Hydrocarbon Signatures of the Ectoparasitoid Sphecophaga vesparum Shows Wasp Host Dependency
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Specimen
2.2. Chemical and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| RT (min) | RI | Identifications | Diagnostic Ions | |
|---|---|---|---|---|
| 1 | 17.92 | 2100 | n-C21 | 296 |
| 2 | 19.01 | 2200 | n-C22 | 310 |
| 3 | 20.09 | 2298 | n-C23 | 324 |
| 4 | 20.48 | 2334 | 11-MeC23 | 168, 169, 196, 197 |
| 5 | 20.64 | 2349 | 5-MeC23 | 84, 85, 280, 281 |
| 6 | 20.71 | 2356 | 2-MeC23 | 42, 43, 322, 323 |
| 7 | 20.88 | 2371 | 3-MeC23 | 56, 57, 308, 309 |
| 8 | 20.99 | 2382 | 5,11-diMeC23 | 84 196, 183, 295 |
| 9 | 21.16 | 2398 | n-C24 | 338 |
| 10 | 21.25 | 2406 | 3,7-diMeC23 | 56, 127, 252, 323 |
| 11 | 21.40 | 2420 | 12-,11-,10-MeC24 | 197, 183/168, 210/154, 224,225 |
| 12 | 21.79 | 2456 | 4-MeC24 | 70, 71, 308, 309 |
| 13 | 21.98 | 2473 | C25:1 | 83, 97, 111 |
| 14 | 22.16 | 2490 | 4,10-; 4,14-; 4,16-diMeC24 | 70, 169, 224, 323/ 168, 225/ 140, 253, 337 |
| 15 | 22.25 | 2499 | n-C25 | 352 |
| 16 | 22.63 | 2533 | 13-,11-MeC25 | 168, 196, 224 |
| 17 | 22.70 | 2540 | 7-MeC25 | 112, 281 |
| 18 | 22.81 | 2550 | 5-MeC25 | 85, 309 |
| 19 | 22.97 | 2564 | 9,13-; 9,15-diMeC25 | 140, 168, 211, 267/239, 267 |
| 20 | 23.06 | 2573 | 3-MeC25 | 57, 337 |
| 21 | 23.16 | 2582 | 5,9-diMeC25 | 85, 155, 252, 323 |
| 22 | 23.36 | 2600 | n-C26 | 366 |
| 23 | 23.44 | 2607 | 3,9-; 3,13-diMeC25 | 155, 252, 351/196, 211 |
| 24 | 23.74 | 2633 | 10-MeC26 | 154, 253 |
| 25 | 23.86 | 2644 | 4-MeC26 | 71, 337 |
| 26 | 24.22 | 2676 | 3-MeC26 | 56, 301, 351 |
| 27 | 24.37 | 2689 | 4,16-; 4,18-diMeC26 | 70, 168, 253, 351/140, 281 |
| 28 | 24.50 | 2700 | n-C27 | 380 |
| 29 | 24.68 | 2716 | 4,8,12-triMeC26 | 141, 224, 211, 295, 365 |
| 30 | 24.87 | 2733 | 13-,11-,9-MeC27 | 197, 224/168, 252/141, 281 |
| 31 | 25.07 | 2750 | 5-MeC27 | 85, 337 |
| 32 | 25.22 | 2763 | 9,13-; 9,19-MeC27 | 140, 211, 295/140, 295 |
| 33 | 25.35 | 2774 | 3-MeC27, 11,17-diMeC27 | 57, 366/ 168, 267 |
| 34 | 25.65 | 2801 | n-C28 | 394 |
| 35 | 25.72 | 2807 | 3,11-; 3,9-diMeC27 | 155, 183 252, 379/155, 281, 380 |
| 36 | 25.88 | 2821 | 14-MeC28 | 210, 211, 224 |
| 37 | 26.04 | 2834 | 10-MeC28 | 155, 280 |
| 38 | 26.38 | 2864 | 4-MeC28 | 70, 364 |
| 39 | 26.65 | 2887 | C29:1 | 83, 97, 111 |
| 40 | 26.81 | 2902 | n-C29 | 408 |
| 41 | 27.17 | 2933 | 15-, 13-, 11-MeC29 | 224, 225/196, 252/168, 281 |
| 42 | 27.53 | 2965 | 9,3-diMeC29 | 140, 211 |
| 43 | 27.60 | 2970 | 11,17-diMeC29 | 168, 295 |
| 44 | 27.68 | 2978 | 3-MeC29 | 56, 393 |
| 45 | 27.97 | 3003 | n-C30 | 422 |
| 46 | 28.03 | 3008 | unknown3 | |
| 47 | 28.34 | 3035 | 5,9,15,19-tetraMeC29 | 84, 155, 182, 239, 253, 337, 407 |
| 48 | 28.89 | 3083 | C31:1 | 83, 97, 111 |
| 49 | 29.12 | 3104 | n-C31 | 436 |
| 50 | 29.47 | 3135 | 15-, 13-, 11-MeC31 | 224, 252/196, 280/ 168, 308 |
| 51 | 29.83 | 3166 | 9,21-diMeC31 | 140, 168, 323, 351 |
| 52 | 29.99 | 3181 | 3-MeC31, 5,21-diMeC31 | 56, 420/84, 168, 323 |
| 53 | 30.19 | 3198 | n-C32 | 450 |
