Can Vibrational Playbacks Disrupt Mating or Influence Other Relevant Behaviours in Bactericera cockerelli (Triozidae: Hemiptera)?
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects’ Rearing
2.2. Recordings
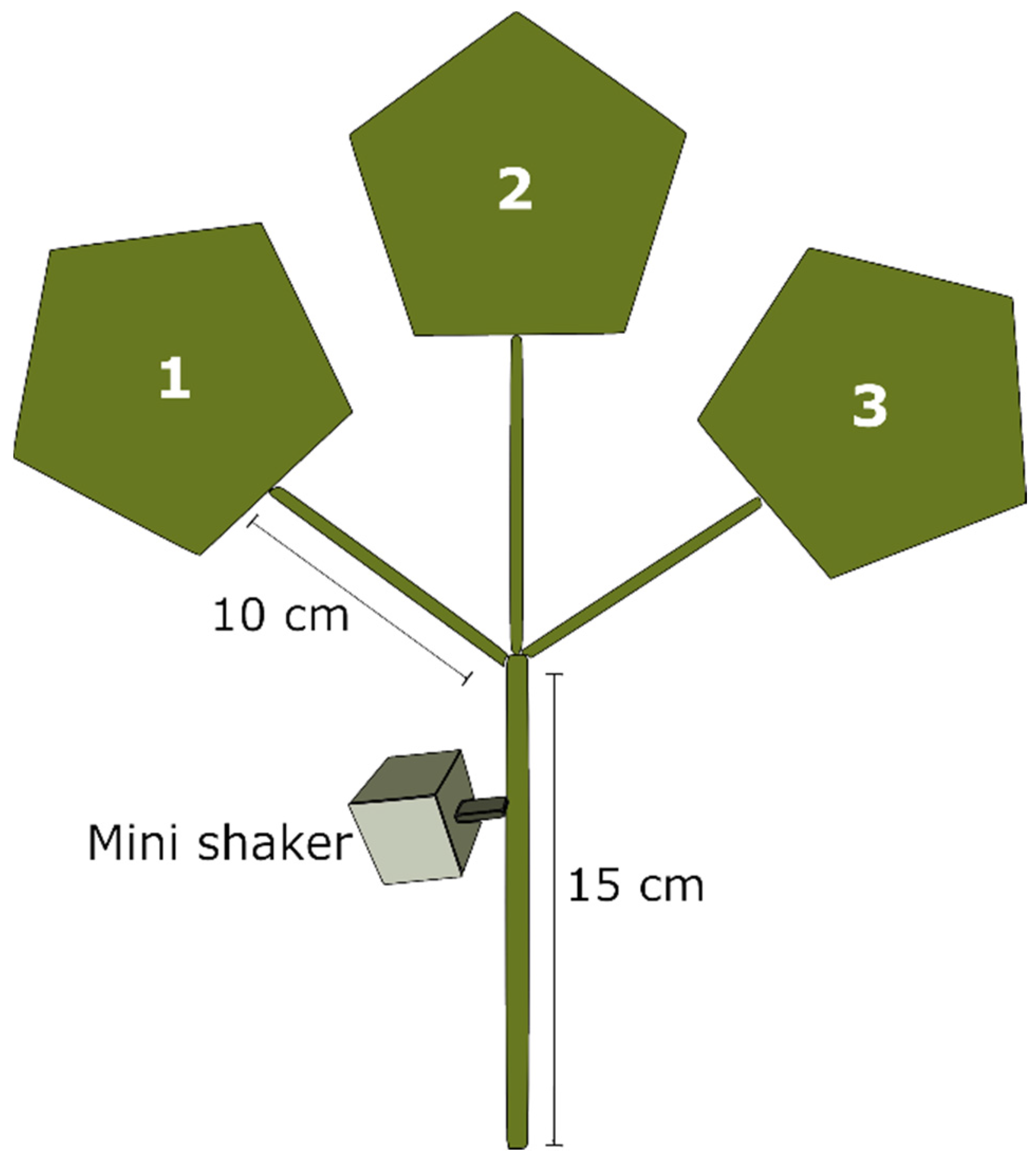
2.3. Playback Trials
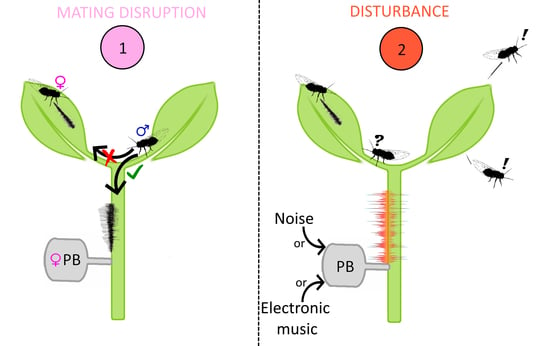
2.3.1. Test 1. Female Playback on Single Males
2.3.2. Test 2. Female Playback on Pairs
2.3.3. Test 3. Unspecific Noise Playbacks
2.4. Data Analysis
3. Results
3.1. Test 1. Female Playback Tested on Single Males
3.2. Test 2. Female Playback Tested on Pairs
3.3. Test 3. Disturbance Playbacks
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hill, P.S.M. Vibration and Animal Communication: A Review 1. Am. Zool. 2001, 41, 1135–1142. [Google Scholar] [CrossRef]
- Virant-Doberlet, M.; Cokl, A. Vibrational Communication in Insects. Neotrop. Entomol. 2004, 33, 121–134. [Google Scholar] [CrossRef]
- Polajnar, J.; Eriksson, A.; Lucchi, A.; Anfora, G.; Virant-Doberlet, M.; Mazzoni, V. Manipulating Behaviour with Substrate-Borne Vibrations—Potential for Insect Pest Control. Pest Manag. Sci. 2015, 71, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, T.; Uechi, N.; Tatsuta, H. Vibrations in Hemipteran and Coleopteran Insects: Behaviors and Application in Pest Management. Appl. Entomol. Zool. 2019, 54, 21–29. [Google Scholar] [CrossRef]
- Polajnar, J.; Eriksson, A.; Virant-Doberlet, M.; Mazzoni, V. Mating Disruption of a Grapevine Pest Using Mechanical Vibrations: From Laboratory to the Field. J. Pest Sci. 2016, 89, 909–921. [Google Scholar] [CrossRef]
- Mazzoni, V.; Nieri, R.; Eriksson, A.; Virant-Doberlet, M.; Polajnar, J.; Anfora, G.; Lucchi, A. Mating Disruption by Vibrational Signals: State of the Field and Perspectives. In Biotremology: Studying Vibrational Behavior; Hill, P.S.M., Lakes-Harlan, R., Mazzoni, V., Narins, P.M., Virant-Doberlet, M., Wessel, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 331–354. [Google Scholar] [CrossRef]
- Lujo, S.; Hartman, E.; Norton, K.; Pregmon, E.A.; Rohde, B.B.; Mankin, R.W. Disrupting Mating Behavior of Diaphorina citri (Liviidae). J. Econ. Entomol. 2016, 109, 2373–2379. [Google Scholar] [CrossRef] [PubMed]
