Effects of Diallyl Trisulfide, an Active Substance from Garlic Essential Oil, on Energy Metabolism in Male Moth Sitotroga cerealella (Olivier)
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Cultures
2.2. Bioassay
2.3. DAT Treatment and Sample Preparation
2.4. Determination of Total Protein and MAGPs Contents
2.5. Determination of Triglyceride (TG) Content
2.6. Determination of Glycogen Content
2.7. Determination of Total Soluble Sugar Content
2.8. Determination of Trehalose Content
2.9. Determination of Trehalase Activity
2.10. Statistical Analysis
3. Results
3.1. Bioassay
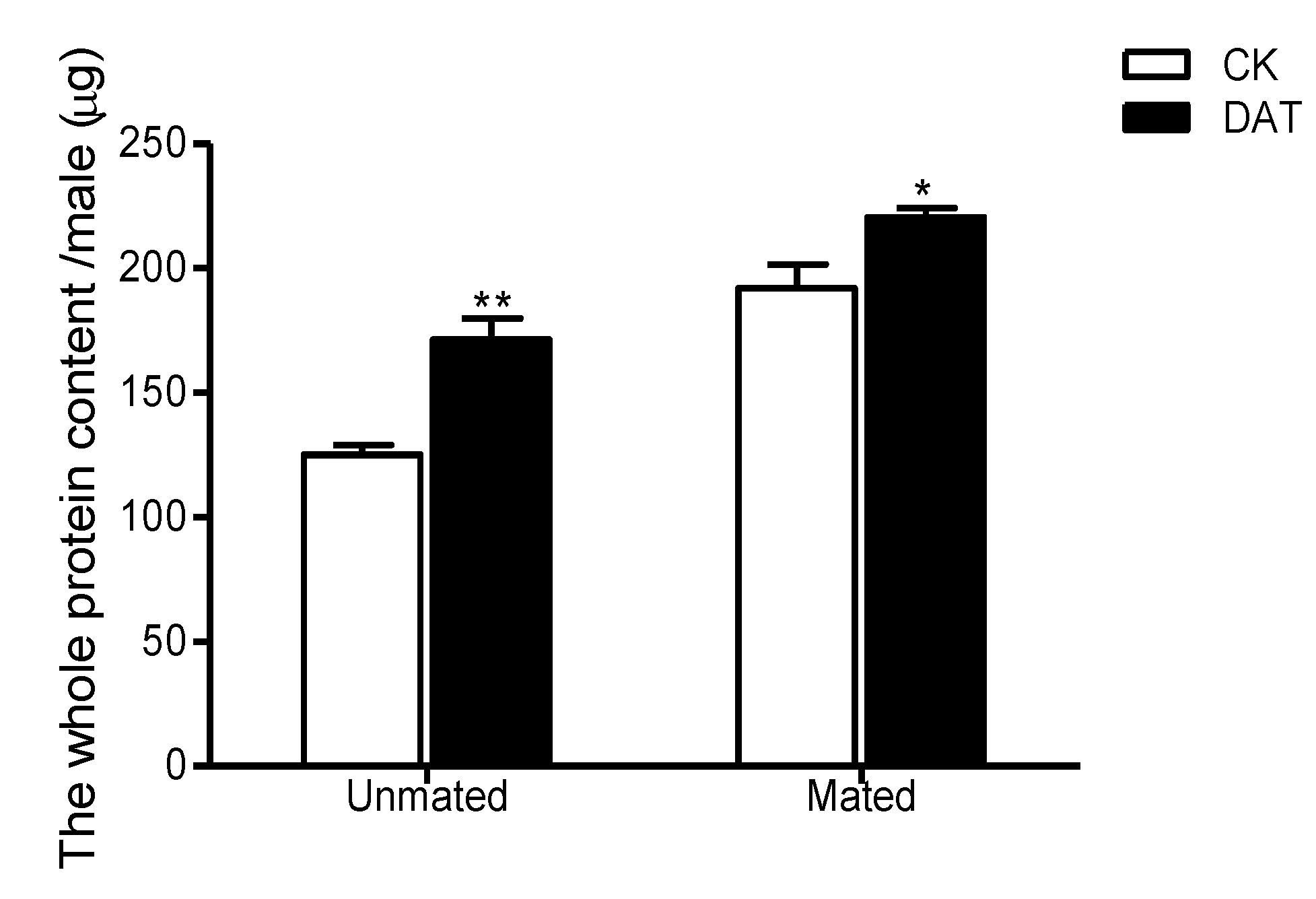
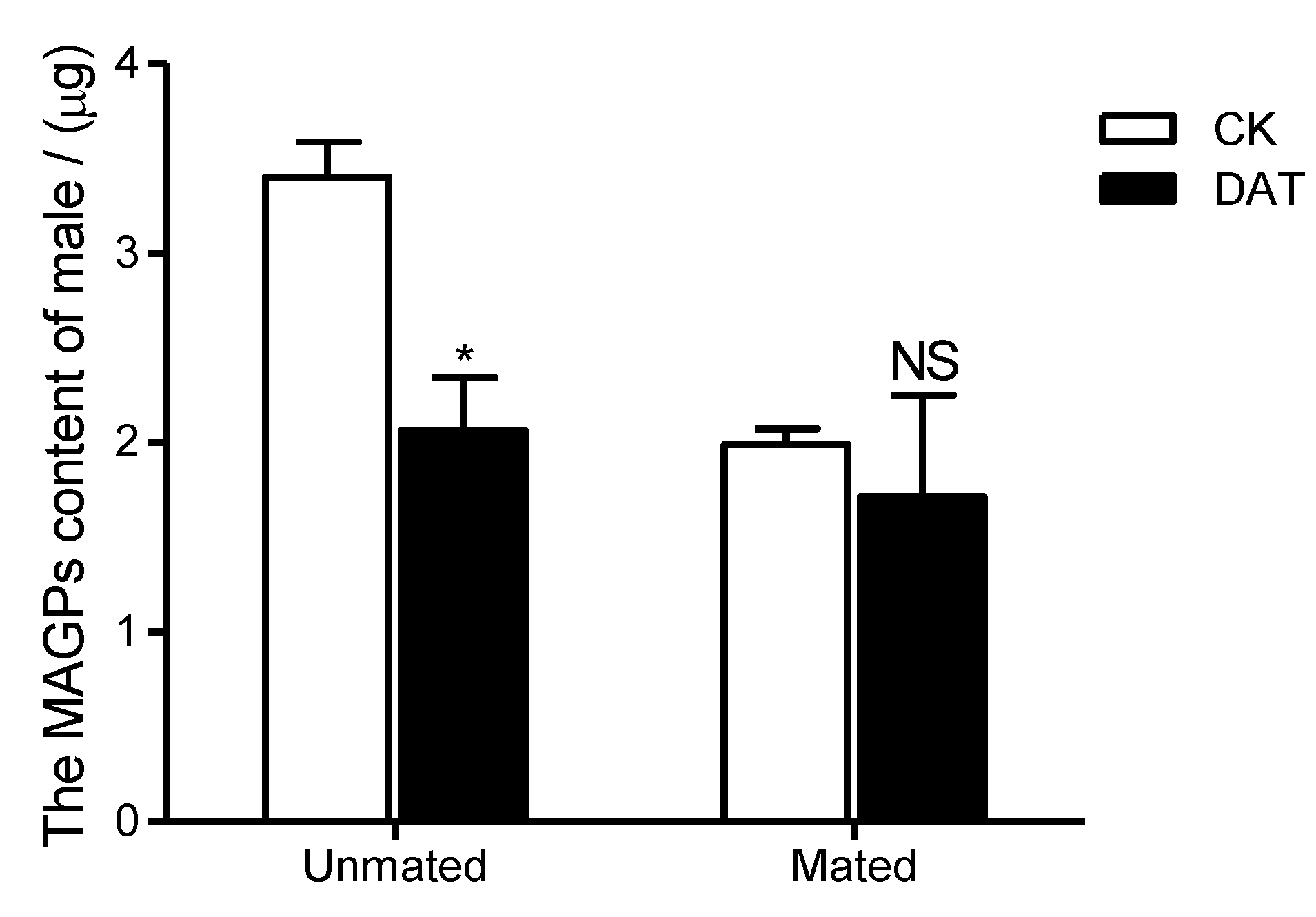
3.2. Effect of DAT on Protein Content in Pre- and Postmating Males
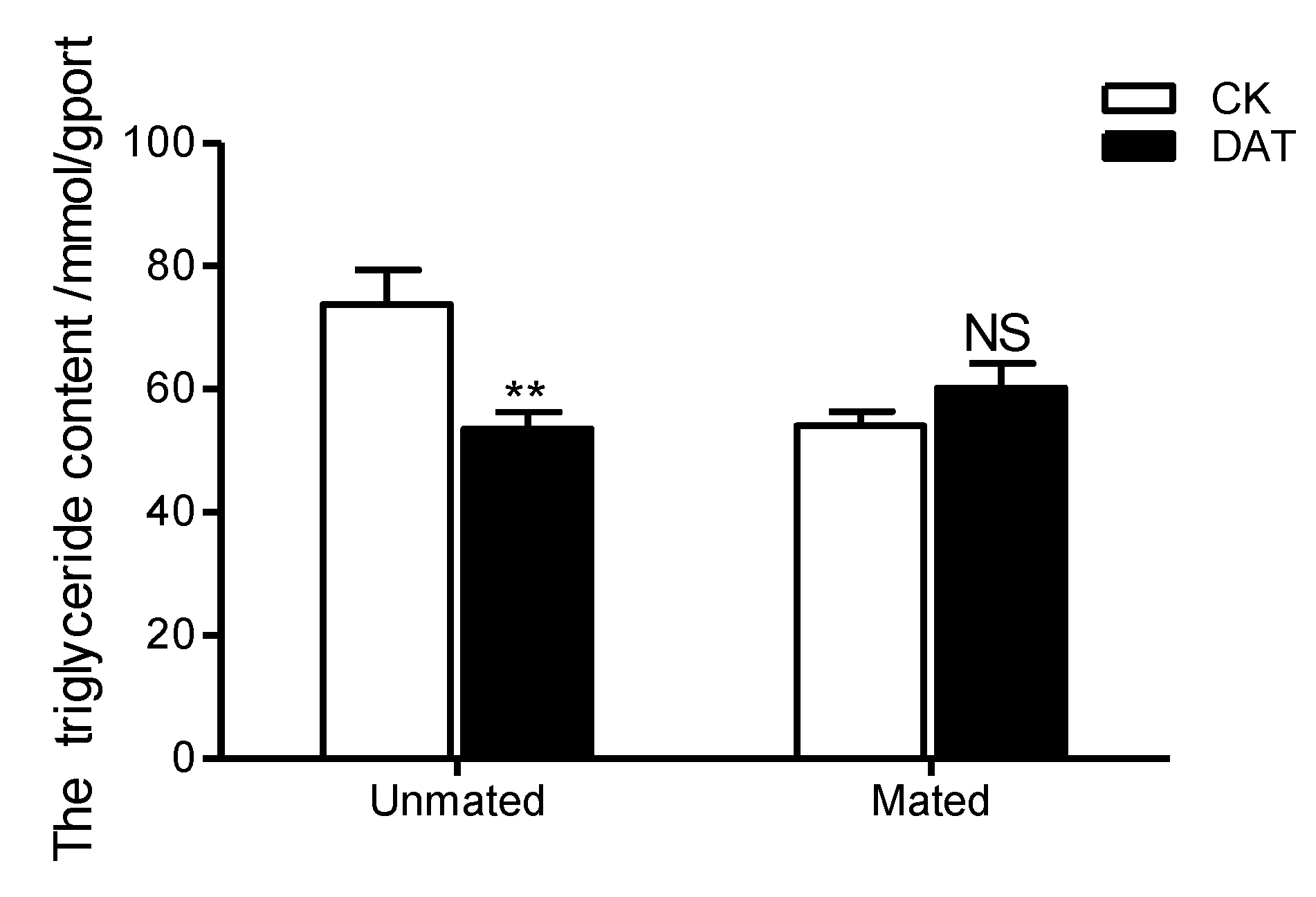
3.3. Effect of DAT on the Triglyceride Content of Pre- and Postmating Males
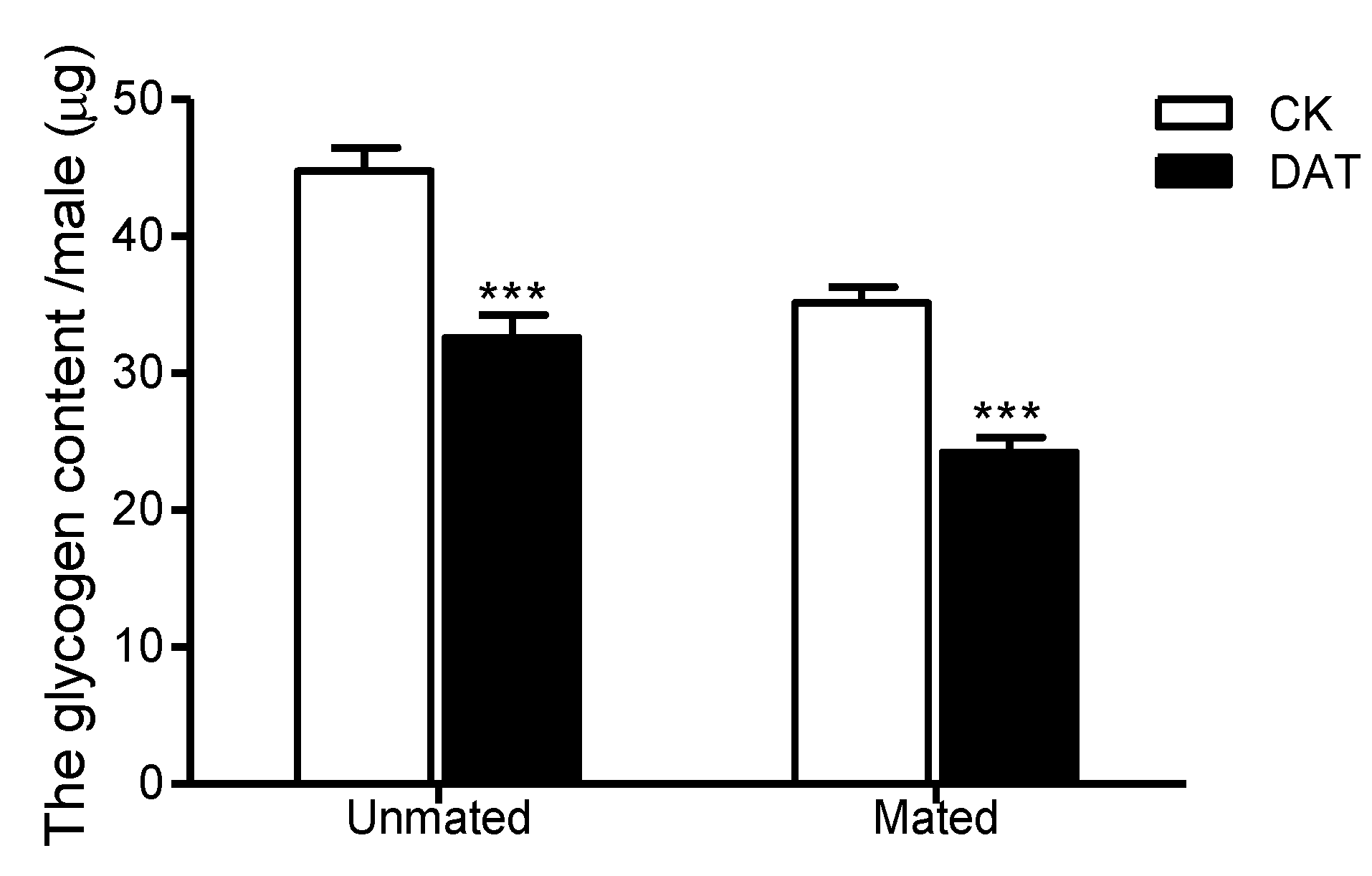
3.4. Effect of DAT on the Glycogen Content of Pre- and Postmating Males
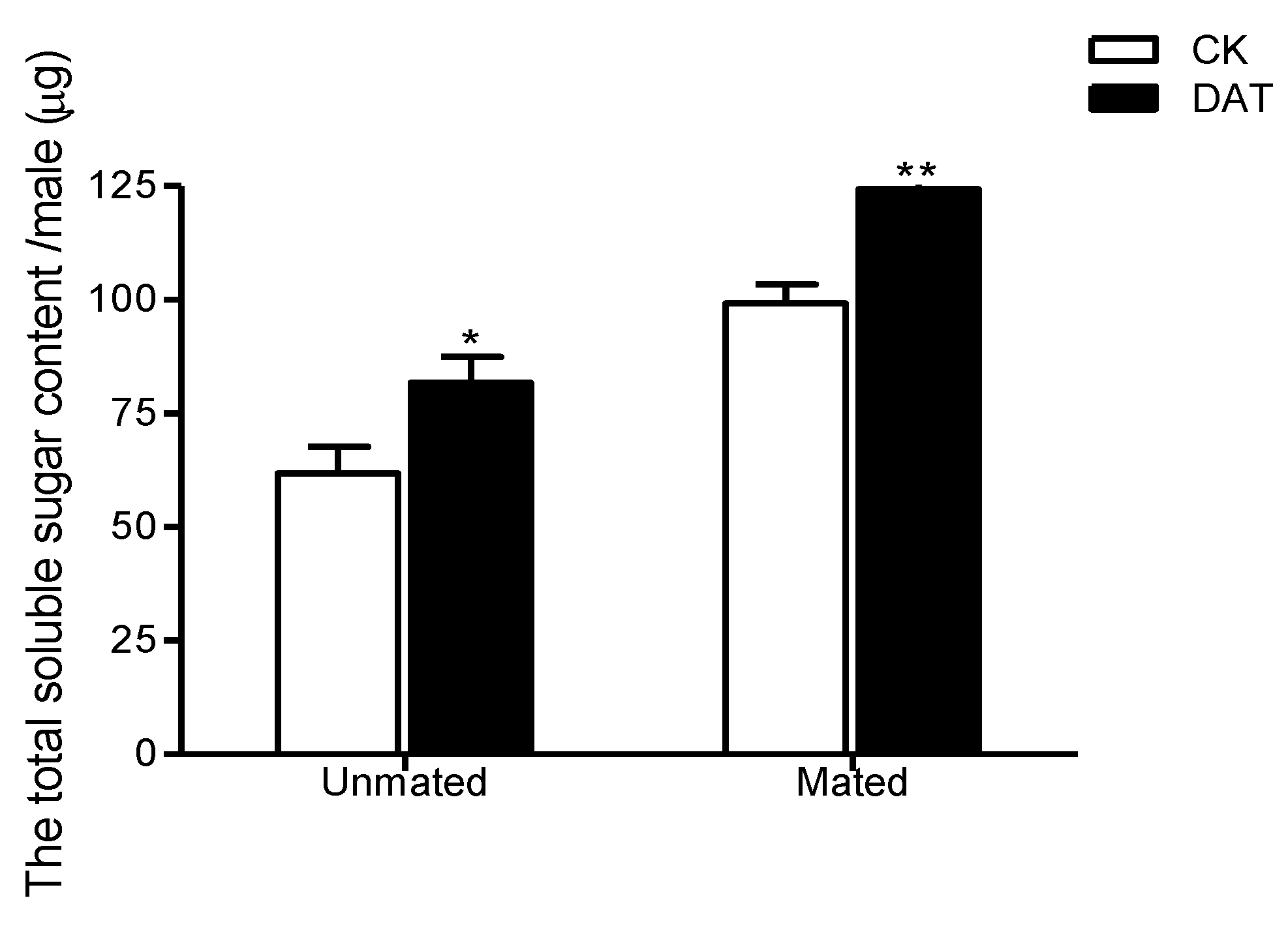
3.5. Effect of DAT on the Total Soluble Sugar Content of Pre- and Postmating Males
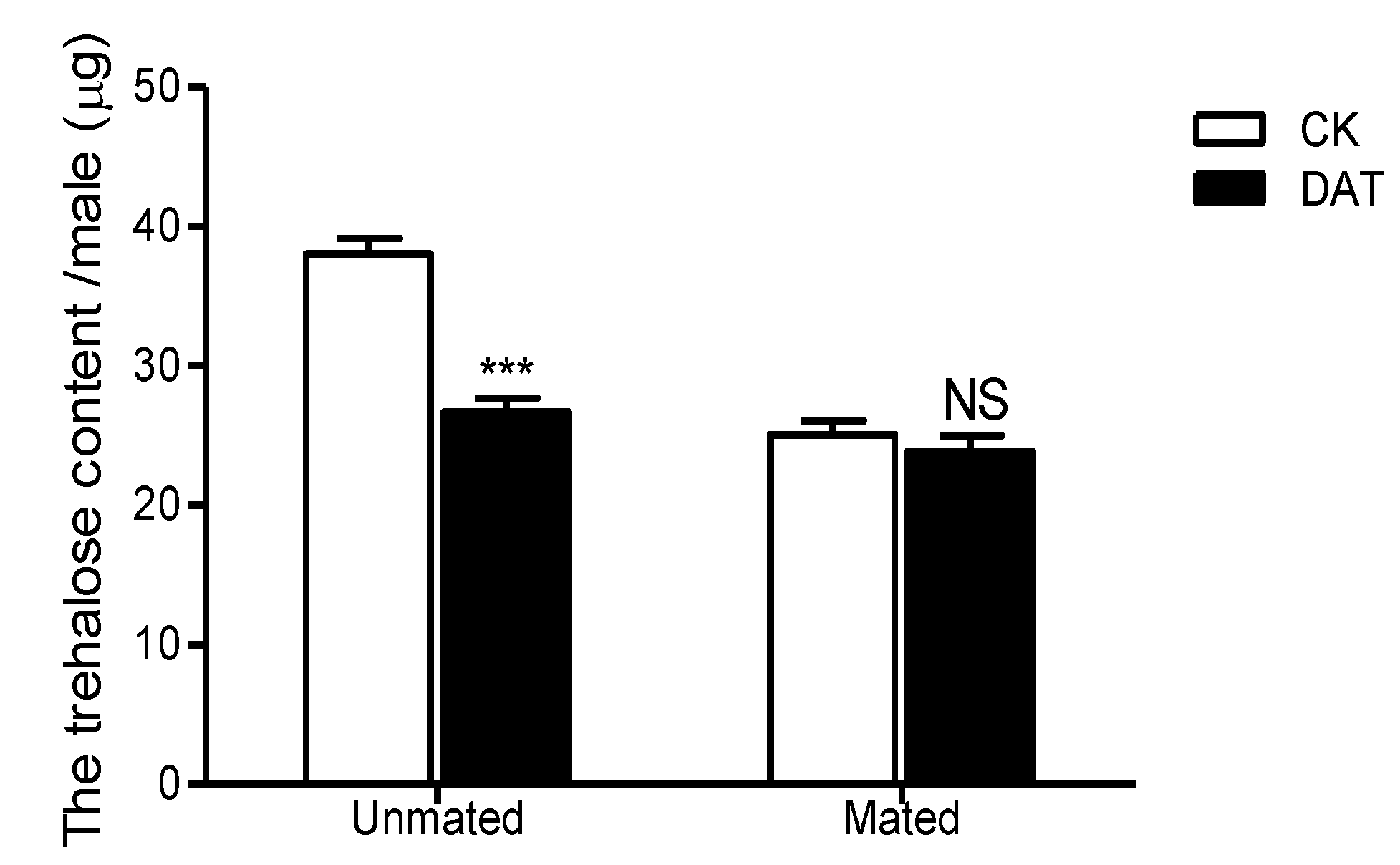
3.6. Effect of DAT on the Trehalose Content of Pre- and Postmating Males
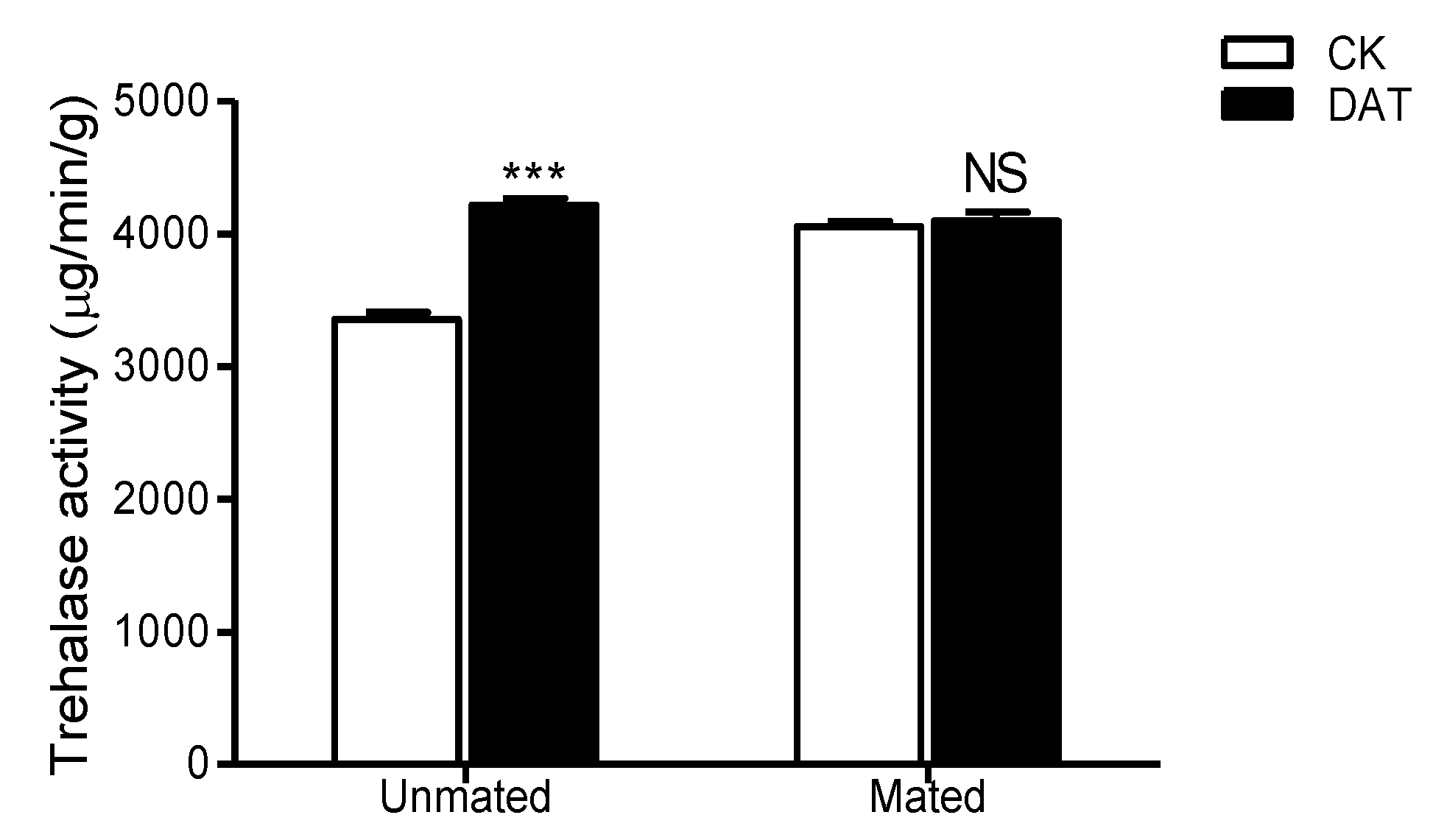
3.7. Effect of DAT on the Trehalase Activity of Pre- and Postmating Males
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Trematerra, P. Adult dispersal of Sitotroga cerealella in a conventional small-farm in Southern Italy. Bull. Insectol. 2015, 68, 111–118. [Google Scholar]