| 54 | 30.21 | 3200 | 3,17-; 3,13-;3,11-diMeC31 | 224, 267, 435/211, 280/ 183, 308 |
| 55 | 30.51 | 3227 | 14-,12-MeC32; 3,11,19-triMeC32 | 183, 196, 309, 323, 450 |
| 56 | 31.40 | 3306 | n-C33 | 464 |
| 57 | 31.72 | 3336 | 15-,13-,11-MeC33 | 224, 280/ 197, 309/ 168, 169, 337 |
| 58 | 32.07 | 3367 | 7,11-; 7,21-diMeC33 | 112, 183, 336/ 112, 196, 323 |
| 59 | 32.43 | 3400 | n-C34 | 478 |
| 60 | 33.52 | 3501 | n-C35 | 492 |
| 61 | 33.85 | 3532 | 15-, 13-, 11-MeC35 | 224, 308/ 196, 337/168, 465 |
| 62 | 34.24 | 3570 | 2,7-diMeC35 | 113, 434 |
| 63 | 34.90 | 3632 | unknown | |
| 64 | 35.58 | 3699 | n-C37 | 520 |
| 65 | 35.93 | 3733 | 19-, 13-, 11-MeC37 | 280, 281/ 196, 365/ 168, 393 |
| 66 | 36.20 | 3759 | 17,21-; 13,25-; 11,21-diMeC37 | 252, 323/196, 379/ 168, 252, 323 |
| 67 | 36.62 | 3801 | n-C38 | 534 |
| 68 | 37.62 | 3901 | n-C39 | 548 |
| 69 | 38.64 | 4003 | n-C40 | 562 |
References
- Lenoir, A.; D’Ettorre, P.; Errard, C.; Hefetz, A. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 2001, 46, 573–599. [Google Scholar] [CrossRef] [PubMed]
- Bagnères, A.; Lorenzi, M.C. Chemical deception/mimicry using cuticular hydrocarbons. In Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology; Blomquist, G., Bagnères, A., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 283–324. [Google Scholar]
- Dettner, K.; Liepert, C. Chemical mimicry and camouflage. Annu. Rev. Entomol. 1994, 39, 129–154. [Google Scholar] [CrossRef]
- Parmentier, T. Guests of Social Insects. In Encyclopedia of Social Insects; Starr, C., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–15. [Google Scholar]
- van Zweden, J.S.; d’Ettorre, P. Nestmate recognition in social insects and the role of hydrocarbons. In Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology; Blomquist, G.J., Bagnères, A.G., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 222–243. [Google Scholar]
- Van Oystaeyen, A.; Oliveira, R.C.; Holman, L.; Van Zweden, J.S.; Romero, C.; Oi, C.A.; d’Ettorre, P.; Khalesi, M.; Billen, J.; Wäckers, F.; et al. Conserved class of queen pheromones stops social insect workers from reproducing. Science 2014, 287, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Uboni, A.; Bagnères, A.G.; Christidès, J.P.; Lorenzi, M.C. Cleptoparasites, social parasites and a common host: Chemical insignificance for visiting host nests, chemical mimicry for living in. J. Insect Physiol. 2012, 58, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Van Oystaeyen, A.; van Zweden, J.S.; Huyghe, H.; Drijfhout, F.; Bonckaert, W.; Wenseleers, T. Chemical strategies of the beetle Metoecus Paradoxus, social parasite of the wasp Vespula vulgaris. J. Chem. Ecol. 2015, 41, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, T.; Dekoninck, W.; Wenseleers, T. Arthropods associate with their red wood ant host without matching nestmate recognition cues. J. Chem. Ecol. 2017, 43, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Nash, D.R.; Boomsma, J.J. Communication between hosts and social parasites. In Sociobiology of Communication: An Interdisciplinary Perspective; d’Ettorre, P., Hughes, D.P., Eds.; Oxford Scholarship Online: Oxford, UK, 2008; pp. 55–79. [Google Scholar]