- Mankin, R.W. Vibrational Trapping and Interference with Mating of Diaphorina citri. In Biotremology: Studying Vibrational Behavior; Hill, P.S.M., Lakes-Harlan, R., Mazzoni, V., Narins, P.M., Virant-Doberlet, M., Wessel, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 399–413. [Google Scholar] [CrossRef]
- Gordon, S.D.; Sandoval, N.; Mazzoni, V.; Krugner, R. Mating Interference of Glassy-Winged Sharpshooters, Homalodisca vitripennis. Entomol. Exp. Appl. 2017, 164, 27–34. [Google Scholar] [CrossRef]
- Gordon, S.D.; Krugner, R. Mating Disruption by Vibrational Signals: Applications for Management of the Glassy-Winged Sharpshooter. In Biotremology: Studying Vibrational Behavior; Hill, P.S.M., Lakes-Harlan, R., Mazzoni, V., Narins, P.M., Virant-Doberlet, M., Wessel, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 355–373. [Google Scholar] [CrossRef]
- Mazzoni, V.; Polajnar, J.; Baldini, M.; Rossi Stacconi, M.V.; Anfora, G.; Guidetti, R.; Maistrello, L. Use of Substrate-Borne Vibrational Signals to Attract the Brown Marmorated Stink Bug, Halyomorpha halys. J. Pest Sci. 2017, 90, 1219–1229. [Google Scholar] [CrossRef]
- Polajnar, J.; Maistrello, L.; Ibrahim, A.; Mazzoni, V. Can Vibrational Playback Improve Control of an Invasive Stink Bug? In Biotremology: Studying Vibrational Behavior; Hill, P.S.M., Lakes-Harlan, R., Mazzoni, V., Narins, P.M., Virant-Doberlet, M., Wessel, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 375–398. [Google Scholar] [CrossRef]
- Munyaneza, J.E.; Crosslin, J.M.; Upton, J.E. Association of Bactericera cockerelli (Homoptera: Psyllidae) with “Zebra Chip,” a New Potato Disease in Southwestern United States and Mexico. J. Econ. Entomol. 2007, 100, 656–663. [Google Scholar] [CrossRef]
- Butler, C.D.; Trumble, J.T. The Potato Psyllid, Bactericera cockerelli (Sulc) (Hemiptera: Triozidae): Life History, Relationship to Plant Diseases, and Management Strategies. Terr. Arthropod Rev. 2012, 5, 87–111. [Google Scholar] [CrossRef]
- Secor, G.A.; Rivera-Varas, V.V. Emerging Diseases of Cultivated Potato and Their Impact on Latin America. Rev. Latinoam. la Papa 2004, 1, 1–8. [Google Scholar]
