First Report Using a Native Lacewing Species to Control Tuta absoluta: From Laboratory Trials to Field Assessment
Abstract
:1. Introduction
2. Material and Methods
2.1. Biological Materials
2.2. Laboratory Predation Trials
2.3. Field Assessment
2.4. Data Analyses
3. Results
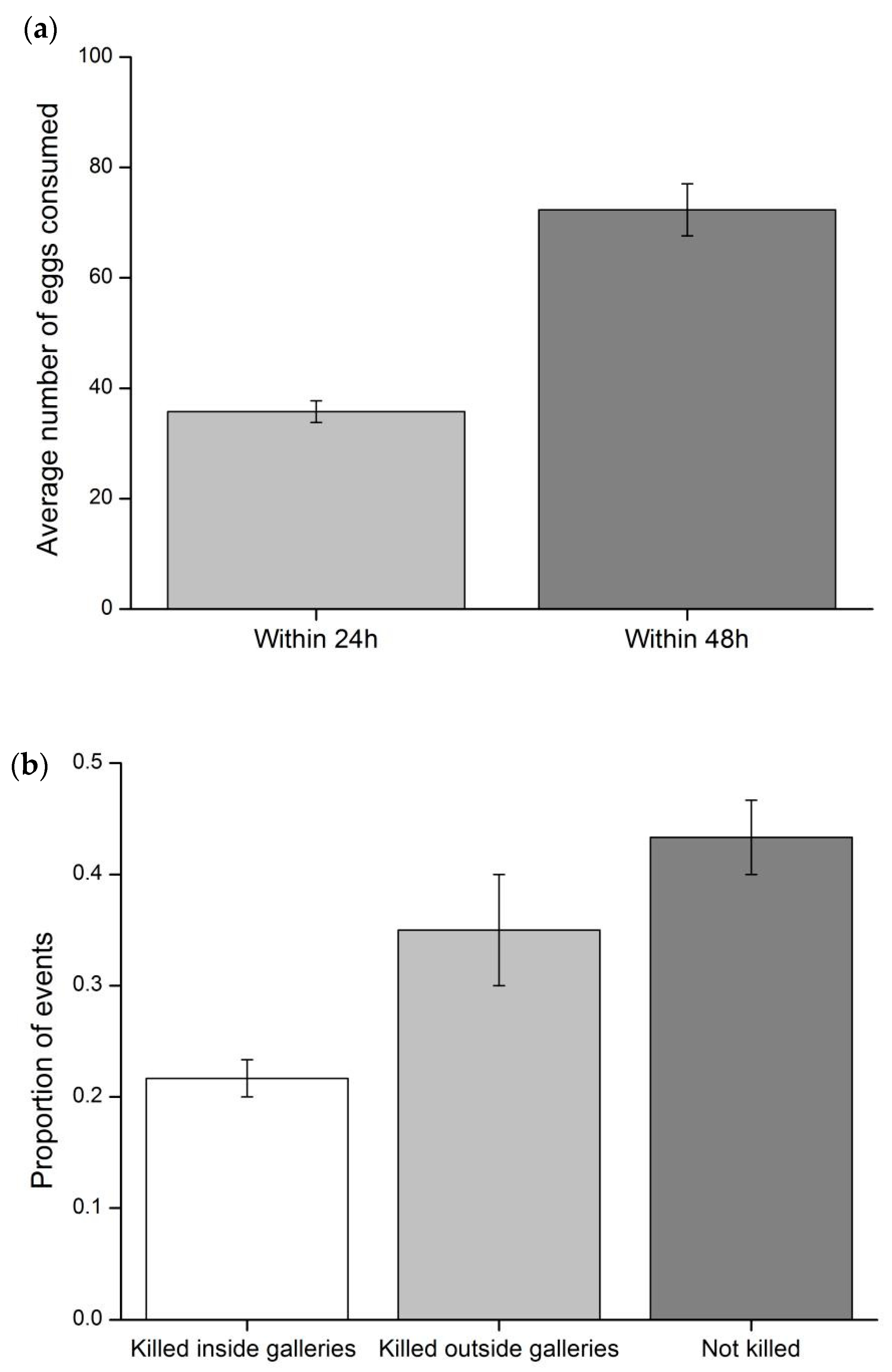
3.1. Predation Rate of C. carnea Larvae on T. absoluta Eggs and Larvae
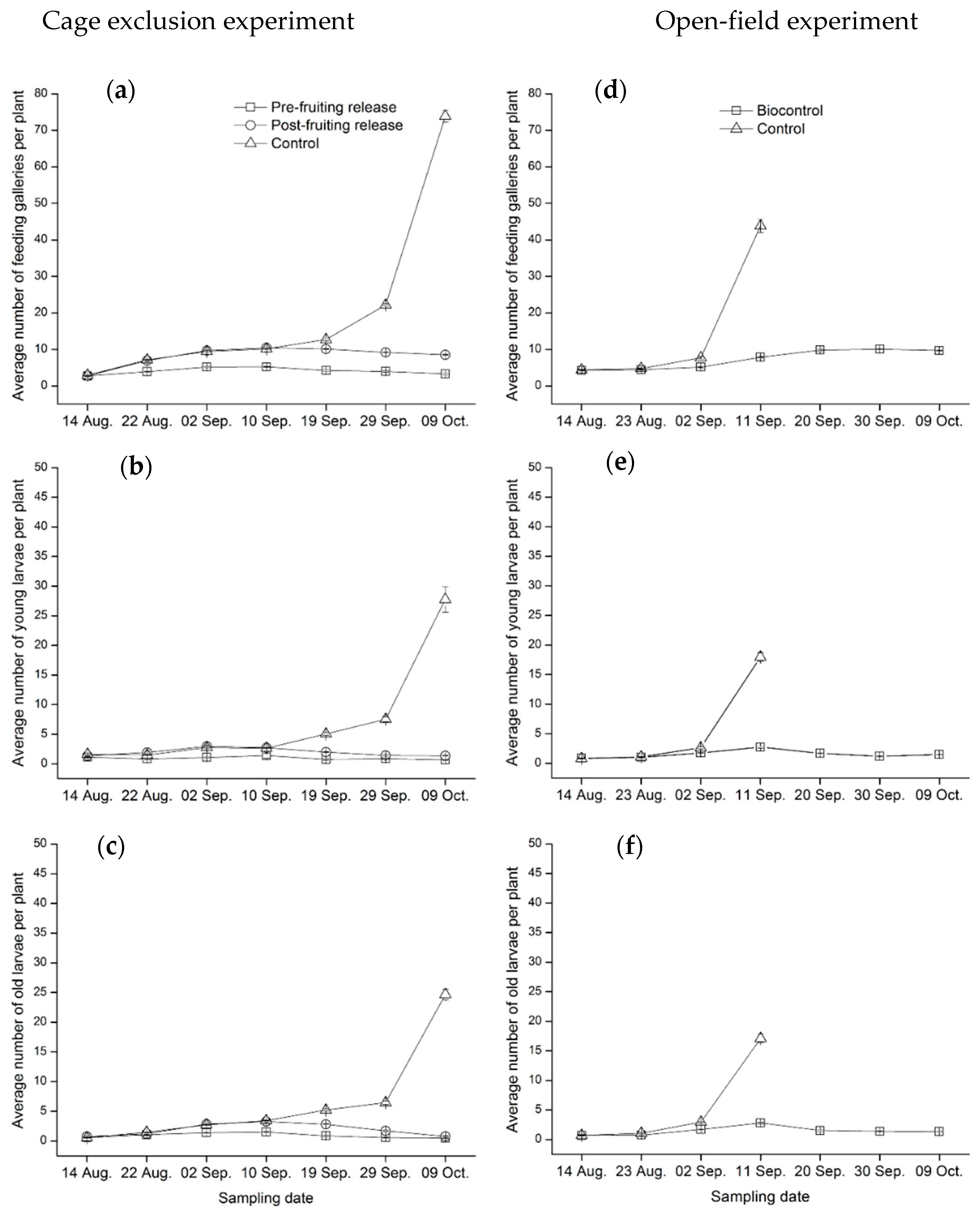
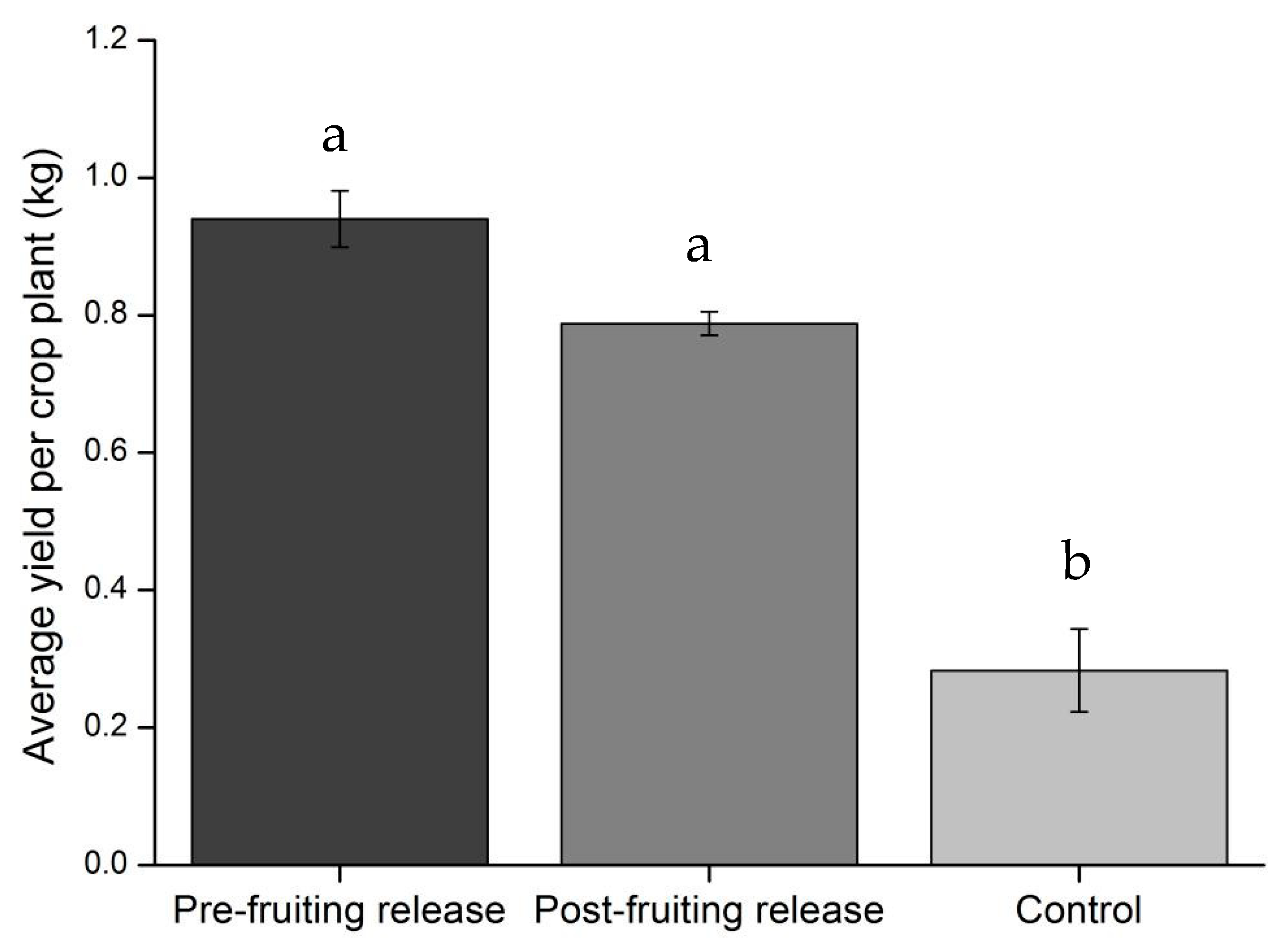
3.2. Field Assessment of C. carnea Controlling T. absoluta
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, C.J.A.; Boris, L.; Bellard, C.; Roiz, D.; Albert, C. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef] [PubMed]
- Witzgall, P.; Stelinski, L.; Gut, L.; Thomson, D. Codling moth management and chemical ecology. Annu. Rev. Entomol. 2008, 53, 503–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desneux, N.; Luna, M.G.; Guillemaud, T.; Urbaneja, A. The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: The new threat to tomato world production. J. Pest Sci. 2011, 84, 403–408. [Google Scholar] [CrossRef]
- Ragsdale, D.W.; Landis, D.A.; Brodeur, J.; Heimpel, G.E.; Desneux, N. Ecology and management of the soybean Aphid in North America. Ann. Rev. Entomol. 2011, 56, 375–399. [Google Scholar] [CrossRef] [Green Version]
- Giorgini, M.; Guerrieri, E.; Cascone, P.; Gontijo, L. Current strategies and future outlook for managing the neotropical tomato pest Tuta absoluta (Meyrick) in the Mediterranean basin. Neotrop. Entomol. 2018, 48, 1–17. [Google Scholar] [CrossRef]
- Gervassio, N.G.S.; Luna, M.G.; Minardi, G.M.; Sanchez, N.E. Assessing inoculative releases of Pseudapanteles dignus (Hymenoptera: Braconidae) for the biological control of Tuta absoluta (Lepidoptera: Gelechiidae). Crop Prot. 2019, 124, 104830. [Google Scholar] [CrossRef]
- Manohar, T.N.; Sharma, P.L.; Verma, S.C.; Chandel, R.S. Demographic parameters of the indigenous egg parasitoids, Trichogramma spp., parasitizing the invasive tomato leafminer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Egypt. J. Biol. Pest Control 2019, 29, 9. [Google Scholar] [CrossRef]