- Liepert, C.; Dettner, K. Recognition of aphid parasitoids by honeydew-collecting ants: The role of cuticular lipids in a chemical mimicry system. J. Chem. Ecol. 1993, 19, 2143–2153. [Google Scholar] [CrossRef] [PubMed]
- Le Conte, Y.; Huang, Z.Y.; Roux, M.; Zeng, Z.J.; Christidès, J.P.; Bagnères, A.G. Varroa destructor changes its cuticular hydrocarbons to mimic new hosts. Biol. Lett. 2015, 11, 20150233. [Google Scholar] [CrossRef] [PubMed]
- Kather, R.; Drijfhout, F.; Martin, S.J. Evidence for colony-specific differences in chemical mimicry in the parasitic mite Varroa destructor. Chemoecology 2015, 25, 215–222. [Google Scholar] [CrossRef]
- Donovan, B.J. Life cycle of Sphecophaga vesparum (Curtis) (Hymenoptera: Ichneumonidae), a parasitoid of some vespid wasps. N. Z. J. Zool. 1991, 18, 181–192. [Google Scholar] [CrossRef]
- MacDonald, J.F.; Akre, R.D.; Hill, W.B. Nest Associates of Vespula atropilosa and V. pensylvanica in Southeastern Washington State (Hymenoptera: Vespidae). J. Kans. Entomol. Soc. 1975, 48, 53–63. [Google Scholar]
- Havron, A.; Margalith, Y. Parasitization of Vespa orientalis nests by Sphecophaga vesparum Curtis in Southern Israel (Hymenoptera: Vespidae, Ichneumonidae). Phytoparasitica 1995, 23, 19–25. [Google Scholar] [CrossRef]
- Field, R.P.; Darby, S.M. Host specificity of the parasitoid, Sphecophaga vesparum (Curtis) (Hymenoptera: Ichneumonidae), a potential biological control agent of the social wasps, Vespula germanica (Fabricius) and V. vulgaris (Linnaeus) (Hymenoptera: Vespidae) in Australia. N. Z. J. Zool. 1991, 18, 193–197. [Google Scholar] [CrossRef]
- Donovan, B.J.; Read, P.E.C. Attempted biological control of social wasps, Vespula spp., (Hymenoptera: Vespidae) with Sphecophaga vesparum (Curtis) (Hymenoptera: Ichneumonidae) in New Zealand. N. Z. J. Zool. 1987, 14, 329–335. [Google Scholar] [CrossRef]
- Akre, R.D. Wasp research: Strengths, weaknesses, and future directions. N. Z. J. Zool. 1991, 18, 223–227. [Google Scholar] [CrossRef]
- Lenoir, A.; Malosse, C.; Yamaoka, R. Chemical mimicry between parasitic ants of the genus Formicoxenus and their host Myrmica (Hymenoptera, Formicidae). Biochem. Syst. Ecol. 1997, 25, 379–389. [Google Scholar] [CrossRef]
- Guerrieri, F.J.; Nehring, V.; Jorgensen, C.G.; Nielsen, J.; Galizia, C.G.; d’Ettorre, P. Ants recognize foes and not friends. Proc. R. Soc. B Biol. Sci. 2009, 276, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Butts, D.P.; Camann, M.A.; Espelie, K.E. Workers and queens of the European hornet Vespa crabro L. have colony-specific cuticular hydrocarbon profiles (Hymenoptera, Vespidae). Insectes Soc. 1995, 42, 45–55. [Google Scholar] [CrossRef]

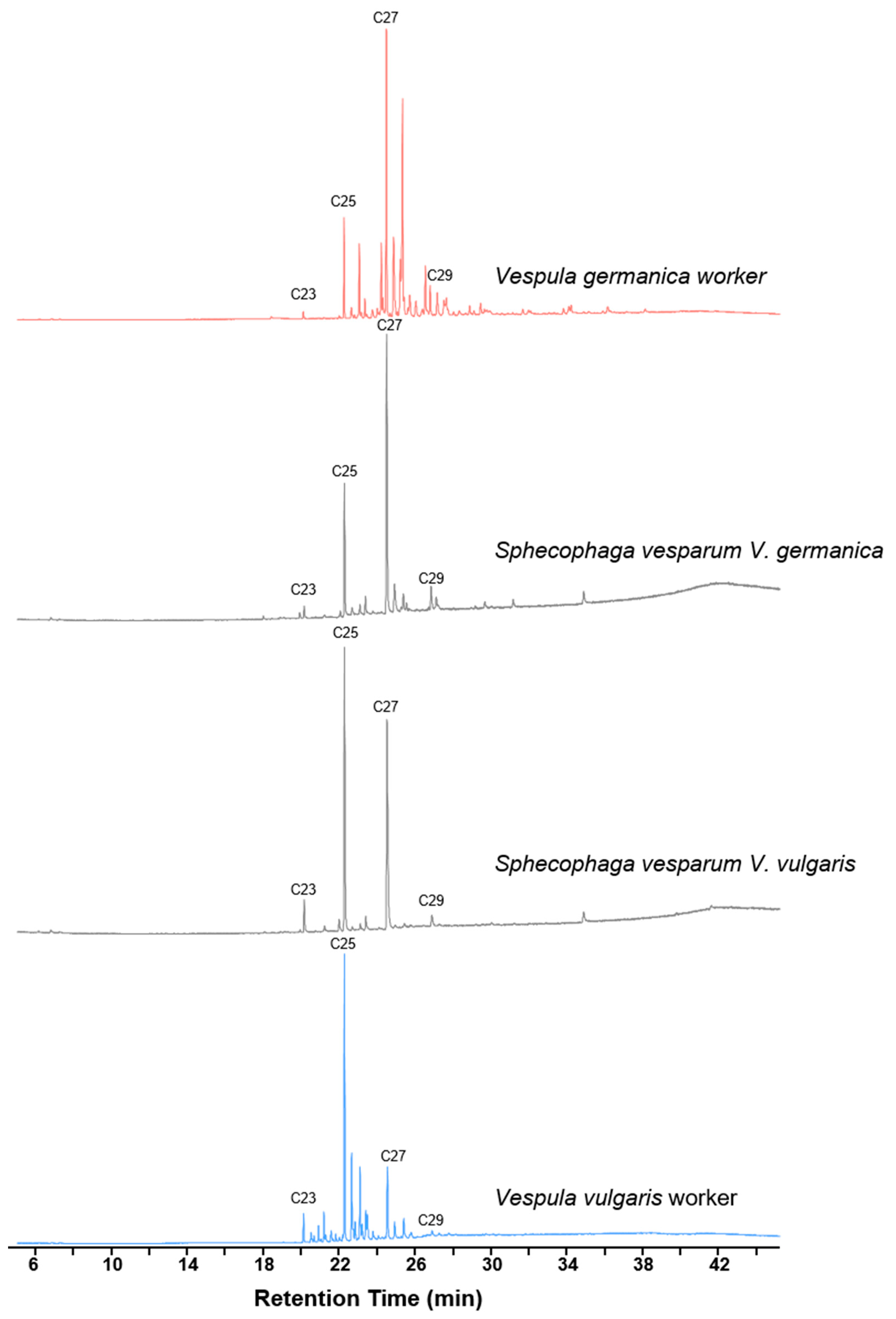
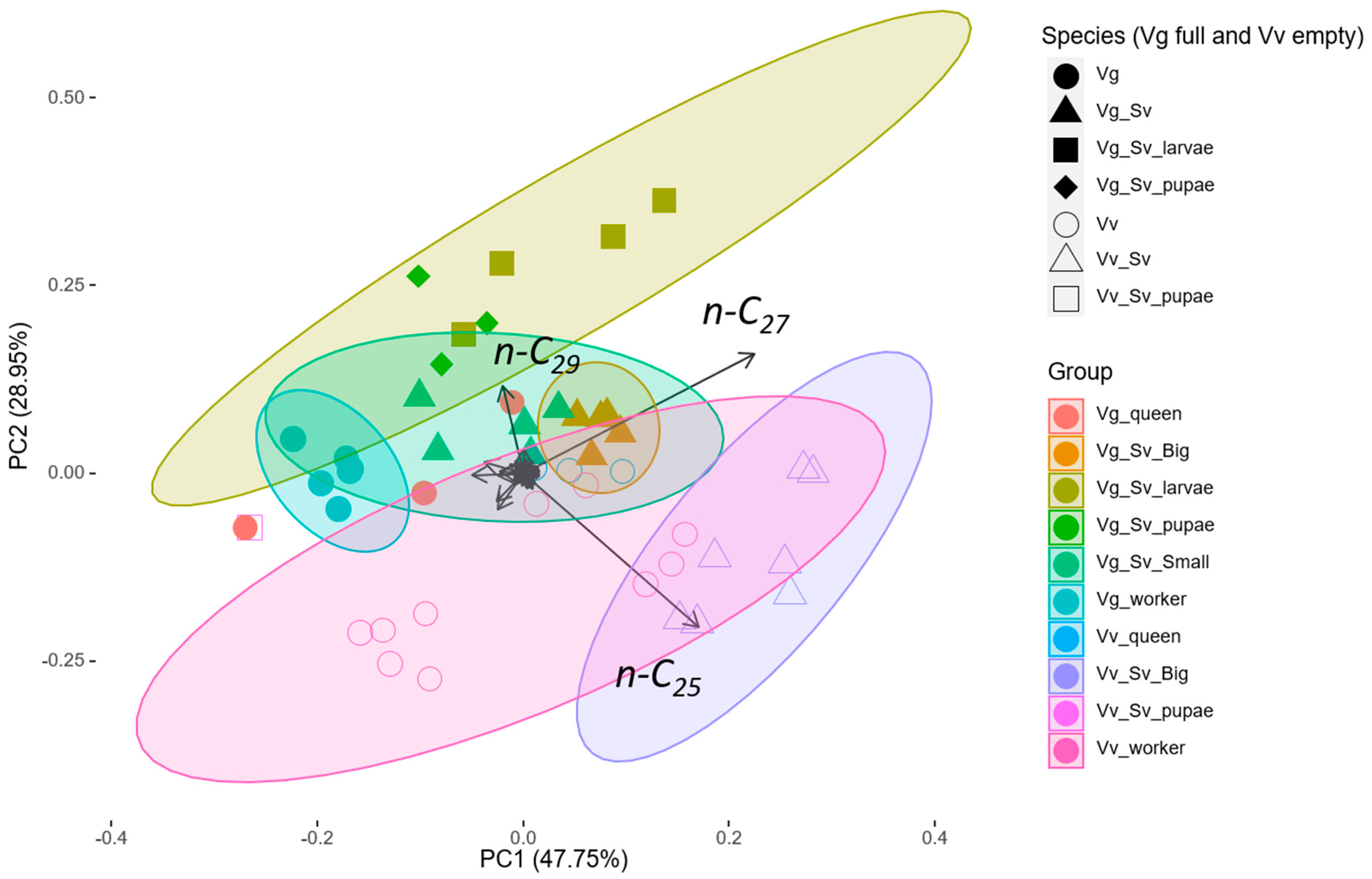
| Vg_Sv_larvae (n = 4) | Vg_Sv_pupae (n = 3) | Vg_Sv_Big (n = 5) | Vg_Sv_Small (n = 5) | Vv_Sv_Big (n = 7) | Vv_Sv_pupae (n = 1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifications | Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | Average |