- Yang, F.L.; Lei, C.L. Insecticidal activities of garlic substances against adults of grain moth, Sitotroga cerealella (Lepidoptera:Gelechiidae). Insect Sci. 2012, 19, 205–212. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Smagghe, G.; Stark, J.D.; Desneux, N. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 2016, 61, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Boyer, S.; Zhang, H.; Lempérière, G. A review of control methods and resistance mechanisms in stored-product insect. Bull. Entomol. Res. 2012, 102, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Chowański, S.; Adamski, Z.; Marciniak, P.; Rosiński, G.; Büyükgüzel, E.; Büyükgüzel, K.; Falabella, P.; Scrano, L.; Ventrella, E.; Lelario, F. A review of bioinsecticidal activity of Solanaceae alkaloids. Toxins 2016, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 40–54. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, W. Control effects of new green-stored grain protectants osthole against stored-grain pests. Grain Process. 2013, 6, 74–78. [Google Scholar]
- Lin, S.F.; Li, T.H.; Chen, K.; Gao, H.T.; Li, G.W. GC-MS analysis of garlic and garlic essential oil for the contents of volatile oil and of chemical components. Phys. Test. Chem. Anal. B 2005, 41, 87–89. [Google Scholar]
- Shahriari, M.; Sahebzadeh, N. Effect of diallyl disulfide on physiological performance of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). Arch. Phytopathol. Plant Protect. 2017, 50, 33–46. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, W.B.; Du, X.Y.; Fa, Y.H.; Wang, X.G. Effects of garlic essential oil on biological activity, physiology and Bio- chemistry of Myzws persicae. Gui Agric. Sci. 2016, 44, 68–71. [Google Scholar]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.N. Advances in Insect Physiology. Trehalose—The Insect ‘Blood’ Sugar; Simpson, S.J., Ed.; Academic Press: New York, NY, USA, 2003; Volume 31, pp. 205–285. [Google Scholar]