- Alikhani, M.; Safavi, S.A.; Iranipour, S. Effect of the entomopathogenic fungus, Metarhizium anisopliae (Metschnikoff) Sorokin, on demographic fitness of the tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Egypt. J. Biol. Pest Control 2019, 29, 23. [Google Scholar] [CrossRef]
- Naik, S.O.; Kannan, G.S.; Chakravarthy, A.K. Impact of integrated pest management modules on natural enemies of whiteflies, Bemisia tabaci (Genn.) in bitter gourd ecosystem. J. Biol. Control 2019, 33, 63–69. [Google Scholar] [CrossRef]
- Sain, S.K.; Monga, D.; Kumar, R.; Nagrale, D.T.; Hiremani, N.S.; Kranth, S. Compatibility of entomopathogenic fungi with insecticides and their efficacy for IPM of Bemisia tabaci in cotton. J. Pestic. Sci. 2019, 44, 97–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.G.; Giovanni, B.; Salvatore, A.; Consuelo, A.N.V.; Joel, G.C.; Diana, C.R.; Elisabeth, T.; Jacques, F.; et al. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Xian, X.Q.; Han, P.; Wang, S.; Zhang, G.F.; Liu, W.X.; Desneux, N.; Wan, F.H. The potential invasion risk and preventive measures against the tomato leafminer Tuta absoluta in China. Entomol. Gen. 2017, 36, 319–333. [Google Scholar] [CrossRef]
- Campos, M.R.; Biondi, A.; Adiga, A.; Guedes, R.N.C.; Desneux, N. From the Western Palaearctic region to beyond: Tuta absoluta 10 years after invading Europe. J. Pest Sci. 2017, 90, 787–796. [Google Scholar] [CrossRef]
- Sankarganesh, E.; Firake, D.M.; Sharma, B.; Verma, V.K.; Behere, G.T. Invasion of South American tomato pinworm, Tuta absoluta (Meyrick) (Lepidoptera: Gelechidae) in Northeastern India: A new challenge and biosecurity concerns. Entomol. Gen. 2017, 36, 335–345. [Google Scholar] [CrossRef]
- Mansour, R.; Brévault, T.; Chailleux, A.; Cherif, A.; Grissa-Lebdi, K.; Haddi, K.; Biondi, A. Occurrence, biology, natural enemies and management of Tuta absoluta in Africa. Entomol. Gen. 2018, 38, 83–111. [Google Scholar] [CrossRef]
- Han, P.; Zhang, Y.N.; Lu, Z.Z.; Wang, S.; Biondi, A.; Desneux, N. Are we ready for the invasion of Tuta absoluta? Unanswered key questions for elaborating an Integrated Pest Management package in Xinjiang, China. Entomol. Gen. 2018, 38, 113–125. [Google Scholar] [CrossRef]
