Using a Two-Sex Life Table Tool to Calculate the Fitness of Orius strigicollis as a Predator of Pectinophora gossypiella
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Cultures
2.1.1. Rearing of O. strigicollis
2.1.2. Collection of P. gossypiella
2.2. Feeding Potential of O. strigicollis
2.3. Selection Pressure and Biological Parameters
2.3.1. Effects of Different Preying Densities on Developmental Period of O. strigicollis
2.3.2. Longevity, Oviposition, and Fecundity of O. strigicollis
2.4. Prey Preference
2.5. Life Table Analysis
3. Results
3.1. Feeding Potential of O. strigicollis
3.2. Biological Parameters of O. strigicollis
3.2.1. Developmental Period
3.2.2. Longevity, Oviposition, and Fecundity of Adults
3.2.3. Population Parameters of O. strigicollis
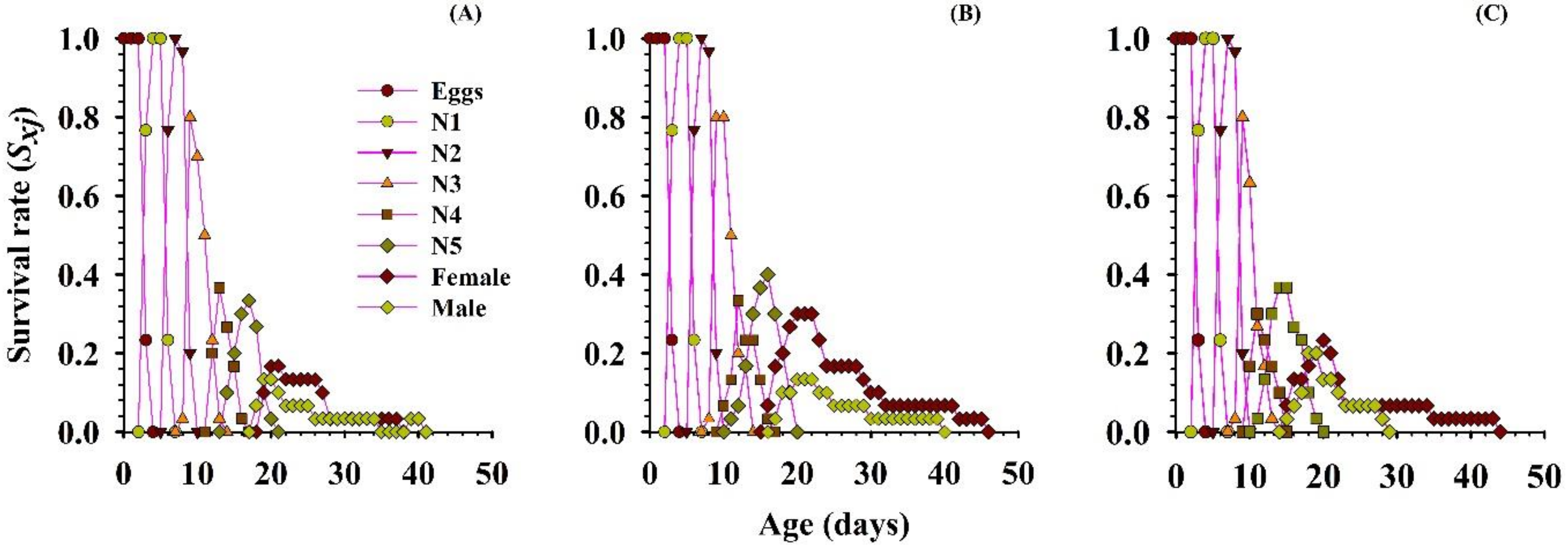
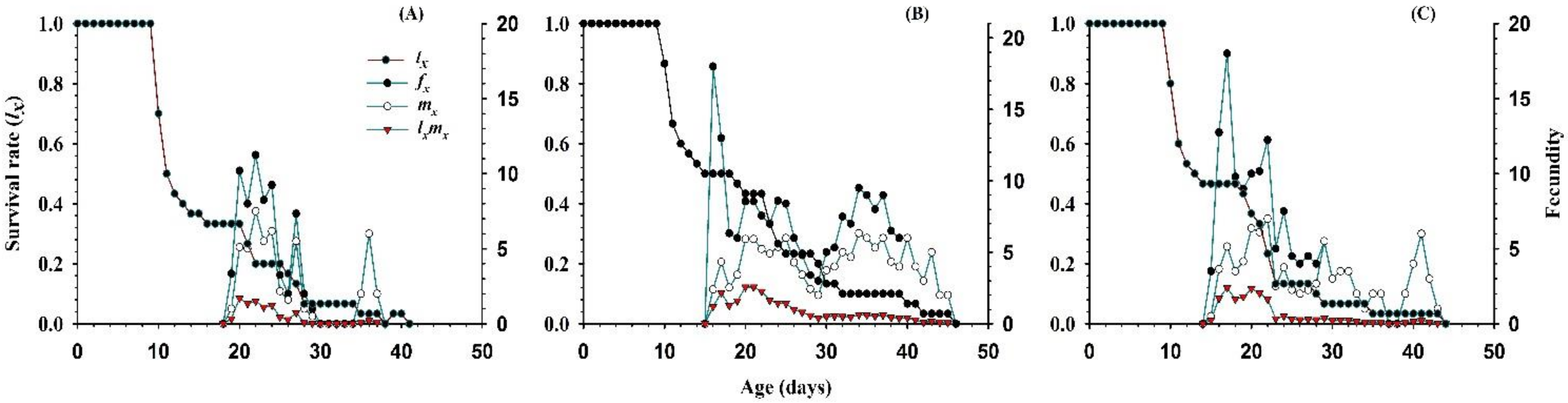
3.2.4. Survival Rate
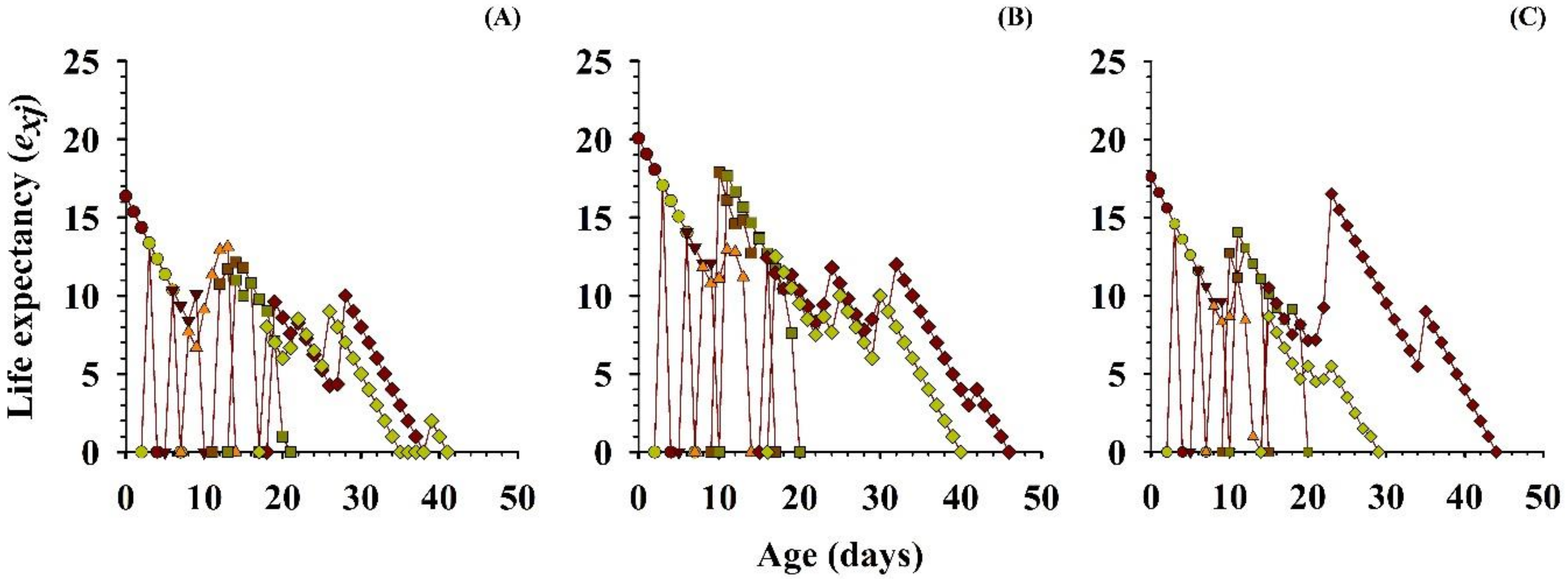
3.2.5. Life Expectancy
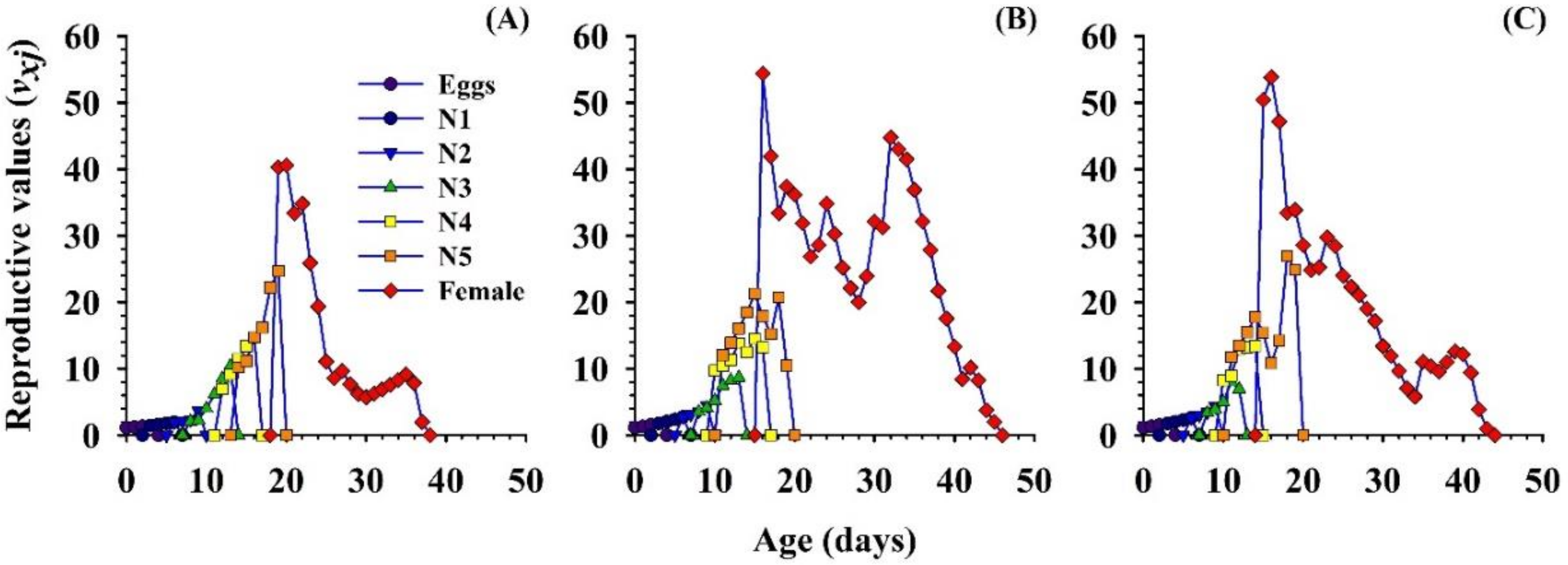
3.2.6. Reproductive Value
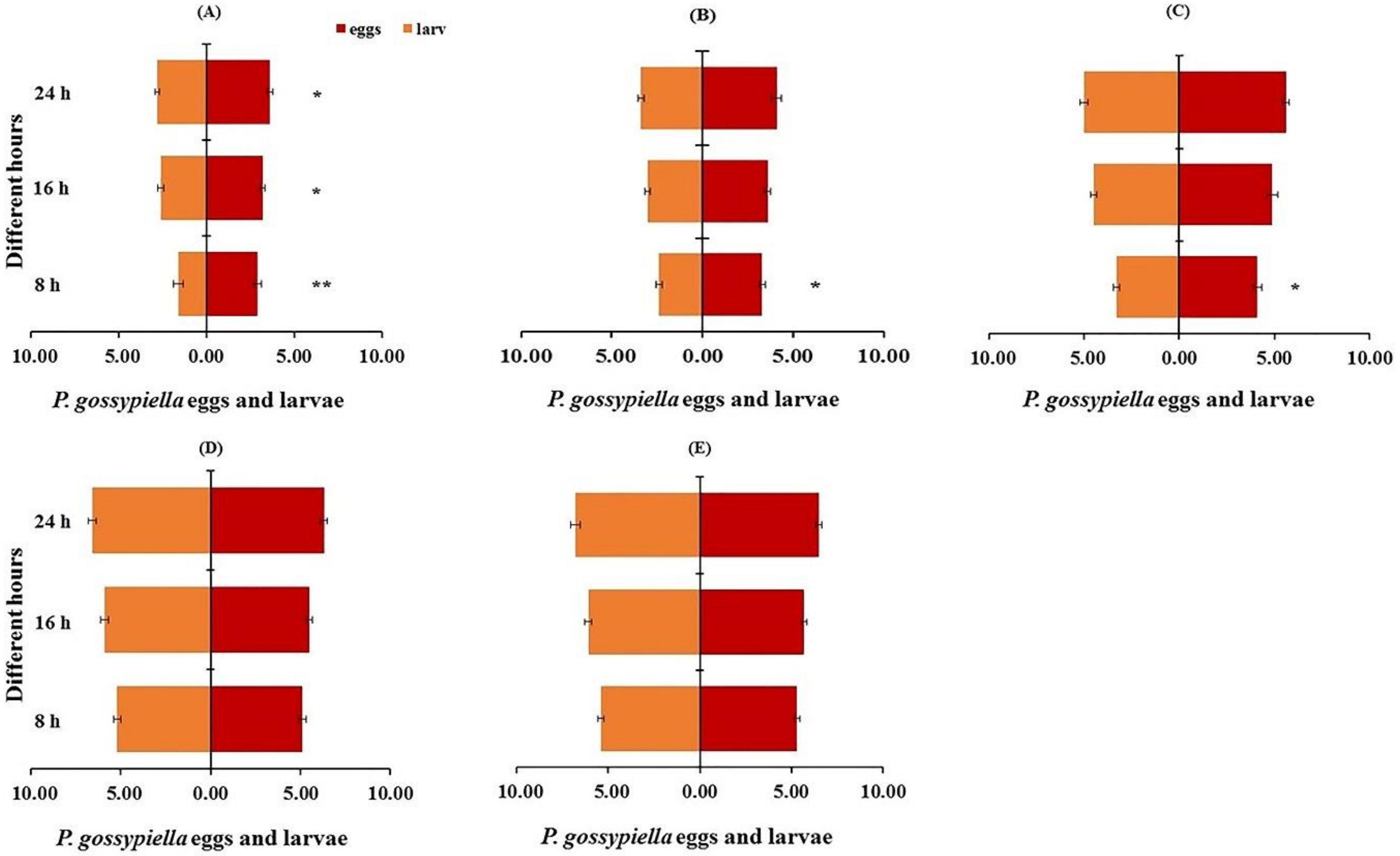
3.3. Prey Preference
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teixeira, I.R.D.V.; Barchuk, A.R.; Zucoloto, F.S. Host preference of the bean weevil Zabrotes subfasciatus. Insect Sci. 2008, 15, 335–341. [Google Scholar] [CrossRef]
- Mooney, K.A.; Pratt, R.T.; Singer, M.S. The tri-trophic interactions hypothesis: Interactive effects of host plant quality, diet breadth and natural enemies on herbivores. PLoS ONE 2012, 7, e34403. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kratina, P. Adding Complexity to Predator-Prey Interactions: Feeding with Conspecifics on Heterogeneous Prey. Ph.D. Thesis, University of Victoria, Victoria, BC, Canada, 2009. [Google Scholar]
- Zimmermann, B. Predatory Behaviour of Wolves in Scandinavia. Ph.D. Thesis, Hedmark University College, Elverum, Norway, 2014. [Google Scholar]
- Sengonca, C.; Ahmadi, K.; Blaeser, P. Biological characteristics of Orius similis Zheng (Heteroptera, Anthocoridae) by feeding on different aphid species as prey. J. Plant. Dis. Prot. 2008, 115, 32–38. [Google Scholar] [CrossRef]
- Zhang, G.; Zong, L.; Duan, H.; Xiong, J.; Lu, R.; Xu, F. Spatial distribution of the flower bug, Orius similis and its interaction with the pink bollworm, Pectinophora gossypiella in cotton fields. Int. J. Pest. Manag. 1994, 40, 309–312. [Google Scholar] [CrossRef]
- Zhou, X.; Lei, C. Utilization efficiency and functional response of Orius similis Zheng (Hemiptera: Anthocoridae) to different preys. Acta Ecol. Sin. 2002, 22, 2085–2090. [Google Scholar]
- Zong, H.L.; Zhong, H.C.; Lei, Z.L.; Peng, H.C.; Wu, H.M. Predation efficacy of Orius similis on the eggs of pink bollworm, Pectinophora gossypiella. Chin. J. Biol. Control. 1987, 3, 87. [Google Scholar]
- Chen, H.; Zheng, X.; Luo, M.; Guo, J.; Solangi, G.S.; Wan, F.; Zhou, Z. Effect of short-term high-temperature exposure on the life history parameters of Ophraella communa. Sci. Rep. 2018, 8, 13969. [Google Scholar] [CrossRef]
- Kutcherov, D.; Lopatina, E.B.; Yermakov, S. Effects of Temperature and Photoperiod on the Immature Development in Cassida rubiginosa Müll. and C. stigmatica Sffr. (Coleoptera: Chrysomelidae). Sci. Rep. 2019, 9, 10047. [Google Scholar] [CrossRef]
- Jaleel, W.; Saeed, S.; Saeed, Q.; Naqqash, M.N.; Sial, M.U.; Aine, Q.U.; Yanyuan, L.; Rui, Z.; He, Y.; Lu, L. Effects of three different cultivars of cruciferous plants on the age-stage, two-sex life table traits of Plutella xylostella (L.) (Lepidoptera: Plutellidae). Entomol. Res. 2019, 49, 151–157. [Google Scholar] [CrossRef]