- Ng, W.C.; Chin, J.S.R.; Tan, K.J.; Yew, J.Y. The fatty acid elongase Bond is essential for Drosophila sex pheromone synthesis and male fertility. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.W. Adipokinetic hormone inhibits the formation of energy stores and egg production in the cricket Gryllus bimaculatus. Comp. Biochem. Phys. B 2003, 136, 197–206. [Google Scholar] [CrossRef]
- Wang, L.P.; Shen, J.; Ge, L.Q.; Wu, J.C.; Yang, G.Q.; Jahn, G.C. Insecticide-induced increase in the protein content of male accessory glands and its effect on the fecundity of females in the brown planthopper Nilaparvata lugens Stål (Hemiptera: Delphacidae). Crop. Prot. 2010, 29, 1280–1285. [Google Scholar] [CrossRef]
- Ma, M.; Chang, M.M.; Lei, C.L.; Yang, F.L. A garlic substance disrupts odorant-binding protein recognition of insect pheromones released from adults of the angoumois grain moth, Sitotroga cerealella (Lepidoptera: Gelechiidae). Insect Mol. Biol. 2016, 25, 530–540. [Google Scholar] [CrossRef]
- Chang, M.M.; Shah, S.; Wu, M.Y.; Zhang, S.S.; Wu, G.; Yang, F.L. Effect of diallyl trisulfide on the reproductive behavior of the grain moth, Sitotroga cerealella (Lepidoptera: Gelechiidae). Insects 2020, 11, 21. [Google Scholar] [CrossRef]
