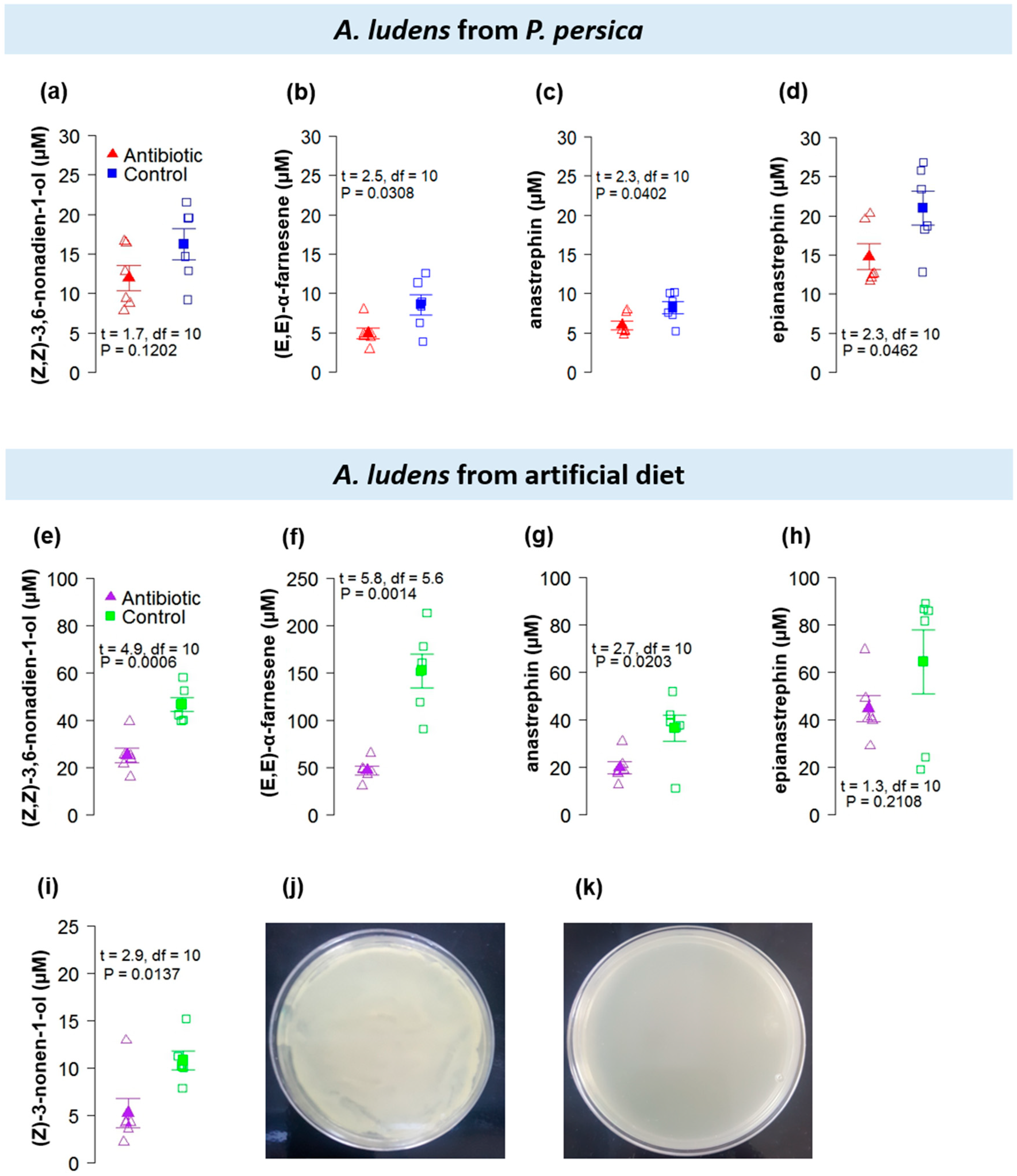
| Fly Species | Plant Species | Plant Family | Type of Infestation |
|---|---|---|---|
| A. ludens | Casimiroa edulis1 | Rutaceae | Naturally infested |
| Citrus aurantium2 | Rutaceae | Naturally infested | |
| C. × paradisi cv. ‘Marsh’ 2 | Rutaceae | Naturally infested | |
| Mangifera indica cv. ‘Manila’ 2,5 | Anacardiaceae | Laboratory forced infestation | |
| Prunus persica cv. ‘Criollo’ 2 | Rosaseae | Forced field infestation | |
| Pyrus communis2 | Rosaceae | Naturally infested | |
| Malus × domestica cv. ‘Rayada’ 2 | Rosaceae | Forced field infestation | |
| Malus × domestica cv. ‘Golden Delicious’ 2 | Rosaceae | Forced field infestation | |
| Punica granatum2 | Lythraceae | Naturally infested | |
| Capsicum pubescens2 | Solanaceae | Forced field infestation | |
| Solanum lycopersicum cv. ‘Saladette’ 3 | Solanaceae | Forced field infestation | |
| Psidium guajava cv. ‘Criolla’ 3 | Myrtaceae | Forced field infestation | |
| A. obliqua | Spondias mombin1 | Anacardiaceae | Naturally infested |
| M. indica cv. ‘Manila’ 2 | Anacardiaceae | Naturally infested | |
| Spondias purpurea4 | Anacardiaceae | Forced field infestation | |
| S. lycopersicum cv. ‘Saladette’ 3 | Solanaceae | Forced field infestation | |
| P. guajava cv. ‘Criolla’ 3 | Myrtaceae | Forced field infestation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aluja, M.; Cabagne, G.; Altúzar-Molina, A.; Pascacio-Villafán, C.; Enciso, E.; Guillén, L. Host Plant and Antibiotic Effects on Scent Bouquet Composition of Anastrepha ludens and Anastrepha obliqua Calling Males, Two Polyphagous Tephritid Pests. Insects 2020, 11, 309. https://doi.org/10.3390/insects11050309
Aluja M, Cabagne G, Altúzar-Molina A, Pascacio-Villafán C, Enciso E, Guillén L. Host Plant and Antibiotic Effects on Scent Bouquet Composition of Anastrepha ludens and Anastrepha obliqua Calling Males, Two Polyphagous Tephritid Pests. Insects. 2020; 11(5):309. https://doi.org/10.3390/insects11050309
Chicago/Turabian StyleAluja, Martín, Gabriela Cabagne, Alma Altúzar-Molina, Carlos Pascacio-Villafán, Erick Enciso, and Larissa Guillén. 2020. "Host Plant and Antibiotic Effects on Scent Bouquet Composition of Anastrepha ludens and Anastrepha obliqua Calling Males, Two Polyphagous Tephritid Pests" Insects 11, no. 5: 309. https://doi.org/10.3390/insects11050309
APA StyleAluja, M., Cabagne, G., Altúzar-Molina, A., Pascacio-Villafán, C., Enciso, E., & Guillén, L. (2020). Host Plant and Antibiotic Effects on Scent Bouquet Composition of Anastrepha ludens and Anastrepha obliqua Calling Males, Two Polyphagous Tephritid Pests. Insects, 11(5), 309. https://doi.org/10.3390/insects11050309