- Verheggen, F.; Fontus, R.B. First record of Tuta absoluta in Haiti. Entomol. Gen. 2019, 38, 349–353. [Google Scholar] [CrossRef]
- Han, P.; Lavoir, A.V.; Le Bot, J.; Amiens-Desneux, E.; Desneux, N. Nitrogen and water availability to tomato plants triggers bottom-up effects on the leafminer Tuta absoluta. Sci. Rep. 2014, 4, 4455. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Desneux, N.; Amiens-Desneux, E.; Le Bot, J.; Bearez, P.; Lavoir, A.V. Does plant cultivar difference modify the bottom-up effects of resource limitation on plant-herbivorous insect interactions? J. Chem. Ecol. 2016, 42, 293–1303. [Google Scholar] [CrossRef]
- Sohrabi, F.; Nooryazdan, H.; Gharati, B.; Saeidi, Z. Evaluation of ten tomato cultivars for resistance against tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) under field infestation conditions. Entomol. Gen. 2016, 36, 163–175. [Google Scholar] [CrossRef]
- Blazhevski, S.; Kalaitzaki, A.P.; Tsagkarakis, A.E. Impact of nitrogen and potassium fertilization regimes on the biology of the tomato leaf miner Tuta absoluta. Entomol. Gen. 2018, 37, 157–174. [Google Scholar] [CrossRef]
- Cherif, A.; Attia-Barhoumi, S.; Mansour, R.; Zappalà, L.; Grissa-Lebdi, K. Elucidating key biological parameters of Tuta absoluta on different host plants and under various temperature and relative humidity regimes. Entomol. Gen. 2019, 39, 1–7. [Google Scholar] [CrossRef]
- Sylla, S.; Brevault, T.; Monticelli, L.S.; Diarra, K.; Desneux, N. Geographic variation of host preference by the invasive tomato leaf miner Tuta absoluta: Implications for host range expansion. J. Pest Sci. 2019, 92, 1387–1396. [Google Scholar] [CrossRef]
- Biondi, A.; Guedes, R.N.C.; Wan, F.; Desneux, N. Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: Past, present, and future. Ann. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Ann. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Desneux, N.; Becker, C.; Larbat, R.; Le Bot, J.; Zhang, J.; Lavoir, A. Bottom-up effects of irrigation, fertilization and plant resistance on Tuta absoluta: Implications for integrated pest management. J. Pest Sci. 2019, 92, 1359–1370. [Google Scholar] [CrossRef]