- Shahriari, M.; Zibaee, A.; Shamakhi, L.; Sahebzadeh, N.; Naseri, D.; Hoda, H. Bio-efficacy and physiological effects of Eucalyptus globulus and Allium sativum essential oils against Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). Toxin Rev. 2018, 1–12. [Google Scholar] [CrossRef]
- Chang, M.M. Study on Ovipitional Inhibition by Active Substances from Garlic Essential Oil against Sitotroga cerealella. Master’s Dissertation, Huazhong Agricultural University, Wuhan, China, 2016. [Google Scholar]
- Chen, J.; Tang, B.; Chen, H.X.; Yao, Q.; Huang, X.F.; Chen, J.; Zhang, D.W.; Zhang, W.Q. Different functions of the insect soluble and membrane-bound trehalase genes in chitin biosynthesis revealed by RNA interference. PLoS ONE 2010, 5, e10133. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhao, X.; Qian, C.; Wei, G.; Zhu, B.; Liu, C. Expression and characterization of a lipase-related protein in the malpighian tubules of the Chinese oak silkworm, Antheraea pernyi. Bull. Entomol. Res. 2016, 106, 615–623. [Google Scholar] [CrossRef]
- Sak, O.; Uçkan, F.; Ergin, E. Effects of cypermethrin on total body weight, glycogen, protein, and lipid contents of Pimpla turionellae (L.)(Hymenoptera: Ichneumonidae). Belg. J. Zool. 2006, 136, 53–58. [Google Scholar]
- Vijayraghavan, C.; Chitra, K.C. Total protein and free amino acid content Spodoptera litura (Fabr.) due to botanicals and conventional insecticides. Indian J. Entomol. 2002, 64, 92–95. [Google Scholar]
- Wang, H.; Xu, Z.X.; Zhao, K.J.; Han, L.L. Effects of Median lethal concentration of imidacloprid on accumulation of energy substances in Aphis glycines (Homoptera: Aphididae). Chin. J. Biol. Control. 2014, 30, 654–659. [Google Scholar]