- Teulon, D.A.J.; Workman, P.J.; Thomas, K.L.; Nielsen, M.C. Bactericera cockerelli: Incursion, Dispersal and Current Distribution on Vegetable Crops in New Zealand. N. Z. Plant Prot. 2009, 62, 136–144. [Google Scholar] [CrossRef]
- Liefting, L.W.; Perez-Egusquiza, Z.C.; Clover, G.R.G.; Anderson, J.A.D. A New ‘Candidatus Liberibacter’ Species in Solanum tuberosum in New Zealand. Plant Dis. 2008, 92, 1474. [Google Scholar] [CrossRef] [PubMed]
- Sengoda, V.G.; Cooper, W.R.; Swisher, K.D.; Henne, D.C.; Munyaneza, J.E. Latent Period and Transmission of “Candidatus Liberibacter Solanacearum” by the Potato Psyllid Bactericera cockerelli (Hemiptera: Triozidae). PLoS ONE 2014, 9, e93475. [Google Scholar] [CrossRef] [PubMed]
- Munyaneza, J.E. Zebra Chip Disease of Potato: Biology, Epidemiology, and Management. Am. J. Potato Res. 2012, 89, 329–350. [Google Scholar] [CrossRef]
- Percy, D.M.; Taylor, G.S.; Kennedy, M. Psyllid Communication: Acoustic Diversity, Mate Recognition and Phylogenetic Signal. Invertebr. Syst. 2006, 20, 431–445. [Google Scholar] [CrossRef]
- Lubanga, U.K.; Guédot, C.; Percy, D.M.; Steinbauer, M.J. Semiochemical and Vibrational Cues and Signals Mediating Mate Finding and Courtship in Psylloidea (Hemiptera): A Synthesis. Insects 2014, 5, 577–595. [Google Scholar] [CrossRef]
- Liao, Y.C.; Yang, M.M. Acoustic Communication of Three Closely Related Psyllid Species: A Case Study in Clarifying Allied Species Using Substrate-Borne Signals (Hemiptera: Psyllidae: Cacopsylla). Ann. Entomol. Soc. Am. 2015, 108, 902–911. [Google Scholar] [CrossRef]
- Avosani, S.; Sullivan, T.E.; Ciolli, M.; Mazzoni, V.; Suckling, D.M. Vibrational Communication and Evidence of Vibrational Behavioural Manipulation of the Tomato Potato Psyllid Bactericera cockerelli. Entomol. Gen. 2020, in press. [Google Scholar]
- Lee, Y.; Kim, H.; Kang, T.J.; Jang, Y. Stress Response to Acoustic Stimuli in an Aphid: A Behavioral Bioassay Model. Entomol. Res. 2012, 42, 320–329. [Google Scholar] [CrossRef]
- Wikipedia Home Page. Available online: https://en.wikipedia.org/wiki/Scary_Monsters_and_Nice_Sprites (accessed on 5 May 2020).