- Saeed, R.; Razaq, M. Effect of Prey Resource on the Fitness of the Predator Chrysoperla carnea (Neuroptera: Chrysopidae). Pak. J. Zool. 2015, 47, 103–109. [Google Scholar]
- Aragón-Sánchez, M.; Román-Fernández, L.R.; Martínez-García, H.; Aragón-García, A.; Pérez-Moreno, I.; Marco-Mancebón, V.S. Rate of consumption, biological parameters, and population growth capacity of Orius laevigatus fed on Spodoptera exigua. BioControl 2018, 63, 785–794. [Google Scholar] [CrossRef]
- Jaleel, W.; Tao, X.; Wang, D.; Lu, L.; He, Y. Using Two-Sex Life Table Traits to Assess the Fruit Preference and Fitness of Bactrocera dorsalis (Diptera: Tephritidae). J. Econ. Entomol. 2018, 111, 2936–2945. [Google Scholar] [CrossRef] [PubMed]
- Jokar, M.; Zarabi, M. Investigation effect three diets on life table parameters Chrysoperla carnea (Steph.)(Neuroptera: Chrysopidae) under Laboratory Conditions. Egypt. Acad. J. Biol. Sci. 2012, 5, 107–114. [Google Scholar] [CrossRef]
- Godin, J.-G.J.; McDonough, H.E. Predator preference for brightly colored males in the guppy: A viability cost for a sexually selected trait. Behav. Ecol. 2003, 14, 194–200. [Google Scholar] [CrossRef]
- Saeed, R.; Sayyed, A.H.; Shad, S.A.; Zaka, S.M. Effect of different host plants on the fitness of diamond-back moth, Plutella xylostella (Lepidoptera: Plutellidae). Crop. Prot. 2010, 29, 178–182. [Google Scholar] [CrossRef]
- Hagley, E.A.; Barber, D.R. Effect of food sources on the longevity and fecundity of Pholetesor ornigis (Weed)(Hymenoptera: Braconidae). Can. Entomol. 1992, 124, 341–346. [Google Scholar] [CrossRef]
- Uckan, F.; Gülel, A. Apanteles galleriae Wilkinson (Hym.; Braconidae)’nin bazı biyolojik özelliklerine konak türün etkileri. Turk. J. Zool. 2000, 24, 105–113. [Google Scholar]
- Sattar, M.; Abro, G.H. Comparative effect of natural and artificial larval diets on biology of Chrysoperla carnea (Stephens)(Neuroptera: Chrysopidae). Pak. J. Zool. 2009, 41, 335–339. [Google Scholar]
- Barbosa, L.; Santos, F.; Soliman, E.; Rodrigues, A.; Wilcken, C.; Campos, J.; Zanuncio, A.; Zanuncio, J. Biological parameters, life table and thermal requirements of Thaumastocoris peregrinus (Heteroptera: Thaumastocoridae) at different temperatures. Sci. Rep. 2019, 9, 10174. [Google Scholar] [CrossRef]
- Spielman, D.J.; Nazli, H.; Ma, X.; Zambrano, P.; Zaidi, F. Technological opportunity, regulatory uncertainty, and Bt cotton in Pakistan. AgBioForum 2015, 18, 98–112. [Google Scholar]
- Majeed, M.Z.; Javed, M.; Riaz, M.A.; Afzal, M. Population dynamics of sucking pest complex on some advanced genotypes of cotton under unsprayed conditions. Pak. J. Zool. 2016, 48, 475–480. [Google Scholar]
- Parmar, V.; Patel, C. Pink Bollworm: A Notorious Pest of Cotton: A Review. Agres Int. E-J. 2016, 5, 88–97. [Google Scholar]
- Bourtzis, K.; Crook, S.; Daffonchio, D.; Durvasula, R.; Hanboonsong, Y.; Infante, F.; Lacava, P.; Miller, T.A.; Vega, F.E. International entomology. Am. Entomol. 2012, 58, 234. [Google Scholar] [CrossRef][Green Version]
- Gassmann, A.J.; Stock, S.P.; Carrière, Y.; Tabashnik, B.E. Effect of entomopathogenic nematodes on the fitness cost of resistance to Bt toxin Cry1Ac in pink bollworm (Lepidoptera: Gelechiidae). J. Econ. Entomol. 2006, 99, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, A.J.; Fabrick, J.A.; Sisterson, M.S.; Hannon, E.R.; Patricia Stock, S.; Carriére, Y.; Tabashnik, B.E. Effects of pink bollworm resistance to Bacillus thuringiensis on phenoloxidase activity and susceptibility to entomopathogenic nematodes. J. Econ. Entomol. 2009, 102, 1224–1232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fabrick, J.; Tabashnik, B.E. Binding of Bacillus thuringiensis toxin Cry1Ac to multiple sites of cadherin in pink bollworm. Insect Biochem. Mol. Biol. 2007, 37, 97–106. [Google Scholar] [CrossRef]