- Xu, C.; Zhang, Z.; Cui, K.; Zhao, Y.H.; Han, J.K.; Liu, F.; Mu, W. Effects of sublethal concentrations of cyantraniliprole on the development, fecundity and nutritional physiology of the black cutworm Agrotis ipsilon (Lepidoptera: Noctuidae). PLoS ONE 2016, 11, e0156555. [Google Scholar] [CrossRef] [PubMed]
- Piri, F.; Sahragard, A.; Ghadamyari, M. Sublethal effects of spinosad on some biochemical and biological parameters of Glyphodes pyloalis Walker (Lepidoptera: Pyralidae). Plant Prot. Sci. 2014, 50, 135–144. [Google Scholar] [CrossRef]
- Sharma, P.; Mohan, L.; Kumar Dua, K.; Srivastava, C.A. Status of carbohydrate, protein and lipid profile in the mosquito larvae treated with certain phytoextracts. Asian Pact. J. Trop. Med. 2011, 4, 301–304. [Google Scholar] [CrossRef]
- Joern, A.; Behmer, S.T. Importance of dietary nitrogen and carbohydrate to survival, growth, and reproduction in adults of the grasshopper Agenotettix deorum. Oecologia 1997, 112, 201–208. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Desneux, N.; Tariq, K.; Ali, A.; Gao, X.W.; Song, D.L. Clothianidin-induced sublethal effects and expression changes of vitellogenin and ecdysone receptors genes in the melon aphid, Aphis gossypii. Entomol. Gen. 2019, 39, 137–149. [Google Scholar] [CrossRef]
- Nie, H.Y.; Zhong, X.W.; Yi, Q.Y.; Zhang, L.P.; Chu, Y.; Zhao, P. Proteome analysis of small heat shock proteins in bombyx mori testis at different developmental stages. Sci. Agric. Sin. 2011, 44, 1923–1930. [Google Scholar]