- Zappalà, L.; Biondi, A.; Alma, A.; Al-Jboory, I.J.; Arnò, J.; Bayram, A.; Chailleux, A.; El-Arnaouty, A.; Gerling, D.; Guenaoui, Y.; et al. Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle East, and their potential use in pest control strategies. J. Pest Sci. 2013, 86, 635–647. [Google Scholar] [CrossRef]
- Miranda, M.; Picanço, M.; Zanuncio, J.; Guedes, R. Ecological life table of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Biocontrol Sci. Technol. 1998, 8, 597–606. [Google Scholar] [CrossRef]
- Picanço, M.C.; Bacci, L.; Queiroz, R.B.; Silva, G.A.; Fabio, S.; Leite, G.L.D.; Motta, M.M.M. Social wasp predators of Tuta absoluta. Sociobiology 2011, 58, 1–13. [Google Scholar]
- Bacci, L.; Silva, É.M.; Silva, G.A.; Silva, L.J.; Rosado, J.F.; Samuels, R.I.; Picanco, M.C. Natural mortality factors of tomato leafminer Tuta absoluta in open-field tomato crops in the South America. Pest Manag. Sci. 2018, 75, 736–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbaneja, A.; Desneux, N.; Gabarra, R.; Arnó, J.; González-Cabrera, J.; Mafra Neto, A.; Stoltman, L.; Pinto, A.D.S.; Parra, J.R.P. Biology, ecology and management of the South American tomato pinworm, Tuta absoluta. In Potential Invasive Pests of Agricultural Crops; Peña, J.E., Ed.; CABI Invasives Series; CABI: Oxfordshire, UK, 2013; pp. 98–125. [Google Scholar]
- Campos, M.R.; Monticelli, L.S.; Béarez, P.; Amiens-Desneux, E.; Wang, Y.S.; Lavoir, A.V.; Zappalà, L.; Biondi, A.; Desneux, N. Impact of a shared sugar food source on biological control of Tuta absoluta by the parasitoid Necremnus tutae. J. Pest Sci. 2020, 93, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Chailleux, A.; Biondi, A.; Han, P.; Tabone, E.; Desneux, N. Suitability of the Pest–Plant System Tuta absoluta (Lepidoptera: Gelechiidae)–Tomato for Trichogramma (Hymenoptera: Trichogrammatidae) Parasitoids and Insights for Biological Control. J. Econ. Entomol. 2013, 106, 2310–2321. [Google Scholar] [CrossRef] [PubMed]