| n-C21 | 0.35 | 0.10 | 0.51 | 0.34 | 0.51 | 0.10 | 0.75 | 0.12 | 0.47 | 0.33 | 2.60 |
| n-C22 | 0.65 | 0.17 | 0.95 | 0.56 | 0.56 | 0.04 | 1.36 | 0.46 | 0.32 | 0.09 | 1.63 |
| n-C23 | 0.93 | 0.24 | 1.81 | 0.95 | 1.57 | 0.14 | 2.47 | 0.35 | 4.89 | 2.68 | 1.87 |
| 11-MeC23 | 0.14 | 0.05 | 0.10 | 0.05 | 0.08 | 0.02 | 0.27 | 0.11 | 0.07 | 0.01 | 0.74 |
| 5-MeC23 | 0.11 | 0.02 | 0.07 | 0.03 | 0.09 | 0.02 | 0.29 | 0.10 | 0.08 | 0.01 | 0.99 |
| 2-MeC23 | 0.17 | 0.04 | 0.15 | 0.04 | 0.13 | 0.02 | 0.33 | 0.08 | 0.07 | 0.02 | 0.48 |
| 3-MeC23 | 0.27 | 0.06 | 0.21 | 0.07 | 0.15 | 0.03 | 0.49 | 0.15 | 0.11 | 0.03 | 1.42 |
| 5,11-diMeC23 | 0.23 | 0.07 | 0.16 | 0.04 | 0.14 | 0.03 | 0.40 | 0.10 | 0.09 | 0.02 | 1.19 |
| n-C24 | 0.61 | 0.12 | 0.58 | 0.21 | 0.61 | 0.04 | 1.40 | 0.27 | 0.74 | 0.09 | 1.34 |
| 3,7-diMeC23 | 0.62 | 0.16 | 0.53 | 0.15 | 0.37 | 0.06 | 1.07 | 0.23 | 0.26 | 0.06 | 1.72 |
| 12-,11-,10-MeC24 | 0.08 | 0.02 | 0.06 | 0.03 | 0.05 | 0.01 | 0.15 | 0.05 | 0.02 | 0.01 | 0.27 |
| 4-MeC24 | 0.36 | 0.08 | 0.26 | 0.08 | 0.17 | 0.05 | 0.59 | 0.12 | 0.12 | 0.05 | 1.58 |
| C25:1 | 0.20 | 0.05 | 0.17 | 0.08 | 0.29 | 0.17 | 0.36 | 0.06 | 0.96 | 1.49 | 1.14 |
| 4,10-; 4,14-; 4,16-diMeC24 | 0.07 | 0.04 | 0.05 | 0.03 | 0.05 | 0.03 | 0.13 | 0.04 | 0.05 | 0.02 | 0.56 |
| n-C25 | 3.21 | 1.94 | 3.83 | 1.64 | 16.14 | 1.57 | 10.16 | 3.33 | 34.33 | 3.46 | 3.49 |
| 13-,11-MeC25 | 0.36 | 0.18 | 0.64 | 0.29 | 1.20 | 0.20 | 1.11 | 0.26 | 0.49 | 0.25 | 1.29 |
| 7-MeC25 | 0.15 | 0.08 | 0.23 | 0.09 | 0.37 | 0.04 | 0.40 | 0.11 | 0.15 | 0.08 | 0.61 |
| 5-MeC25 | 0.25 | 0.08 | 0.20 | 0.04 | 0.28 | 0.05 | 0.50 | 0.15 | 0.22 | 0.12 | 1.25 |
| 9,13-; 9,15-diMeC25 | 0.18 | 0.05 | 0.12 | 0.03 | 0.14 | 0.03 | 0.29 | 0.06 | 0.13 | 0.10 | 0.80 |
| 3-MeC25 | 0.39 | 0.25 | 0.86 | 0.36 | 1.67 | 0.23 | 1.26 | 0.28 | 0.80 | 0.38 | 1.31 |
| 5,9-diMeC25 | 0.26 | 0.15 | 0.27 | 0.08 | 0.28 | 0.05 | 0.48 | 0.16 | 0.32 | 0.19 | 1.56 |
| n-C26 | 1.31 | 0.13 | 1.35 | 0.18 | 2.19 | 0.27 | 1.80 | 0.13 | 1.96 | 0.33 | 1.36 |
| 3,9-; 3,13-diMeC25 | 0.85 | 0.23 | 1.01 | 0.20 | 0.85 | 0.20 | 1.30 | 0.15 | 0.78 | 0.24 | 2.08 |
| 10-MeC26 | 0.48 | 0.15 | 0.65 | 0.11 | 0.74 | 0.15 | 0.84 | 0.17 | 0.28 | 0.11 | 2.11 |
| 4-MeC26 | 0.22 | 0.06 | 0.23 | 0.04 | 0.17 | 0.05 | 0.37 | 0.08 | 0.13 | 0.07 | 1.01 |
| 3-MeC26 | 0.17 | 0.03 | 0.28 | 0.13 | 0.44 | 0.15 | 0.41 | 0.06 | 0.19 | 0.09 | 1.59 |
| 4,16-; 4,18-diMeC26 | 0.07 | 0.03 | 0.06 | 0.04 | 0.08 | 0.08 | 0.11 | 0.04 | 0.02 | 0.01 | 0.48 |
| n-C27 | 40.07 | 11.07 | 27.81 | 3.02 | 35.68 | 1.63 | 26.62 | 5.00 | 37.68 | 7.75 | 2.76 |
| 4,8,12-triMeC26 | 0.82 | 0.25 | 0.31 | 0.06 | 0.34 | 0.04 | 0.88 | 0.21 | 0.65 | 0.12 | 1.17 |
| 13-,11-,9-MeC27 | 1.99 | 1.37 | 6.08 | 1.56 | 8.60 | 1.62 | 3.90 | 0.96 | 0.81 | 0.17 | 2.61 |
| 5-MeC27 | 0.35 | 0.07 | 0.31 | 0.14 | 0.51 | 0.12 | 0.46 | 0.03 | 0.13 | 0.05 | 0.98 |
| 9,13-; 9,19-MeC27 | 0.60 | 0.30 | 1.21 | 0.22 | 0.76 | 0.10 | 0.51 | 0.09 | 0.17 | 0.05 | 1.27 |