- Wang, H.H.; Reitz, S.R.; Wang, L.X.; Wang, S.Y.; Xue, L.I.; Lei, Z.R. The mRNA Expression Profiles of Five heat shock protein genes from frankliniella occidentalis at different stages and their responses to temperatures and insecticides. J. Integr. Agric. 2014, 13, 2196–2210. [Google Scholar] [CrossRef]
- Guo, Y.J.; Liang, P.; Gao, X.W. Induced expression of hsp70 mRNA by nitenpyram, chlorpyrifos and beta-cypermethrin in Bemisia tabaci Mediterranean (Hemiptera: Aleyrodidae). Acta Entomol. Sin. 2013, 56, 29–38. [Google Scholar]
- Lu, K.; Chen, X.; Liu, W.T.; Zhang, Z.C.; Wang, Y.; You, K.K.; Li, Y.; Zhang, R.B.; Zhou, Q. Characterization of heat shock protein 70 transcript from Nilaparvata lugens (Stål): Its response to temperature and insecticide stresses. Pest. Biochem. Physiol. 2017, 142, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ji, B.Z.; Liu, S.W.; Tian, L.; Jin, F. The advances of insect male accessory gland functional proteins. Chin. Bull. Life Sci. 2008, 20, 618–624. [Google Scholar]
- Gillott, C. Male accessory gland secretions: Modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 2003, 48, 163–184. [Google Scholar] [CrossRef]
- Ge, L.Q.; Wang, L.P.; Zhao, K.F.; Wu, J.C.; Huang, L.J. Mating pair combinations of insecticide-treated male and female Nilaparvata lugens Stål (Hemiptera: Delphacidae) planthoppers influence protein content in the male accessory glands (MAGs) and vitellin content in both fat bodies and ovaries of adult females. Pest. Biochem. Physiol. 2010, 98, 279–288. [Google Scholar] [CrossRef]
- Yaginuma, T.; Happ, G.M. Trehalase from the bean-shaped accessory glands and the spermatophore of the male mealworm beetle, Tenebrio molitor. J. Comp. Physiol. B 1988, 157, 765. [Google Scholar] [CrossRef] [PubMed]