- Wang, L.; Ma, Y.; Wan, P.; Liu, K.; Xiao, Y.; Wang, J.; Cong, S.; Xu, D.; Wu, K.; Fabrick, J.A. Resistance to Bacillus thuringiensis linked with a cadherin transmembrane mutation affecting cellular trafficking in pink bollworm from China. Insect Biochem. Mol. Biol. 2018, 94, 28–35. [Google Scholar] [CrossRef]
- Zhang, S.-C.; Zhu, F.; Zheng, X.-L.; Lei, C.-L.; Zhou, X.-M. Survival and developmental characteristics of the predatory bug Orius similis (Hemiptera: Anthocoridae) fed on Tetranychus cinnabarinus (Acari: Tetranychidae) at three constant temperatures. Eur. J. Entomol. 2012, 109, 503–508. [Google Scholar] [CrossRef]
- Hiltunen, T.; Kaitala, V.; Laakso, J.; Becks, L. Evolutionary contribution to coexistence of competitors in microbial food webs. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170415. [Google Scholar] [CrossRef]
- Calixto, A.; Bueno, V.; Montes, F.; Silva, A.; Van Lenteren, J. Effect of different diets on reproduction, longevity and predation capacity of Orius insidiosus (Say) (Hemiptera: Anthocoridae). Biocontrol Sci. Technol. 2013, 23, 1245–1255. [Google Scholar] [CrossRef]
- Amer, M.E.S.; Fu, Y.; Niu, L. Biological Aspects of Orius similis Zheng Reared on Two Preys at Three Constant Temperatures. J. Agric. Sci. Technol. A 2018, 8, 350–363. [Google Scholar]
- Liu, P.; Jia, W.; Zheng, X.; Zhang, L.; Sangbaramou, R.; Tan, S.; Liu, Y.; Shi, W. Predation functional response and life table parameters of Orius sauteri (Hemiptera: Anthocoridae) feeding on Megalurothrips usitatus (Thysanoptera: Thripidae). Fla. Entomol. 2018, 101, 254–259. [Google Scholar] [CrossRef]
- Jalali, M.A.; Tirry, L.; De Clercq, P. Effect of temperature on the functional response of Adalia bipunctata to Myzus persicae. BioControl 2010, 55, 261–269. [Google Scholar] [CrossRef]
- Sarmento, R.A.; Pallini, A.; Venzon, M.; Souza, O.F.F.D.; Molina-Rugama, A.J.; Oliveira, C.L.D. Functional response of the predator Eriopis connexa (Coleoptera: Coccinellidae) to different prey types. Braz. Arch. Biol. Technol. 2007, 50, 121–126. [Google Scholar] [CrossRef]
- El-Basha, N.A.; Salman, M.; Osman, M. Functional response of Orius albidipennis (Hemiptera: Anthocoridae) to the two-spotted spider mite Tetranychus urticae (Acari: Tetranychidae). J. Entomol. 2012, 9, 248–256. [Google Scholar] [CrossRef]
- Zarghami, S.; Mossadegh, M.S.; Kocheili, F.; Allahyari, H.; Rasekh, A. Functional responses of Nephus arcuatus Kapur (Coleoptera: Coccinellidae), the most important predator of spherical mealybug Nipaecoccus viridis (Newstead). Psyche A J. Entomol. 2016, 2016, 9. [Google Scholar] [CrossRef]
- Sharma, P.; Verma, S.; Chandel, R.; Shah, M.; Gavkare, O. Functional response of Harmonia dimidiata (fab.) to melon aphid, Aphis gossypii Glover under laboratory conditions. Phytoparasitica 2017, 45, 373–379. [Google Scholar] [CrossRef]
- Butt, A.; Talib, R.; Khan, M.X. Effects of Insecticides on the Functional Response of Spider Oxyopes javanus against Aphid Sitobion avenae. Int. J. Agric. Biol. 2019, 22, 503–509. [Google Scholar]
- Butler, C.D.; O’Neil, R.J. Life history characteristics of Orius insidiosus (Say) fed diets of soybean aphid, Aphis glycines Matsumura and soybean thrips, Neohydatothrips variabilis (Beach). Biol. Control. 2007, 40, 339–346. [Google Scholar] [CrossRef]