- Gebiola, M.; Bernardo, U.; Ribes, A.; Gibson, G.A.P. An integrative study of Necremnus Thomson (Hymenoptera: Eulophidae) associated with invasive pests in Europe and North America: Taxonomic and ecological implications. Zool. J. Linn. Soc. Lond. 2015, 173, 352–423. [Google Scholar] [CrossRef] [Green Version]
- Biondi, A.; Zappalà, L.; Di Mauro, A.; Garzia, G.T.; Russo, A.; Desneux, N.; Siscaro, G. Can alternative host plant and prey affect phytophagy and biological control by the zoophytophagous mirid Nesidiocoris tenuis? BioControl 2016, 61, 79–90. [Google Scholar] [CrossRef]
- Sylla, S.; Brevault, T.; Streito, J.C.; Diarra, K. First Record of Nesidiocoris tenuis (Reuter) (Heteroptera: Miridae), as a Predator of the Tomato Leaf Miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), in Senegal. Egypt. J. Biol. Pest Control 2016, 26, 851–853. [Google Scholar]
- Bompard, A.; Jaworski, C.C.; Bearez, P.; Desneux, N. Sharing a predator: Can an invasive alien pest affect the predation on a local pest? Popul. Ecol. 2013, 55, 433–440. [Google Scholar] [CrossRef]
- Han, P.; Dong, Y.C.; Lavoir, A.V.; Adamowicz, S.; Bearez, P.; Wajnberg, E.; Desneux, N. Effect of plant nitrogen and water status on the foraging behavior and fitness of an omnivorous arthropod. Ecol. Evol. 2015, 5, 5468–5477. [Google Scholar] [CrossRef] [Green Version]
- Jaworski, C.C.; Bompard, A.; Genies, L.; Amiens-Desneux, E.; Desneux, N. Preference and prey switching in a generalist predator attacking local and invasive alien pests. PLoS ONE 2013, 8, e82231. [Google Scholar] [CrossRef]
- Castañé, C.; Arnó, J.; Gabarra, R.; Alomar, O. Plant damage to vegetable crops by zoophytophagous mirid predators. Biol. Control 2011, 59, 22–29. [Google Scholar] [CrossRef]
- Han, P.; Bayram, Y.; Shaltiel-Harpaz, L.; Sohrabi, F.; Saji, A.; Uulu, T.E.; Jalilov, A.; Ali, A.; Shashank, P.R.; Ismoilov, K.; et al. Tuta absoluta continues to disperse in Asia: Damage, ongoing management and future challenges. J. Pest Sci. 2019, 92, 1317–1327. [Google Scholar] [CrossRef]