- Nath, B.S. Changes in carbohydrate metabolism in hemolymph and fat body of the silkworm, Bombyx mori L., exposed to organophosphorus insecticides. Pest. Biochem. Physiol. 2000, 68, 127–137. [Google Scholar] [CrossRef]
- Canavoso, L.E.; Jouni, Z.E.; Karnas, K.J.; Pennington, J.E.; Wells, M.A. Fat metabolism in insects. Annu. Rev. Nutr. 2001, 21, 23–46. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Lohar, M.K.; Wright, D.J. Changes in the lipid content in heamolymph, fat body and oocytes of malathion treated Tenebrio molitor L. adult females. Pak. J. Zool. 1993, 25, 57–60. [Google Scholar]
- Senthilkumar, N.; Varma, P.; Gurusubramaniam, G. Larvicidal and adulticidal activities of some medicinal plants against the malaria vector, Anoheles stephensi (Liston). Parasitol. Res. 2009, 104, 237–244. [Google Scholar] [CrossRef]
- Murthy, P.S.; Ramalakshmi, K.; Srinivas, P. Fungitoxic activity of Indian borage (Plectranthus amboinicus) volatiles. Food Chem. 2009, 114, 1014–1018. [Google Scholar] [CrossRef]
- Kumar, A.; Shukla, R.; Singh, P.; Dubey, N.K. Chemical composition, antifungal and antiaflatoxigenic activities of Ocimum sanctum L. essential oil and its safety assessment as plant based antimicrobia. Food Chem. Toxicol. 2009, 48, 539–543. [Google Scholar] [CrossRef] [PubMed]
| ∆CK | ∆DAT | F | df | p | Significance of Difference | |
|---|---|---|---|---|---|---|
| Protein | 66.9 ±5.62 | 49.18 ± 5.22 | 0.017 | 8 | 0.490 | * |
| MAGPs | −1.41 ± 0.10 | −0.35 ± 0.36 | 5.336 | 4 | 0.047 | * |
| Triglyceride | −23.54 ± 6.55 | 3.77 ± 3.48 | 2.226 | 4 | 0.021 | * |