- Dieng, H.; The, C.C.; Satho, T.; Miake, F.; Wydiamala, E.; Kassim, N.F.A.; Hashim, N.A.; Morales Vargas, R.E.; Morales, N.P. The Electronic Song “Scary Monsters and Nice Sprites” Reduces Host Attack and Mating Success in the Dengue Vector Aedes aegypti. Acta Trop. 2019, 194, 93–99. [Google Scholar] [CrossRef] [PubMed]
- De Groot, M.; Čokl, A.; Virant-Doberlet, M. Search Behaviour of Two Hemipteran Species Using Vibrational Communication. Cent. Eur. J. Biol. 2011, 6, 756–769. [Google Scholar] [CrossRef]
- Guédot, C.; Horton, D.R.; Landolt, P.J.; Munyaneza, J.E. Effect of Mating on Sex Attraction in Bactericera cockerelli with Evidence of Refractoriness. Entomol. Exp. Appl. 2013, 149, 27–35. [Google Scholar] [CrossRef]
- Michelsen, A.; Fink, F.; Gogala, M.; Traue, D. Plants as Transmission Channels for Insect Vibrational Songs. Behav. Ecol. Sociobiol. 1982, 11, 269–281. [Google Scholar] [CrossRef]
- Mankin, R.W.; Stanaland, D.; Haseeb, M.; Rohde, B.; Menocal, O.; Carrillo, D. Assessment of Plant Structural Characteristics, Health, and Ecology Using Bioacoustic Tools. Proc. Meet. Acoust 2018, 33, 010003. [Google Scholar]
- Wenninger, E.J.; Hall, D.G.; Mankin, R.W. Vibrational Communication Between the Sexes in Diaphorina citri (Hemiptera: Psyllidae). Ann. Entomol. Soc. Am. 2009, 102, 547–555. [Google Scholar] [CrossRef]
- Hodge, S.; Bennett, J.; Merfield, C.N.; Hofmann, R.W. Effects of Sticky Trap Colour, UV Illumination and within-Trap Variation on Tomato Potato Psyllid Captures in Glasshouses. N. Z. J. Crop Hortic. Sci. 2019, 47, 48–62. [Google Scholar] [CrossRef]
- Gwynne, D.T. Sexual Competition among Females: What Causes Courtship-Role Reversal? Trends Ecol. Evol. 1991, 6, 118–121. [Google Scholar] [CrossRef]
- Mazzoni, V.; Polajnar, J.; Virant-Doberlet, M. Secondary Spectral Components of Substrate-Borne Vibrational Signals Affect Male Preference. Behav. Process. 2015, 115, 53–60. [Google Scholar] [CrossRef]
- Mazzoni, V.; Gordon, S.D.; Nieri, R.; Krugner, R. Design of a Candidate Vibrational Signal for Mating Disruption against the Glassy-Winged Sharpshooter, Homalodisca vitripennis. Pest Manag. Sci. 2017, 73, 2328–2333. [Google Scholar] [CrossRef]
- Čokl, A.; Dias, A.M.; Moraes, M.C.B.; Borges, M.; Laumann, R.A. Rivalry between Stink Bug Females in a Vibrational Communication Network. J. Insect Behav. 2017, 30, 741–758. [Google Scholar] [CrossRef]
- Svensson, B.G.; Petersson, E.; Forsgren, E. Why Do Males of the Dance Fly Empis borealis Refuse to Mate? The Importance of Female Age and Size. J. Insect Behav. 1989, 2, 387–395. [Google Scholar] [CrossRef]
- Smith, R.L. Paternity Assurance and Altered Roles in the Mating Behaviour of a Giant Water Bug, Abedus herberti (Heteroptera: Belostomatidae). Anim. Behav. 1979, 27, 716–725. [Google Scholar] [CrossRef]
- Greenfield, M.D. Cooperation and Conflict in the Evolution of Signal Interactions. Ann. Rev. Ecol. Syst. 1994, 25, 97–126. [Google Scholar] [CrossRef]
- Greenfield, M.D. Signal Interactions and Interference in Insect Choruses: Singing and Listening in the Social Environment. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2014, 201, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Tishechkin, D.Y.; Drosopoulos, S.; Claridge, M.F. Vibratory Communication in Psylloidea (Hemiptera). In Insect Sounds and Communication: Physiology, Behaviour, Ecology and Evolution; Dros, S., Claridge, M.F., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 357–363. [Google Scholar]