- Saidov, N.; Srinivasan, R.; Mavlyanova, R.; Qurbonov, Z. First report of invasive South American tomato leaf miner Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in Tajikistan. Fla. Entomol. 2018, 101, 147–149. [Google Scholar] [CrossRef] [Green Version]
- Guedes, R.N.C.; Picanço, M.C. The tomato borer Tuta absoluta in South America: Pest status, management and insecticide resistance. Bull. OEPP 2012, 42, 211–216. [Google Scholar] [CrossRef]
- Larbat, R.; Adamowicz, S.; Robin, C.; Han, P.; Desneux, N.; Le Bot, J. Interrelated responses of tomato plants and the leaf miner Tuta absoluta to nitrogen supply. Plant Biol. 2016, 18, 495–504. [Google Scholar] [CrossRef]
- Yao, Y.S.; Han, P.; Niu, C.Y.; Dong, Y.C.; Gao, X.W.; Cui, J.J.; Desneux, N. Transgenic Bt cotton does not disrupt the Top-down forces regulating the cotton Aphid in Central China. PLoS ONE 2016, 11, e0166771. [Google Scholar] [CrossRef] [Green Version]
- Chailleux, A.; Bearez, P.; Pizzol, J.; Amiens-Desneux, E.; Ramirez-Romero, R.; Desneux, N. Potential for combined use of parasitoids and generalist predators for biological control of the key invasive tomato pest, Tuta absoluta. J. Pest Sci. 2013, 86, 533–541. [Google Scholar] [CrossRef]
- Mollá, O.; Biondi, A.; Alonso-Valiente, M.; Urbaneja, A. A comparative life history study of two mirid bugs preying on Tuta absoluta and Ephestia kuehniella eggs on tomato crops: Implications for biological control. BioControl 2014, 59, 175–183. [Google Scholar] [CrossRef]
- Urbaneja, A.; Montón, H.; Mollá, O. Suitability of the tomato borer Tuta absoluta as prey for Macrolophus pygmaeus and Nesidiocoris tenuis. J. Appl. Entomol. 2009, 133, 292–296. [Google Scholar] [CrossRef]
- Tabone, E.; Bardon, C.; Desneux, N. Study of dispersal as a selection criterion for Trichogrammatidae for biological controlin cauliflower greenhouses. Acta Hortic. 2012, 927, 227–235. [Google Scholar] [CrossRef]