| 3-MeC27, 11,17-diMeC27 | 1.57 | 1.74 | 4.41 | 0.88 | 4.83 | 0.77 | 2.42 | 0.55 | 0.80 | 0.16 | 2.44 |
| n-C28 | 0.99 | 0.22 | 1.29 | 0.15 | 0.39 | 0.09 | 0.65 | 0.21 | 0.14 | 0.05 | 1.28 |
| 3,11-; 3,9-diMeC27 | 0.61 | 0.14 | 0.72 | 0.20 | 0.54 | 0.07 | 0.91 | 0.27 | 0.49 | 0.09 | 1.76 |
| 14-MeC28 | 0.14 | 0.03 | 0.11 | 0.05 | 0.07 | 0.03 | 0.22 | 0.07 | 0.07 | 0.02 | 0.55 |
| 10-MeC28 | 0.84 | 0.16 | 0.79 | 0.24 | 0.64 | 0.06 | 1.18 | 0.36 | 0.35 | 0.13 | 2.59 |
| 4-MeC28 | 0.54 | 0.17 | 0.55 | 0.05 | 0.49 | 0.11 | 0.99 | 0.25 | 0.35 | 0.12 | 2.06 |
| C29:1 | 0.17 | 0.07 | 0.14 | 0.02 | 0.27 | 0.16 | 0.41 | 0.10 | 0.11 | 0.05 | 0.85 |
| n-C29 | 15.79 | 3.60 | 17.71 | 8.92 | 3.25 | 0.99 | 4.57 | 2.82 | 2.07 | 0.63 | 1.71 |
| 15-, 13-, 11-MeC29 | 1.89 | 0.49 | 3.17 | 0.38 | 2.24 | 0.80 | 2.76 | 0.90 | 0.69 | 0.21 | 2.25 |
| 9,3-diMeC29 | 0.90 | 0.19 | 0.99 | 0.28 | 0.42 | 0.09 | 0.99 | 0.22 | 0.33 | 0.12 | 1.37 |
| 11,17-diMeC29 | 0.10 | 0.04 | 0.15 | 0.01 | 0.09 | 0.02 | 0.13 | 0.04 | 0.05 | 0.02 | 0.21 |
| 3-MeC29 | 1.11 | 0.18 | 1.00 | 0.12 | 0.63 | 0.09 | 1.08 | 0.20 | 0.37 | 0.10 | 1.82 |
| n-C30 | 0.47 | 0.10 | 0.50 | 0.11 | 0.19 | 0.04 | 0.48 | 0.16 | 0.11 | 0.04 | 0.61 |
| unknown3 | 0.56 | 0.13 | 0.47 | 0.03 | 0.39 | 0.07 | 0.87 | 0.14 | 0.27 | 0.07 | 0.97 |
| 5,9,15,19-tetraMeC29 | 0.53 | 0.11 | 0.48 | 0.04 | 0.36 | 0.12 | 0.82 | 0.24 | 0.19 | 0.06 | 1.20 |
| C31:1 | 0.69 | 0.17 | 0.73 | 0.04 | 0.53 | 0.07 | 1.06 | 0.17 | 0.31 | 0.07 | 2.71 |
| n-C31 | 2.32 | 0.82 | 2.62 | 1.32 | 0.64 | 0.11 | 1.36 | 0.39 | 0.35 | 0.08 | 2.43 |
| 15-, 13-, 11-MeC31 | 0.95 | 0.25 | 1.20 | 0.07 | 0.49 | 0.07 | 0.93 | 0.10 | 0.33 | 0.13 | 1.52 |
| 9,21-diMeC31 | 0.85 | 0.23 | 0.52 | 0.12 | 0.49 | 0.13 | 0.71 | 0.10 | 0.34 | 0.11 | 1.32 |
| 3-MeC31, 5,21-diMeC31 | 2.47 | 1.07 | 0.79 | 0.04 | 0.68 | 0.18 | 1.17 | 0.18 | 0.71 | 0.25 | 1.63 |
| n-C32 | 0.10 | 0.04 | 0.06 | 0.02 | 0.04 | 0.01 | 0.09 | 0.03 | 0.03 | 0.01 | 0.24 |
| 3,17-; 3,13-;3,11-diMeC31 | 0.93 | 0.30 | 0.53 | 0.09 | 0.54 | 0.10 | 1.13 | 0.29 | 0.31 | 0.09 | 2.41 |
| 14-,12-MeC32; 3,11,19-triMeC32 | 0.08 | 0.03 | 0.05 | 0.02 | 0.05 | 0.01 | 0.13 | 0.04 | 0.03 | 0.01 | 0.25 |
| n-C33 | 0.20 | 0.06 | 0.17 | 0.01 | 0.12 | 0.02 | 0.26 | 0.06 | 0.07 | 0.02 | 0.54 |
| 15-,13-,11-MeC33 | 0.85 | 0.06 | 0.95 | 0.11 | 0.46 | 0.08 | 1.02 | 0.13 | 0.29 | 0.09 | 2.44 |
| 7,11-; 7,21-diMeC33 | 1.35 | 0.42 | 0.75 | 0.22 | 0.48 | 0.06 | 1.13 | 0.12 | 0.38 | 0.14 | 2.64 |
| n-C34 | 0.10 | 0.02 | 0.12 | 0.02 | 0.08 | 0.02 | 0.16 | 0.03 | 0.05 | 0.01 | 0.41 |
| n-C35 | 0.08 | 0.04 | 0.09 | 0.00 | 0.05 | 0.01 | 0.13 | 0.01 | 0.03 | 0.01 | 0.32 |
| 15-, 13-, 11-MeC35 | 0.67 | 0.21 | 0.99 | 0.07 | 0.40 | 0.04 | 0.85 | 0.16 | 0.28 | 0.09 | 2.59 |
| 2,7-diMeC35 | 0.91 | 0.28 | 0.92 | 0.21 | 0.65 | 0.13 | 1.37 | 0.35 | 0.42 | 0.10 | 4.58 |
| unknown | 3.64 | 1.06 | 4.74 | 1.14 | 3.40 | 0.14 | 6.76 | 1.17 | 1.72 | 0.56 | 2.59 |
| n-C37 | 0.09 | 0.02 | 0.10 | 0.04 | 0.06 | 0.02 | 0.15 | 0.03 | 0.04 | 0.02 | 0.30 |
| 19-, 13-, 11-MeC37 | 0.29 | 0.10 | 0.40 | 0.04 | 0.27 | 0.05 | 0.49 | 0.18 | 0.12 | 0.06 | 1.43 |