- Tsubaki, R.; Hosoda, N.; Kitajima, H.; Takanashi, T. Substrate-Borne Vibrations Induce Behavioral Responses in the Leaf-Dwelling Cerambycid, Paraglenea fortunei. Zoolog. Sci. 2014, 31, 789–794. [Google Scholar] [CrossRef]
- Caorsi, V.Z.; Both, C.; Cechin, S.; Antunes, R.; Borges-Martins, M. Effects of Traffic Noise on the Calling Behavior of Two Neotropical Hylid Frogs. PLoS ONE 2017, 12, e0183342. [Google Scholar] [CrossRef]
- Gilsdorf, J.M.; Hygnstrom, S.E.; VerCauteren, K.C. Use of Frightening Devices in Wildlife Damage Management. Integr. Pest Manag. Rev. 2002, 7, 29–45. [Google Scholar] [CrossRef]
- McNett, G.D.; Luan, L.H.; Cocroft, R.B. Wind-Induced Noise Alters Signaler and Receiver Behavior in Vibrational Communication. Behav. Ecol. Sociobiol. 2010, 64, 2043–2051. [Google Scholar] [CrossRef]
- Samarra, F.I.P.; Klappert, K.; Brumm, H.; Miller, P.J.O. Background Noise Constrains Communication: Acoustic Masking of Courtship Song in the Fruit Fly Drosophila montana. Behaviour 2009, 146, 1635–1648. [Google Scholar] [CrossRef]
- Nieri, R.; Mazzoni, V. Vibrational Mating Disruption of Empoasca Vitis by Natural or Artificial Disturbance Noises. Pest Manag. Sci. 2019, 75, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Čokl, A.; Virant-Doberlet, M.; Zorović, M. Sense Organs Involved in the Vibratory Communication of Bugs. In Insect Sounds and Communication; CRC Press: Boca Raton, FL, USA, 2005; pp. 80–89. [Google Scholar]
- Henry, C.S. Sexual Behavior of Green Lacewings [Chrysopidae] [1984]. Ser. Entomol. 2013, 27, 101–110. [Google Scholar]
- Devetak, D.; Amon, T. Substrate Vibration Sensitivity of the Leg Scolopidial Organs in the Green Lacewing, Chrysoperla carnea. J. Insect Physiol. 1997, 43, 433–437. [Google Scholar] [CrossRef]
- Senior, L.J.; McEwen, P.K. The Use of Lacewings in Biological Control. In Lacewings in the Crop Environment; McEwan, P.K., New, T.R., Whittington, A.E., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 296–302. [Google Scholar]
- Harrewijn, P.; Kayser, H. Pymetrozine, a Fast-Acting and Selective Inhibitor of Aphid Feeding. In-Situ Studies with Electronic Monitoring of Feeding Behaviour. Pestic. Sci. 1997, 49, 130–140. [Google Scholar] [CrossRef]
- Tishechkin, D.Y. New Data on Vibratory Communication in Jumping Plant Lice of the Families Aphalaridae and Triozidae (Homoptera, Psyllinea). Entomol. Rev. 2007, 87, 394–400. [Google Scholar] [CrossRef]
- Lubanga, U.K.; Peters, R.A.; Steinbauer, M.J. Substrate-Borne Vibrations of Male Psyllids Vary with Body Size and Age but Females Are Indifferent. Anim. Behav. 2016, 120, 173–182. [Google Scholar] [CrossRef]
- Oppedisano, T.; Polajnar, J.; Kostanjšek, R.; De Cristofaro, A.; Ioriatti, C.; Virant-Doberlet, M.; Mazzoni, V. Substrate-Borne Vibrational Communication in the Vector of Apple Proliferation Disease Cacopsylla picta (Hemiptera: Psyllidae). J. Econ. Entomol. 2020, 113, 596–603. [Google Scholar] [CrossRef]
- Nuhardiyati, M.; Bailey, W. Calling and Duetting Behavior in the Leafhopper Balclutha incisa (Hemiptera: Cicadellidae: Deltocephalinae): Opportunity for Female Choice? J. Insect Behav. 2005, 18, 259–280. [Google Scholar] [CrossRef]
- Polajnar, J.; Eriksson, A.; Rossi Stacconi, M.V.; Lucchi, A.; Anfora, G.; Virant-Doberlet, M.; Mazzoni, V. The Process of Pair Formation Mediated by Substrate-Borne Vibrations in a Small Insect. Behav. Process. 2014, 107, 68–78. [Google Scholar] [CrossRef]