- Zhou, X.-M.; Zhu, F.; Li, H.; Lei, C.-L. Effect of temperature on development of Orius similis Zheng (Hemiptera: Anthocoridae) and on its predation activity against Aphis gossypii Glover (Hemiptera: Aphididae). Pan Pac. Entomol. 2006, 82, 97–102. [Google Scholar]
- Butler, C.D.; O’neil, R.J. Defensive response of soybean aphid (Hemiptera: Aphididae) to predation by insidious flower bug (Hemiptera: Anthocoridae). Ann. Entomol. Soc. Am. 2006, 99, 317–320. [Google Scholar] [CrossRef]
- Bonte, J.; De Hauwere, L.; Conlong, D.; De Clercq, P. Predation capacity, development and reproduction of the southern African flower bugs Orius thripoborus and Orius naivashae (Hemiptera: Anthocoridae) on various prey. Biol. Control. 2015, 86, 52–59. [Google Scholar] [CrossRef]
- Yasunaga, T. The Flower Bug Genus Orius WOLFF (Heteroptera: Anthocoridae) from Japan and Taiwan, Part II. Appl. Entomol. Zool. 1997, 32, 379–386. [Google Scholar] [CrossRef][Green Version]
- Jung, S.; Yamada, K.; Lee, S. Annotated catalog, biological notes and diagnoses of the flower bugs (Heteroptera: Anthocoridae sensu lato) in the Korean Peninsula. J. Asia-Pac. Entomol. 2013, 16, 421–427. [Google Scholar] [CrossRef]
- Huang, Y.B.; Chi, H. Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci. 2012, 19, 263–273. [Google Scholar] [CrossRef]
- Tuan, S.J.; Lee, C.C.; Chi, H. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest. Manag. Sci. 2014, 70, 805–813. [Google Scholar] [CrossRef]
- Kiman, Z.; Yeargan, K. Development and reproduction of the predator Orius insidiosus (Hemiptera: Anthocoridae) reared on diets of selected plant material and arthropod prey. Ann. Entomol. Soc. Am. 1985, 78, 464–467. [Google Scholar] [CrossRef]
- Kim, J. Development and oviposition of Orius strigicollis (Poppius) (Hemiptera: Anthocoridae) reared on three different insect preys. Korean J. Appl. Entomol. 1997, 36, 166–171. [Google Scholar]
- Kim, J. Effect of temperature on the development and oviposition of minute pirate bug, Orius strigicollies (Hemiptera: Anthocoridae). Korean J. Appl. Entomol. 1999, 38, 29–33. [Google Scholar]
- Eubanks, M.D.; Denno, R.F. Health food versus fast food: The effects of prey quality and mobility on prey selection by a generalist predator and indirect interactions among prey species. Ecol. Entomol. 2000, 25, 140–146. [Google Scholar] [CrossRef]
- Lins Jr, J.C.; Bueno, V.H.; Silva, D.B.; van Lenteren, J.C.; Calixto, A.M.; Sidney, L.A. Tuta absoluta egg predation by Orius insidiosus. Iobc Wprs Bull. 2011, 68, 101–104. [Google Scholar]
- Ahmadi, K.; Sengonca, C.; Blaeser, P. Effect of two different temperatures on the biology of predatory flower bug Orius similis Zheng (Heteroptera: Anthocoridae) with two different aphid species as prey. Turk. Entomol. Derg 2007, 31, 253–268. [Google Scholar]
- Le-yi, Z. Two new species of orius wolff from china (hemiptera: Anthocoridae). Acta Entomol. Sin. 1982, 25, 191–194. [Google Scholar]
- Wan, P.; Huang, Y.; Wu, H.; Huang, M.; Cong, S.; Tabashnik, B.E.; Wu, K. Increased frequency of pink bollworm resistance to Bt toxin Cry1Ac in China. PLoS ONE 2012, 7, e29975. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Jaleel, W.; Naqqash, M.N.; Saeed, Q.; Zaka, S.M.; Sarwar, Z.M.; Ishtiaq, M.; Qayyum, M.A.; Sial, M.U.; Batool, M. Fitness parameters of Plutella xylostella (L.) (Lepidoptera; Plutellidae) at four constant temperatures by using age-stage, two-sex life tables. Saudi J. Biol. Sci. 2018, 26, 1661–1667. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. National Chung Hsing University, Taichung, Taiwan. Available online: http://140.120.197.173/Ecology/Download/Twosex-MSChart.rar (accessed on 1 May 2019).