| 17,21-; 13,25-; 11,21-diMeC37 | 0.43 | 0.01 | 0.48 | 0.03 | 0.36 | 0.06 | 0.67 | 0.14 | 0.19 | 0.06 | 1.57 |
| n-C38 | 0.07 | 0.05 | 0.08 | 0.01 | 0.06 | 0.02 | 0.12 | 0.03 | 0.03 | 0.01 | 0.23 |
| n-C39 | 0.05 | 0.02 | 0.05 | 0.01 | 0.05 | 0.02 | 0.11 | 0.04 | 0.04 | 0.01 | 0.28 |
| n-C40 | 0.12 | 0.06 | 0.12 | 0.03 | 0.11 | 0.04 | 0.24 | 0.08 | 0.06 | 0.02 | 0.61 |
| Vg_worker (n = 6) | Vv_worker (n = 10) | Vg_queen (n = 3) | Vv_queen (n = 3) | |||||
|---|---|---|---|---|---|---|---|---|
| Identifications | Average | SD | Average | SD | Average | SD | Average | SD |
| n-C21 | 0.19 | 0.38 | 0.10 | 0.03 | 0.72 | 1.19 | 0.04 | 0.02 |
| n-C22 | 0.13 | 0.14 | 0.13 | 0.04 | 0.58 | 0.94 | 0.05 | 0.03 |
| n-C23 | 1.74 | 3.63 | 1.89 | 0.65 | 0.79 | 1.02 | 0.48 | 0.07 |
| 11-MeC23 | 0.03 | 0.04 | 0.54 | 0.57 | 0.26 | 0.43 | 0.08 | 0.03 |
| 5-MeC23 | 0.02 | 0.02 | 0.36 | 0.35 | 0.24 | 0.40 | 0.06 | 0.02 |
| 2-MeC23 | 0.01 | 0.01 | 0.03 | 0.02 | 0.18 | 0.30 | 0.01 | 0.01 |
| 3-MeC23 | 0.06 | 0.02 | 1.02 | 0.93 | 0.44 | 0.62 | 0.21 | 0.06 |
| 5,11-diMeC23 | 0.03 | 0.02 | 0.26 | 0.24 | 0.35 | 0.56 | 0.08 | 0.04 |
| n-C24 | 0.14 | 0.12 | 1.76 | 0.48 | 0.39 | 0.41 | 0.64 | 0.05 |
| 3,7-diMeC23 | 0.06 | 0.06 | 0.60 | 0.54 | 0.51 | 0.83 | 0.17 | 0.07 |
| 12-,11-,10-MeC24 | 0.01 | 0.01 | 0.01 | 0.00 | 0.10 | 0.17 | 0.01 | 0.00 |
| 4-MeC24 | 0.08 | 0.01 | 0.57 | 0.45 | 0.66 | 0.95 | 0.12 | 0.02 |
| C25:1 | 0.34 | 0.14 | 0.33 | 0.27 | 0.57 | 0.52 | 0.12 | 0.03 |
| 4,10-; 4,14-; 4,16-diMeC24 | 0.03 | 0.01 | 0.37 | 0.34 | 0.22 | 0.33 | 0.08 | 0.02 |
| n-C25 | 6.62 | 1.07 | 23.09 | 5.69 | 7.99 | 4.06 | 18.47 | 2.14 |
| 13-,11-MeC25 | 1.44 | 0.59 | 6.07 | 5.36 | 0.79 | 0.49 | 1.42 | 0.37 |
| 7-MeC25 | 0.26 | 0.12 | 0.76 | 0.59 | 0.32 | 0.32 | 0.22 | 0.02 |
| 5-MeC25 | 0.30 | 0.04 | 1.68 | 0.93 | 0.56 | 0.61 | 0.41 | 0.11 |
| 9,13-; 9,15-diMeC25 | 0.22 | 0.09 | 0.16 | 0.12 | 0.31 | 0.32 | 0.09 | 0.02 |
| 3-MeC25 | 5.40 | 2.06 | 6.04 | 1.69 | 6.22 | 4.98 | 3.45 | 0.36 |
| 5,9-diMeC25 | 0.50 | 0.15 | 1.38 | 1.11 | 0.69 | 0.53 | 0.49 | 0.05 |
| n-C26 | 1.14 | 0.39 | 3.64 | 0.97 | 1.99 | 0.80 | 4.84 | 0.50 |
| 3,9-; 3,13-diMeC25 | 0.41 | 0.12 | 2.41 | 1.90 | 0.92 | 0.91 | 0.96 | 0.12 |
| 10-MeC26 | 0.99 | 0.26 | 1.06 | 0.86 | 0.93 | 0.85 | 0.40 | 0.11 |
| 4-MeC26 | 0.07 | 0.02 | 0.16 | 0.08 | 0.34 | 0.51 | 0.05 | 0.01 |
| 3-MeC26 | 6.18 | 3.09 | 0.62 | 0.41 | 3.03 | 1.58 | 0.60 | 0.29 |
| 4,16-; 4,18-diMeC26 | 0.27 | 0.11 | 0.17 | 0.13 | 0.23 | 0.14 | 0.07 | 0.02 |
| n-C27 | 15.29 | 4.11 | 20.00 | 11.69 | 19.13 | 15.69 | 30.89 | 3.28 |
| 4,8,12-triMeC26 | 0.29 | 0.09 | 0.10 | 0.03 | 0.55 | 0.61 | 0.12 | 0.05 |
| 13-,11-,9-MeC27 | 9.34 | 0.59 | 2.81 | 1.96 | 3.67 | 0.95 | 1.53 | 0.49 |
| 5-MeC27 | 0.32 | 0.06 | 0.32 | 0.15 | 0.48 | 0.53 | 0.13 | 0.04 |
| 9,13-; 9,19-MeC27 | 5.51 | 2.17 | 0.35 | 0.18 | 2.28 | 0.85 | 0.19 | 0.06 |
| 3-MeC27, 11,17-diMeC27 | 15.46 | 6.00 | 5.47 | 2.57 | 12.35 | 8.37 | 7.68 | 0.81 |
| n-C28 | 0.87 | 0.08 | 0.94 | 0.65 | 1.16 | 0.22 | 1.76 | 0.27 |