| Glycogen | −7.11 ± 0.63 | −8.94 ± 0.92 | 0.009 | 11 | 0.119 | NS |
| Total soluble sugar | 37.36 ± 2.08 | 42.68 ± 2.53 | 0.552 | 8 | 0.143 | NS |
| Trehalose | −11.34 ± 0.44 | −0.02 ± 0.28 | 1.497 | 14 | <0.001 | *** |
| Trehalase | 702.19 ± 69.71 | −110.18 ± 117.89 | 1.954 | 14 | <0.001 | *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.-Y.; Ying, Y.-Y.; Zhang, S.-S.; Li, X.-G.; Yan, W.-H.; Yao, Y.-C.; Shah, S.; Wu, G.; Yang, F.-L. Effects of Diallyl Trisulfide, an Active Substance from Garlic Essential Oil, on Energy Metabolism in Male Moth Sitotroga cerealella (Olivier). Insects 2020, 11, 270. https://doi.org/10.3390/insects11050270
Wu M-Y, Ying Y-Y, Zhang S-S, Li X-G, Yan W-H, Yao Y-C, Shah S, Wu G, Yang F-L. Effects of Diallyl Trisulfide, an Active Substance from Garlic Essential Oil, on Energy Metabolism in Male Moth Sitotroga cerealella (Olivier). Insects. 2020; 11(5):270. https://doi.org/10.3390/insects11050270
Chicago/Turabian StyleWu, Meng-Ya, Yi-Yi Ying, Su-Su Zhang, Xue-Gang Li, Wen-Han Yan, Yu-Chen Yao, Sakhawat Shah, Gang Wu, and Feng-Lian Yang. 2020. "Effects of Diallyl Trisulfide, an Active Substance from Garlic Essential Oil, on Energy Metabolism in Male Moth Sitotroga cerealella (Olivier)" Insects 11, no. 5: 270. https://doi.org/10.3390/insects11050270
APA StyleWu, M.-Y., Ying, Y.-Y., Zhang, S.-S., Li, X.-G., Yan, W.-H., Yao, Y.-C., Shah, S., Wu, G., & Yang, F.-L. (2020). Effects of Diallyl Trisulfide, an Active Substance from Garlic Essential Oil, on Energy Metabolism in Male Moth Sitotroga cerealella (Olivier). Insects, 11(5), 270. https://doi.org/10.3390/insects11050270