- Gontijo, L.M.; Nechols, J.R.; Margolies, D.C.; Cloyd, R.A. Plant architecture and prey distribution influence foraging behavior of the predatory mite Phytoseiulus persimilis (Acari: Phytoseiidae). Exp. Appl. Acarol. 2012, 56, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Madadi, H.; Parizi, E.M.; Allahyari, H.; Enkegaard, A. Assessment of the biological control capability of Hippodamia variegata (Col.: Coccinellidae) using functional response experiments. J. Pest Sci. 2011, 84, 447–455. [Google Scholar] [CrossRef]
- Desneux, N.; Kaplan, I.; Yoo, H.J.S.; Wang, S.; O’Neil, R.J. Temporal synchrony mediates the outcome of indirect effects between prey via a shared predator. Entomol. Gen. 2019, 39, 127–136. [Google Scholar] [CrossRef]
- Han, P.; Becker, C.; Le Bot, J.; Larbat, R.; Lavoir, A.V.; Desneux, N. Plant nutrient supply alters the magnitude of indirect interactions between insect herbivores: From foliar chemistry to community dynamics. J. Ecol. 2019, in press. [Google Scholar] [CrossRef]
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M.S. Habitat management to suppress pest populations: Progress and prospects. Annu. Rev. Entomol. 2017, 62, 91–109. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismoilov, K.; Wang, M.; Jalilov, A.; Zhang, X.; Lu, Z.; Saidov, A.; Sun, X.; Han, P. First Report Using a Native Lacewing Species to Control Tuta absoluta: From Laboratory Trials to Field Assessment. Insects 2020, 11, 286. https://doi.org/10.3390/insects11050286
Ismoilov K, Wang M, Jalilov A, Zhang X, Lu Z, Saidov A, Sun X, Han P. First Report Using a Native Lacewing Species to Control Tuta absoluta: From Laboratory Trials to Field Assessment. Insects. 2020; 11(5):286. https://doi.org/10.3390/insects11050286
Chicago/Turabian StyleIsmoilov, Khasan, Minghui Wang, Anvar Jalilov, Xin Zhang, Zhaozhi Lu, Abdusattor Saidov, Xiao Sun, and Peng Han. 2020. "First Report Using a Native Lacewing Species to Control Tuta absoluta: From Laboratory Trials to Field Assessment" Insects 11, no. 5: 286. https://doi.org/10.3390/insects11050286
APA StyleIsmoilov, K., Wang, M., Jalilov, A., Zhang, X., Lu, Z., Saidov, A., Sun, X., & Han, P. (2020). First Report Using a Native Lacewing Species to Control Tuta absoluta: From Laboratory Trials to Field Assessment. Insects, 11(5), 286. https://doi.org/10.3390/insects11050286