| 3,11-; 3,9-diMeC27 | 1.86 | 0.64 | 1.59 | 0.57 | 1.36 | 0.14 | 0.81 | 0.10 |
| 14-MeC28 | 0.02 | 0.02 | 0.04 | 0.02 | 0.15 | 0.17 | 0.02 | 0.02 |
| 10-MeC28 | 1.62 | 0.44 | 0.39 | 0.21 | 1.38 | 1.11 | 0.35 | 0.06 |
| 4-MeC28 | 0.86 | 0.27 | 0.56 | 0.10 | 1.01 | 0.60 | 0.39 | 0.14 |
| C29:1 | 0.53 | 0.24 | 0.35 | 0.18 | 0.56 | 0.33 | 0.27 | 0.16 |
| n-C29 | 5.64 | 9.47 | 3.07 | 1.51 | 2.48 | 0.37 | 6.08 | 1.28 |
| 15-, 13-, 11-MeC29 | 2.37 | 1.01 | 1.09 | 0.67 | 1.61 | 0.88 | 0.85 | 0.21 |
| 9,3-diMeC29 | 1.41 | 0.36 | 0.48 | 0.27 | 1.62 | 0.21 | 0.49 | 0.09 |
| 11,17-diMeC29 | 0.36 | 0.13 | 0.07 | 0.06 | 0.35 | 0.14 | 0.14 | 0.02 |
| 3-MeC29 | 1.51 | 0.62 | 1.27 | 0.39 | 2.05 | 0.12 | 4.55 | 0.89 |
| n-C30 | 0.13 | 0.06 | 0.18 | 0.14 | 0.57 | 0.31 | 0.24 | 0.05 |
| unknown3 | 0.32 | 0.05 | 0.57 | 0.20 | 0.85 | 0.61 | 0.28 | 0.04 |
| 5,9,15,19-tetraMeC29 | 0.45 | 0.13 | 0.30 | 0.19 | 0.94 | 0.85 | 0.26 | 0.13 |
| C31:1 | 0.69 | 0.36 | 0.57 | 0.23 | 1.19 | 1.34 | 0.73 | 0.37 |
| n-C31 | 0.81 | 1.11 | 0.49 | 0.20 | 1.39 | 1.63 | 0.60 | 0.06 |
| 15-, 13-, 11-MeC31 | 1.16 | 0.59 | 0.36 | 0.18 | 1.16 | 0.93 | 0.58 | 0.16 |
| 9,21-diMeC31 | 0.72 | 0.22 | 0.34 | 0.28 | 0.87 | 0.57 | 1.09 | 0.18 |
| 3-MeC31, 5,21-diMeC31 | 0.55 | 0.19 | 0.51 | 0.22 | 1.00 | 0.82 | 2.07 | 0.49 |
| n-C32 | 0.03 | 0.01 | 0.03 | 0.02 | 0.08 | 0.09 | 0.04 | 0.01 |
| 3,17-; 3,13-;3,11-diMeC31 | 0.32 | 0.17 | 0.39 | 0.16 | 0.83 | 0.98 | 0.30 | 0.07 |
| 14-,12-MeC32; 3,11,19-triMeC32 | 0.03 | 0.02 | 0.03 | 0.02 | 0.12 | 0.17 | 0.01 | 0.01 |
| n-C33 | 0.08 | 0.13 | 0.06 | 0.02 | 0.20 | 0.29 | 0.04 | 0.00 |
| 15-,13-,11-MeC33 | 0.69 | 0.61 | 0.27 | 0.10 | 1.05 | 1.24 | 0.31 | 0.04 |
| 7,11-; 7,21-diMeC33 | 0.80 | 0.42 | 0.38 | 0.25 | 1.31 | 1.44 | 1.74 | 0.42 |
| n-C34 | 0.04 | 0.05 | 0.04 | 0.02 | 0.15 | 0.19 | 0.06 | 0.01 |
| n-C35 | 0.02 | 0.02 | 0.02 | 0.01 | 0.14 | 0.21 | 0.02 | 0.01 |
| 15-, 13-, 11-MeC35 | 0.60 | 0.30 | 0.27 | 0.08 | 0.85 | 0.89 | 0.21 | 0.07 |
| 2,7-diMeC35 | 1.37 | 0.35 | 0.39 | 0.19 | 1.81 | 1.67 | 1.15 | 0.26 |
| unknown | 0.39 | 0.46 | 0.30 | 0.09 | 1.01 | 1.35 | 0.22 | 0.10 |
| n-C37 | 0.02 | 0.02 | 0.03 | 0.01 | 0.09 | 0.12 | 0.02 | 0.01 |
| 19-, 13-, 11-MeC37 | 0.15 | 0.05 | 0.10 | 0.05 | 0.43 | 0.64 | 0.05 | 0.04 |
| 17,21-; 13,25-; 11,21-diMeC37 | 0.62 | 0.17 | 0.18 | 0.07 | 0.97 | 1.05 | 0.10 | 0.06 |
| n-C38 | 0.03 | 0.03 | 0.03 | 0.02 | 0.15 | 0.21 | 0.02 | 0.01 |
| n-C39 | 0.02 | 0.02 | 0.03 | 0.02 | 0.13 | 0.20 | 0.01 | 0.01 |
| n-C40 | 0.04 | 0.04 | 0.05 | 0.04 | 0.20 | 0.29 | 0.02 | 0.02 |
| (A) | Vg_Sv versus Vv_Sv | (B) | Vg versus Vv | ||||||
| Compound | Cumulative | p-Value | Compound | Cumulative | p-Value | ||||
| 1 | n-C25 | 0.31 | 0.001 | *** | 1 | n-C25 | 0.15 | 0.007 | ** |
| 2 | n-C27 | 0.45 | 0.991 | 2 | n-C27 | 0.29 | 0.584 | ||
| 3 | 13-, 11-, 9-MeC27 | 0.53 | 0.004 | ** | 3 | 3-MeC27, 11-17-diMeC27 | 0.39 | 0.001 | *** |
| 4 | unknown | 0.58 | 0.008 | ** | 4 | 13-, 11-, 9-MeC27 | 0.45 | 0.003 | ** |
| 5 | n-C23 | 0.62 | 0.056 | 5 | 3-meC26 | 0.50 | 0.001 | *** | |