- Tuan, S.-J.; Yeh, C.-C.; Atlihan, R.; Chi, H. Linking life table and predation rate for biological control: A comparative study of Eocanthecona furcellata (Hemiptera: Pentatomidae) fed on Spodoptera litura (Lepidoptera: Noctuidae) and Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 2015, 109, 13–24. [Google Scholar] [CrossRef]
- Ortigosa, A.; Rowe, L. The effect of hunger on mating behaviour and sexual selection for male body size in Gerris buenoi. Anim. Behav. 2002, 64, 369–375. [Google Scholar] [CrossRef]
- Jaleel, W.; Saeed, S.; Naqqash, M.N.; Sial, M.U.; Ali, M.; Zaka, S.M.; Sarwar, Z.M.; Ishtiaq, M.; Qayyum, M.A.; Aine, Q.U. Effects of temperature on baseline susceptibility and stability of insecticide resistance against Plutella xylostella (Lepidoptera: Plutellidae) in the absence of selection pressure. Saudi J. Biol. Sci. 2019, 27, 1–5. [Google Scholar] [CrossRef]
- Chen, Q.; Li, N.; Wang, X.; Ma, L.; Huang, J.-B.; Huang, G.-H. Age-stage, two-sex life table of Parapoynx crisonalis (Lepidoptera: Pyralidae) at different temperatures. PLoS ONE 2017, 12, e0173380. [Google Scholar] [CrossRef]
- Huang, Y.B.; Chi, H. Life tables of Bactrocera cucurbitae (Diptera: Tephritidae): With an invalidation of the jackknife technique. J. Appl. Entomol. 2013, 137, 327–339. [Google Scholar] [CrossRef]
- Jaleel, W.; Lu, L.; He, Y. Biology, taxonomy, and IPM strategies of Bactrocera tau Walker and complex species (Diptera; Tephritidae) in Asia: A comprehensive review. Environ. Sci. Pollut. Res. 2018, 25, 19346–19361. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Akköprü, E.P.; Atlıhan, R.; Okut, H.; Chi, H. Demographic assessment of plant cultivar resistance to insect pests: A case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J. Econ. Entomol. 2015, 108, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.-Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Xie, W.; Wu, Q.; Xu, B.; Wang, S.; Li, C.; Zhang, Y. Development of Bradysiaodoriphaga (Diptera: Sciaridae) as affected by humidity: An age–stage, two-sex, life-table study. Appl. Entomol. Zool. 2015, 50, 3–10. [Google Scholar] [CrossRef]
- Aljetlawi, A.A.; Sparrevik, E.; Leonardsson, K. Prey–predator size-dependent functional response: Derivation and rescaling to the real world. J. Anim. Ecol. 2004, 73, 239–252. [Google Scholar] [CrossRef]
- Kalinoski, R.M.; DeLong, J.P. Beyond body mass: How prey traits improve predictions of functional response parameters. Oecologia 2016, 180, 543–550. [Google Scholar] [CrossRef]
- Pasandideh, A.; Talebi, A.A.; Hajiqanbar, H.; Tazerouni, Z. Host stage preference and age-specific functional response of Praon volucre (Hymenoptera: Braconidae, Aphidiinae) a parasitoid of Acyrthosiphon pisum (Hemiptera: Aphididae). J. Crop. Prot. 2015, 4, 563–575. [Google Scholar]
- Tazerouni, Z.; Talebi, A.; Fathipour, Y.; Soufbaf, M. Age-specific functional response of Aphidius matricariae and Praon volucre (Hymenoptera: Braconidae) on Myzus persicae (Hemiptera: Aphididae). Neotrop. Entomol. 2016, 45, 642–651. [Google Scholar] [CrossRef]
- Lawton, J.; Thompson, B.; Thompson, D. The effects of prey density on survival and growth of damselfly larvae. Ecol. Entomol. 1980, 5, 39–51. [Google Scholar] [CrossRef]