- Burckhardt, D.; Ouvrard, D.; Queiroz, D.; Percy, D. Psyllid Host-Plants (Hemiptera: Psylloidea): Resolving a Semantic Problem. Fla. Entomol. 2014, 97, 242–246. [Google Scholar] [CrossRef]
- Scharf, I.; Martin, O.Y. Same-Sex Sexual Behavior in Insects and Arachnids: Prevalence, Causes, and Consequences. Behav. Ecol. Sociobiol. 2013, 67, 1719–1730. [Google Scholar] [CrossRef]
- Guédot, C.; Horton, D.R.; Landolt, P.J. Sex Attraction in Bactericera cockerelli (Hemiptera: Triozidae). Environ. Entomol. 2010, 39, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Soroker, V.; Talebaev, S.; Harari, A.R.; Wesley, S.D. The Role of Chemical Cues in Host and Mate Location in the Pear Psylla Cacopsylla bidens (Homoptera: Psyllidae). J. Insect Behav. 2004, 17, 613–626. [Google Scholar] [CrossRef]
- Horton, D.R.; Landolt, P.J. Attraction of Male Pear Psylla, Cacopsylla pyricola, to Female-Infested Pear Shoots. Entomol. Exp. Appl. 2007, 123, 177–183. [Google Scholar] [CrossRef]
- Saxena, K.N.; Kumar, H. Interruption of Acoustic Communication and Mating in a Leafhopper and a Planthopper by Aerial Sound Vibrations Picked up by Plants. Experientia 1980, 36, 933–936. [Google Scholar] [CrossRef]
| Measured Parameters | Number of Males Analysed for Each Parameter | χ2 | df | p | |
|---|---|---|---|---|---|
| Control | Playback | ||||
| Number of searching males | 27 | 26 | 0.16 | 1 | 1 |
| Number of mating | 17 | 7 | 6.94 | 1 | ≤0.001 |
| Number of males that reached the mini shaker | 0 | 8 | 7.5 | 1 | 0.006 |
| Measured Parameters | Number of Males Analysed for Each Parameter | Median | U | p | ||
|---|---|---|---|---|---|---|
| Control | Playback | Control | Playback | |||
| Start of male search | 27 | 26 | 180 s | 365 s | 250 | 0.07 |
| Time to reach the female | 17 | 7 | 980 s | 960 s | 56.5 | 0.8 |
| Time to reach the node | 17 | 7 | 660 s | 712 s | 56.5 | 0.8 |
| Number of times the male visited the female leaf | 25 | 27 | 1 | 1 | 274 | 0.19 |
| Number of times the male visited other leaves | 25 | 27 | 1 | 2 | 213 | 0.01 |
| Number of times the male went towards the stem | 25 | 27 | 0 | 1 | 195.5 | 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avosani, S.; Sullivan, T.E.; Ciolli, M.; Mazzoni, V.; Suckling, D.M. Can Vibrational Playbacks Disrupt Mating or Influence Other Relevant Behaviours in Bactericera cockerelli (Triozidae: Hemiptera)? Insects 2020, 11, 299. https://doi.org/10.3390/insects11050299
Avosani S, Sullivan TE, Ciolli M, Mazzoni V, Suckling DM. Can Vibrational Playbacks Disrupt Mating or Influence Other Relevant Behaviours in Bactericera cockerelli (Triozidae: Hemiptera)? Insects. 2020; 11(5):299. https://doi.org/10.3390/insects11050299
Chicago/Turabian StyleAvosani, Sabina, Thomas E. Sullivan, Marco Ciolli, Valerio Mazzoni, and David Maxwell Suckling. 2020. "Can Vibrational Playbacks Disrupt Mating or Influence Other Relevant Behaviours in Bactericera cockerelli (Triozidae: Hemiptera)?" Insects 11, no. 5: 299. https://doi.org/10.3390/insects11050299
APA StyleAvosani, S., Sullivan, T. E., Ciolli, M., Mazzoni, V., & Suckling, D. M. (2020). Can Vibrational Playbacks Disrupt Mating or Influence Other Relevant Behaviours in Bactericera cockerelli (Triozidae: Hemiptera)? Insects, 11(5), 299. https://doi.org/10.3390/insects11050299