| 6 | 3-MeC27, 11-17-diMeC27 | 0.67 | 1.000 | 6 | 13-, 11-MeC25 | 0.54 | 0.021 | * | |
| 7 | n-C29 | 0.69 | 0.692 | 7 | n-C29 | 0.59 | 0.088 | ||
| 8 | 15-, 13-, 11-MeC29 | 0.72 | 0.001 | *** | 8 | 9,13-; 9,19-diMeC27 | 0.63 | 0.001 | *** |
| 9 | C25:1 | 0.73 | 0.054 | 9 | 3-MeC25 | 0.66 | 0.892 | ||
| 10 | 3-MeC25 | 0.74 | 0.974 | 10 | n-C26 | 0.69 | 0.001 | *** | |
| (C) | Vg versus Vg_Sv | (D) | Vg versus Vv_Sv | ||||||
| Compound | Cumulative | p-Value | Compound | Cumulative | p-Value | ||||
| 1 | n-C27 | 0.18 | 0.068 | 1 | n-C25 | 0.23 | 0.001 | *** | |
| 2 | 3-MeC27, 11-17-diMeC27 | 0.31 | 0.001 | *** | 2 | n-C27 | 0.41 | 0.001 | *** |
| 3 | n-C25 | 0.39 | 1.000 | 3 | 3-MeC27, 11-17-diMeC27 | 0.53 | 0.001 | *** | |
| 4 | 3-meC26 | 0.45 | 0.001 | *** | 4 | 13-, 11-, 9-MeC27 | 0.59 | 0.001 | *** |
| 5 | unknown | 0.50 | 0.001 | *** | 5 | 3-meC26 | 0.63 | 0.001 | *** |
| 6 | 3-MeC25 | 0.55 | 0.003 | ** | 6 | 3-MeC26 | 0.67 | 0.002 | ** |
| 7 | n-C29 | 0.60 | 0.210 | 7 | n-C23 | 0.71 | 0.001 | *** | |
| 8 | 9,13-; 9,19-diMeC27 | 0.64 | 0.001 | *** | 8 | 9,13-; 9,19-diMeC27 | 0.74 | 0.001 | *** |
| 9 | 13-, 11-, 9-MeC27 | 0.68 | 0.929 | 9 | n-C29 | 0.77 | 0.445 | ||
| 10 | n-C23 | 0.71 | 0.486 | 10 | 15-, 13-, 11-MeC29 | 0.78 | 0.058 | ||
| (E) | Vv versus Vg_Sv | (F) | Vv versus Vv_Sv | ||||||
| Compound | Cumulative | p-Value | Compound | Cumulative | p-Value | ||||
| 1 | n-C27 | 0.16 | 0.844 | 1 | n-C27 | 0.21 | 0.041 | * | |
| 2 | n-C25 | 0.29 | 0.995 | 2 | n-C25 | 0.38 | 0.312 | ||
| 3 | unknown | 0.36 | 0.001 | *** | 3 | 3-MeC27, 11-17-diMeC27 | 0.45 | 0.860 | |
| 4 | 13-, 11-meC25 | 0.42 | 0.009 | ** | 4 | 3-MeC25 | 0.52 | 0.001 | *** |
| 5 | 13-, 11-, 9-meC27 | 0.48 | 0.206 | 5 | 13-, 11-meC25 | 0.58 | 0.038 | * | |
| 6 | 3-MeC25 | 0.53 | 0.005 | ** | 6 | n-C23 | 0.63 | 0.008 | ** |
| 7 | 3-MeC27, 11-17-diMeC27 | 0.58 | 1.000 | 7 | n-C29 | 0.65 | 0.639 | ||
| 8 | n-C29 | 0.61 | 0.657 | 8 | n-C26 | 0.68 | 0.004 | ** | |
| 9 | n-C26 | 0.63 | 0.001 | *** | 9 | 15-, 13-, 11-MeC29 | 0.70 | 1.000 | |
| 10 | 15-, 13-, 11-MeC29 | 0.65 | 0.003 | ** | 10 | 3-MeC29 | 0.73 | 0.015 | * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oi, C.A.; Brown, R.L.; Stevens, I.; Wenseleers, T. Hydrocarbon Signatures of the Ectoparasitoid Sphecophaga vesparum Shows Wasp Host Dependency. Insects 2020, 11, 268. https://doi.org/10.3390/insects11050268
Oi CA, Brown RL, Stevens I, Wenseleers T. Hydrocarbon Signatures of the Ectoparasitoid Sphecophaga vesparum Shows Wasp Host Dependency. Insects. 2020; 11(5):268. https://doi.org/10.3390/insects11050268
Chicago/Turabian StyleOi, Cintia Akemi, Robert L. Brown, Ian Stevens, and Tom Wenseleers. 2020. "Hydrocarbon Signatures of the Ectoparasitoid Sphecophaga vesparum Shows Wasp Host Dependency" Insects 11, no. 5: 268. https://doi.org/10.3390/insects11050268
APA StyleOi, C. A., Brown, R. L., Stevens, I., & Wenseleers, T. (2020). Hydrocarbon Signatures of the Ectoparasitoid Sphecophaga vesparum Shows Wasp Host Dependency. Insects, 11(5), 268. https://doi.org/10.3390/insects11050268