- Hafeez, M.; Jan, S.; Nawaz, M.; Ali, E.; Ali, B.; Qasim, M.; Fernández-Grandon, G.M.; Shahid, M.; Wang, M. Sub-lethal effects of lufenuron exposure on spotted bollworm Earias vittella (Fab): Key biological traits and detoxification enzymes activity. Environ. Sci. Pollut. Res. 2019, 26, 14300–14312. [Google Scholar] [CrossRef] [PubMed]
- Garrad, R.; Booth, D.; Furlong, M. The effect of rearing temperature on development, body size, energetics and fecundity of the diamondback moth. Bull. Entomol. Res. 2016, 106, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Baldwin, I.T. Manduca quinquemaculata’s optimization of intra-plant oviposition to predation, food quality, and thermal constraints. Ecology 2002, 83, 2346–2354. [Google Scholar] [CrossRef]
- Bonte, M.; De Clercq, P. Influence of predator density, diet and living substrate on developmental fitness of Orius laevigatus. J. Appl. Entomol. 2011, 135, 343–350. [Google Scholar] [CrossRef]
- Azher, F.; Khan, M.M.; Bilal, M.; Asghar, I.; Rasheed, M.A.; Ali, S. The Development of Antilochus coquebertii Fabr.(Heteroptera: Pyrrhocoridae) on Different Artificial Diets. J. Kansa. Entomol. Soc. 2019, 91, 192–208. [Google Scholar] [CrossRef]
- Kakimoto, K.; Urano, S.; Noda, T.; Matuo, K.; Sakamaki, Y.; Tsuda, K.; Kusigemati, K. Comparison of the reproductive potential of three Orius species, O. strigicollis, O. sauteri, and O. minutus (Heteroptera: Anthocoridae), using eggs of the Mediterranean flour moth as a food source. Appl. Entomol. Zool. 2005, 40, 247–255. [Google Scholar] [CrossRef]
- Yosoff, S.F.; Mohamed, M.T.M.; Parvez, A.; Ahmad, S.H.; Ghazali, F.M.; Hassan, H. Production system and harvesting stage influence on nitrate content and quality of butterhead lettuce. Bragantia 2015, 74, 322–330. [Google Scholar] [CrossRef][Green Version]
- Schroeder, L.A. Consumer growth efficiencies: Their limits and relationships to ecological energetics. J. Biol. 1981, 93, 805–828. [Google Scholar] [CrossRef]
- Duffey, S.S.; Stout, M.J. Antinutritive and toxic components of plant defense against insects. Arch. Insect Biochem. Physiol. Publ. Collab. Entomol. Soc. Am. 1996, 32, 3–37. [Google Scholar] [CrossRef]
- Kindlmann, P.; Dixon, A. Insect predator-prey dynamics and the biological control of aphids by ladybirds. In Proceedings of the International Symposium on Biological Control of Arthropods, Honolulu, HI, USA, 14–18 January 2002; pp. 118–124. [Google Scholar]
- Rondon, S.I.; Cantliffe, D.J.; Price, J.F. The feeding behavior of the bigeyed bug, minute pirate bug, and pink spotted lady beetle relative to main strawberry pests. Environ. Entomol. 2004, 33, 1014–1019. [Google Scholar] [CrossRef]
- Syed, A.N.; Ashfaq, M.; Khan, S. Comparison of development and predation of Chrysoperla carnea (Neuroptera: Chrysopidae) on different densities of two hosts (Bemisia tabaci, and Amrasca devastans). Pak. Entomol. 2005, 27, 231–234. [Google Scholar]
- Notter-Hausmann, C.; Dorn, S. Relationship between behavior and physiology in an invasive pest species: Oviposition site selection and temperature-dependent development of the oriental fruit moth (Lepidoptera: Tortricidae). Environ. Entomol. 2010, 39, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Clancy, K.M.; Price, P.W. Effect of plant resistance, competition, and enemies on a leaf-galling sawfly (Hymenoptera: Tenthredinidae). Environ. Entomol. 1989, 18, 284–290. [Google Scholar] [CrossRef]
- Abrahamson, W.G.; Weis, A.E. Evolutionary Ecology across Three Trophic Levels: Goldenrods, Gallmakers, and Natural Enemies; Princeton University Press: Princeton, NJ, USA, 1997; Volume 29. [Google Scholar]
- Sharpe, P.J.; DeMichele, D.W. Reaction kinetics of poikilotherm development. J. Biol. 1977, 64, 649–670. [Google Scholar] [CrossRef]
- Pak, G. Behavioural variations among strains of Trichogramma spp. A review of the literature on host-age selection 1. J. Appl. Entomol. 1986, 101, 55–64. [Google Scholar] [CrossRef]
- Varley, G.; Gradwell, G. Recent advances in insect population dynamics. Annu. Rev. Entomol. 1970, 15, 1–24. [Google Scholar] [CrossRef]
- Bonte, J.; Vangansbeke, D.; Maes, S.; Bonte, M.; Conlong, D.; De Clercq, P.; Cohen, A. Moisture source and diet affect development and reproduction of Orius thripoborus and Orius naivashae, two predatory anthocorids from southern Africa. J. Insect Sci. 2012, 12. [Google Scholar] [CrossRef]
- Musa, P.D.; Ren, S.X. Development and reproduction of Bemisia tabaci (Homoptera: Aleyrodidae) on three bean species. Insect Sci. 2005, 12, 25–30. [Google Scholar] [CrossRef]
- Southwood, T.R.E.; Henderson, P.A. Ecological Methods; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Bonte, J.; De Ro, M.; Conlong, D.; De Clercq, P. Thermal biology of the predatory bugs Orius thripoborus and O. naivashae (Hemiptera: Anthocoridae). Environ. Entomol. 2012, 41, 989–996. [Google Scholar] [CrossRef]
- Sayyed, A.H.; Saeed, S.; Noor-Ul-Ane, M.; Crickmore, N. Genetic, biochemical, and physiological characterization of spinosad resistance in Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 2008, 101, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Cocuzza, G.; De Clercq, P.; Lizzio, S.; Van De Veire, M.; Tirry, L.; Degheele, D.; Vacante, V. Life tables and predation activity of Orius laevigatus and O. albidipennis at three constant temperatures. Entomol. Exp. Appl. 1997, 85, 189–198. [Google Scholar] [CrossRef]
- Khaliq, A.; Attique, M.; Sayyed, A. Evidence for resistance to pyrethroids and organophosphates in Plutella xylostella (Lepidoptera: Plutellidae) from Pakistan. Bull. Entomol. Res. 2007, 97, 191–200. [Google Scholar] [CrossRef] [PubMed]
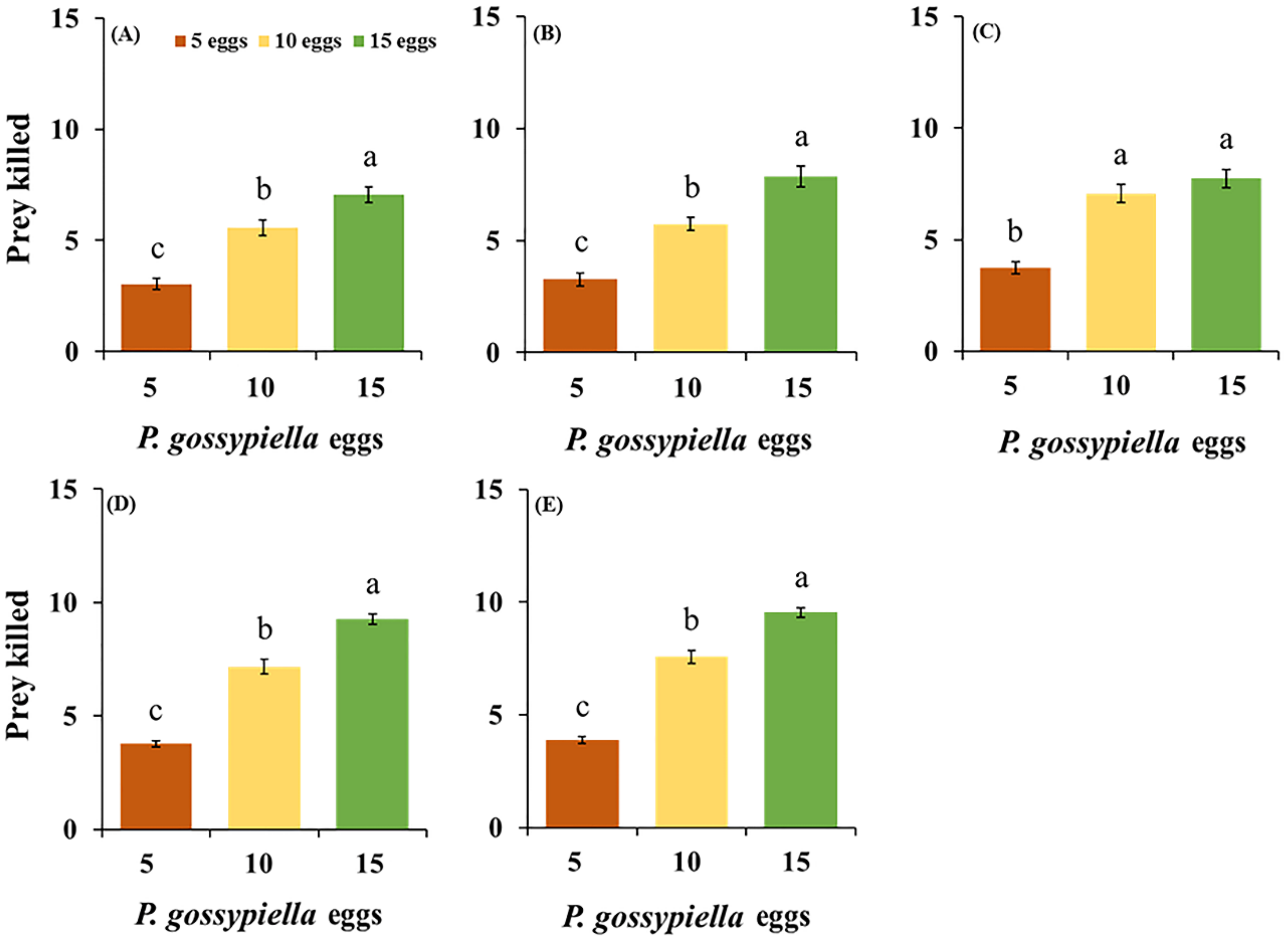
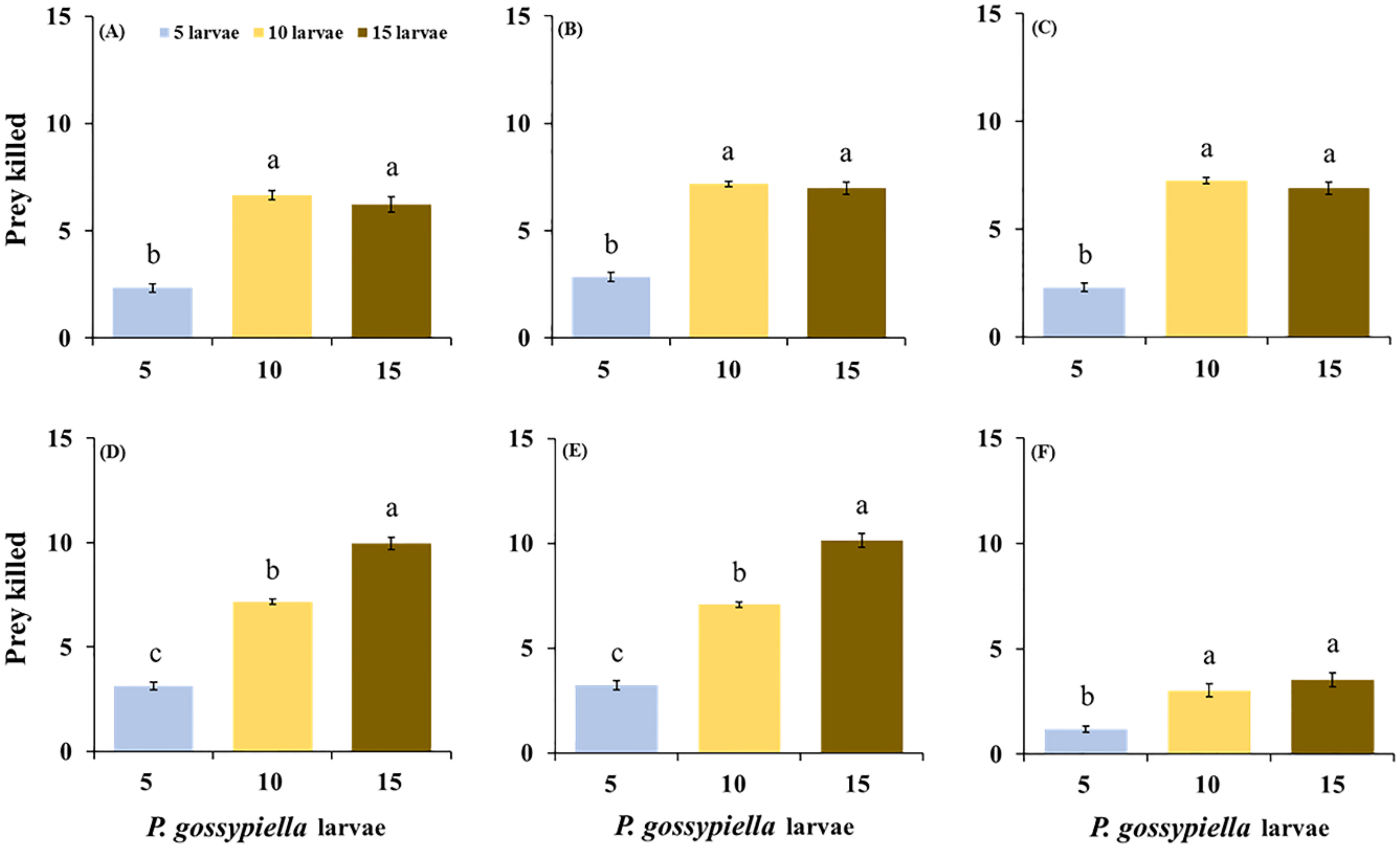
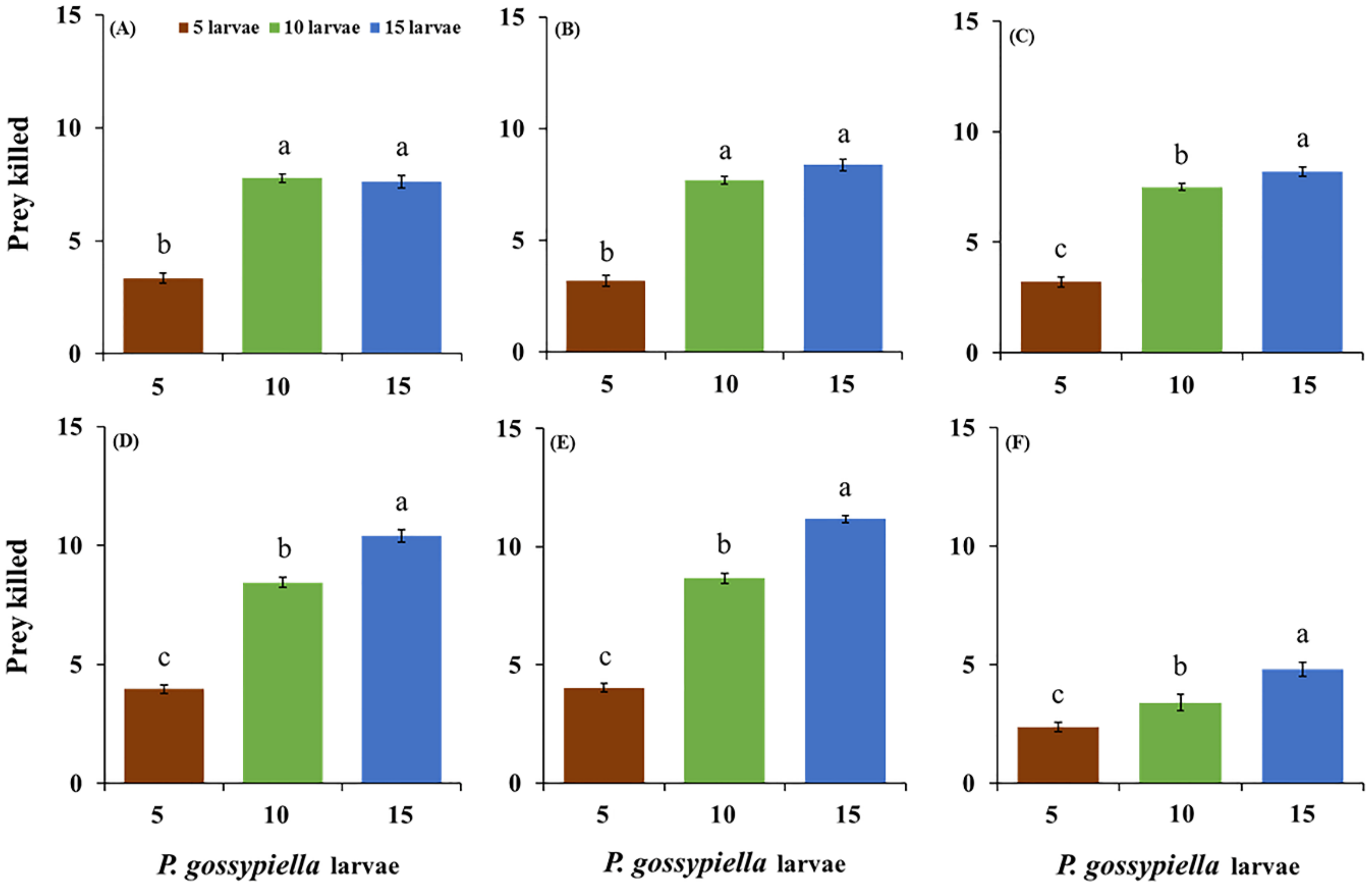
| Stages | Prey Densities | ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | 5 Eggs | n | 10 Eggs | n | 15 Eggs | df | F | p | |
| 1st instar nymph | 27 | 3.33 ± 0.29 b | 28 | 3.89 ± 0.29 a,b | 29 | 4.41 ± 0.28 a | 2, 81 | 3.56 | 0.03 |
| 2nd instar nymph | 26 | 4.58 ± 0.38 a | 28 | 5.04 ± 0.37 a | 28 | 5.29 ± 0.37 a | 2, 79 | 0.93 | 0.40 |
| 3rd instar nymph | 26 | 12.54 ± 1.12 b | 28 | 16.86 ± 1.08 a | 28 | 19.21 ± 1.08 a | 2, 79 | 9.43 | 0.00 |
| 4th instar nymph | 22 | 10.46 ± 1.08 b | 24 | 10.92 ± 1.03 b | 23 | 15.65 ± 1.05 a | 2, 66 | 7.44 | 0.00 |
| 5th instar nymph | 18 | 13.56 ± 1.58 b | 23 | 20.78 ± 1.40 a | 20 | 21.70 ± 1.50 a | 2, 58 | 8.35 | 0.00 |
| Male | 8 | 75.00 ± 13.46 a | 8 | 126.50 ± 28.06 a | 8 | 117.00 ± 30.67 a | 2, 21 | 1.18 | 0.33 |
| Female | 10 | 67.40 ± 12.20 b | 15 | 159.40 ± 30.11 a,b | 12 | 188.33 ± 33.66 a | 2, 34 | 3.16 | 0.05 |
| Stages (d) | Prey Densities | ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | 5 Eggs | n | 10 Eggs | n | 15 Eggs | df | F | p | |
| Egg duration | 60 | 3.23 ± 0.06 a | 60 | 3.23 ± 0.06 a | 60 | 3.23 ± 0.06 a | 2,177 | 0.00 | 1.00 |
| 1st instar nymph | 60 | 3.00 ± 0.00 a | 60 | 3.00 ± 0.00 a | 60 | 3.00 ± 0.00 a | 2,177 | 0.00 | 1.00 |
| 2nd instar nymph | 60 | 2.93 ± 0.03 a | 60 | 2.93 ± 0.03 a | 60 | 2.93 ± 0.03 a | 2,177 | 0.00 | 1.00 |
| 3rd instar nymph | 26 | 3.38 ± 0.12 a | 34 | 3.00 ± 0.19 a | 32 | 2.31 ± 0.20 b | 2,89 | 8.70 | 0.00 |
| 4th instar nymph | 22 | 2.55 ± 0.11 a | 30 | 2.07 ± 0.14 b | 28 | 1.93 ± 0.11 b | 2,77 | 5.83 | 0.00 |
| 5th instar nymph | 18 | 3.67 ± 0.11 a | 28 | 3.93 ± 0.15 a | 28 | 3.93 ± 0.15 a | 2,71 | 0.84 | 0.44 |
| Male | 8 | 7.50 ± 1.94 a | 8 | 11.25 ± 2.09 a | 12 | 6.67 ± 1.25 a | 2,25 | 2.01 | 0.16 |
| Female | 10 | 9.20 ± 1.64 a | 20 | 11.40 ± 1.84 a | 16 | 8.88 ± 2.13 a | 2,43 | 0.54 | 0.59 |
| Total longevity of male adult | 8 | 26.00 ± 2.09 a | 8 | 29.50 ± 2.49 a | 12 | 23.67 ± 1.08 a | 2,25 | 2.74 | 0.08 |
| Total longevity of female adult | 10 | 28.60 ± 1.73 a | 20 | 29.20 ± 1.91 a | 16 | 26.12 ± 2.10 a | 2,43 | 0.70 | 0.50 |
| Stages (d) | Prey Densities | ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | 5 Eggs | n | 10 Eggs | n | 15 Eggs | df | F | p | |
| TPOP/TPRP a (d) | 10 | 19.80 ± 0.13 a | 18 | 18.00 ± 0.36 b | 16 | 17.50 ± 0.50 b | 2,43 | 6.81 | 0.00 |
| APOP/APRP b (d) | 10 | 0.40 ± 0.16 a | 18 | 0.70 ± 0.17 a | 16 | 0.25 ± 0.11 a | 2,43 | 1.58 | 0.22 |
| Oviposition (d) | 10 | 7.20 ± 1.18 a | 18 | 10.22 ± 1.98 a | 16 | 7.88 ± 1.92 a | 2,43 | 0.12 | 0.89 |
| Post-Oviposition (d) | 10 | 0.60 ± 0.40 a | 18 | 1.40 ± 0.48 a | 16 | 0.50 ± 0.34 a | 2,43 | 1.39 | 0.26 |
| Fecundity (eggs/female) | 10 | 54.40 ± 9.67 a | 18 | 90.44 ± 17.87 a | 16 | 66.12 ± 9.38 a | 2,43 | 0.84 | 0.44 |
| Hatchability (%) | 10 | 29.63 ± 2.73 a | 18 | 31.46 ± 2.03 a | 16 | 26.44 ± 2.16 a | 2,41 | 1.45 | 0.25 |
| Population Parameters | Prey Densities | ||
|---|---|---|---|
| 5 Eggs | 10 Eggs | 15 Eggs | |
| Intrinsic rate of increase (r) (d−1) | 0.09 ± 0.02 a | 0.14 ± 0.01 a | 0.14 ± 0.01 a |
| Finite rate of increase (λ) (d−1) | 1.10 ± 0.02 a | 1.15 ± 0.01 a | 1.15 ± 0.02 a |
| Net reproductive rate (R0) (offspring) | 9.07 ± 3.05 a | 27.13 ± 7.47 a | 17.63 ± 4.50 a |
| Mean generation time (T) (d) | 23.57 ± 0.39 a | 23.21 ± 1.04 a,b | 20.89 ± 0.79 b |
| Gross reproductive rate (GRR) | 51.03 ± 11.68 a | 129.61 ± 27.26 a | 90.32 ± 16.91 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.; Li, S.; Jaleel, W.; Musa Khan, M.; Wang, J.; Zhou, X. Using a Two-Sex Life Table Tool to Calculate the Fitness of Orius strigicollis as a Predator of Pectinophora gossypiella. Insects 2020, 11, 275. https://doi.org/10.3390/insects11050275
Ali S, Li S, Jaleel W, Musa Khan M, Wang J, Zhou X. Using a Two-Sex Life Table Tool to Calculate the Fitness of Orius strigicollis as a Predator of Pectinophora gossypiella. Insects. 2020; 11(5):275. https://doi.org/10.3390/insects11050275
Chicago/Turabian StyleAli, Shahzaib, Sizhe Li, Waqar Jaleel, Muhammad Musa Khan, Jintao Wang, and Xingmiao Zhou. 2020. "Using a Two-Sex Life Table Tool to Calculate the Fitness of Orius strigicollis as a Predator of Pectinophora gossypiella" Insects 11, no. 5: 275. https://doi.org/10.3390/insects11050275
APA StyleAli, S., Li, S., Jaleel, W., Musa Khan, M., Wang, J., & Zhou, X. (2020). Using a Two-Sex Life Table Tool to Calculate the Fitness of Orius strigicollis as a Predator of Pectinophora gossypiella. Insects, 11(5), 275. https://doi.org/10.3390/insects11050275







