Comparative Mortality and Adaptation of a Smurf Assay in Two Species of Tenebrionid Beetles Exposed to Bacillus thuringiensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Establishment of a Positive Control for Gut Disruption in T. molitor and T. castaneum
2.3. Bioassays
2.3.1. Insects
2.3.2. Bacterial Cultures
2.3.3. Bacterial Exposure
2.4. Statistics
3. Results
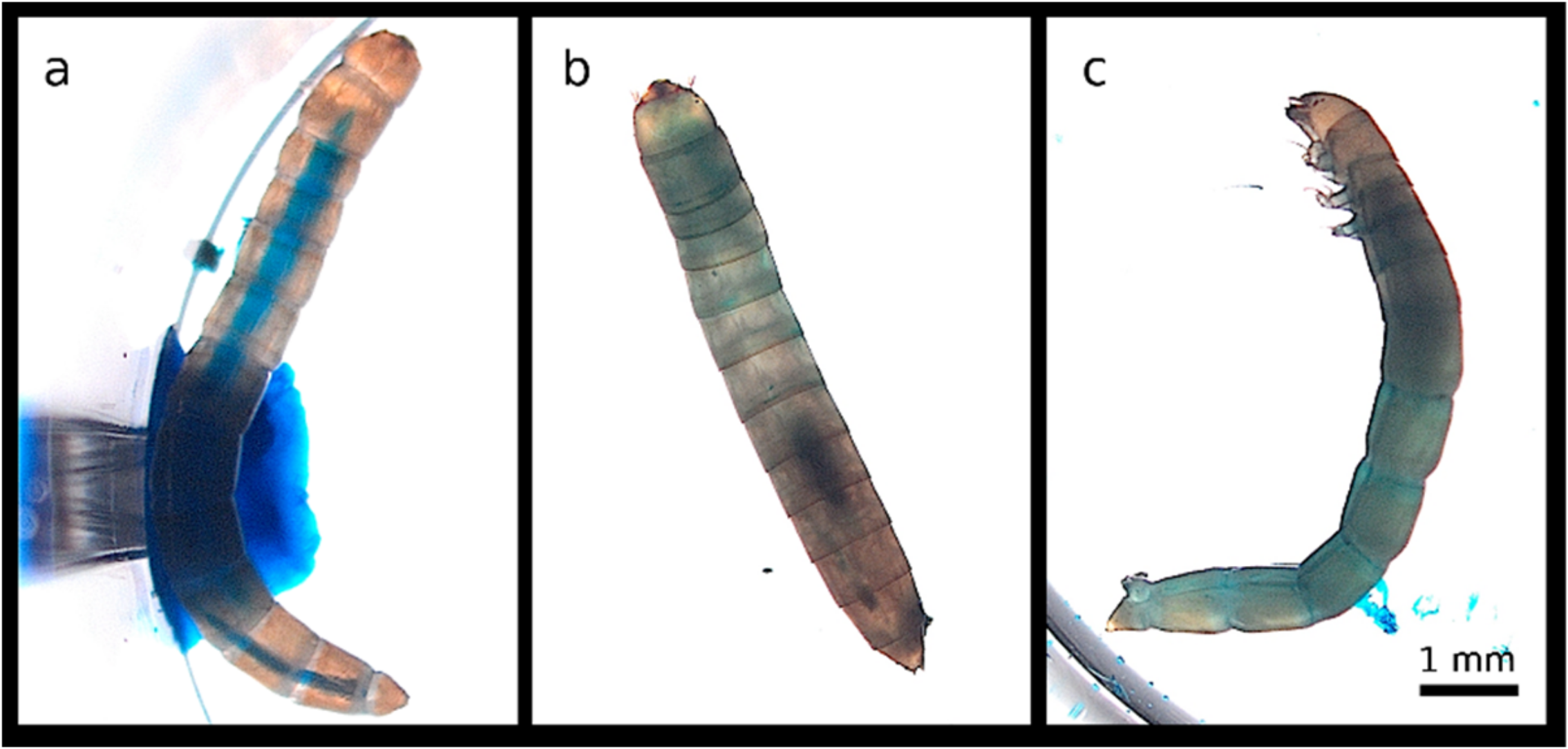
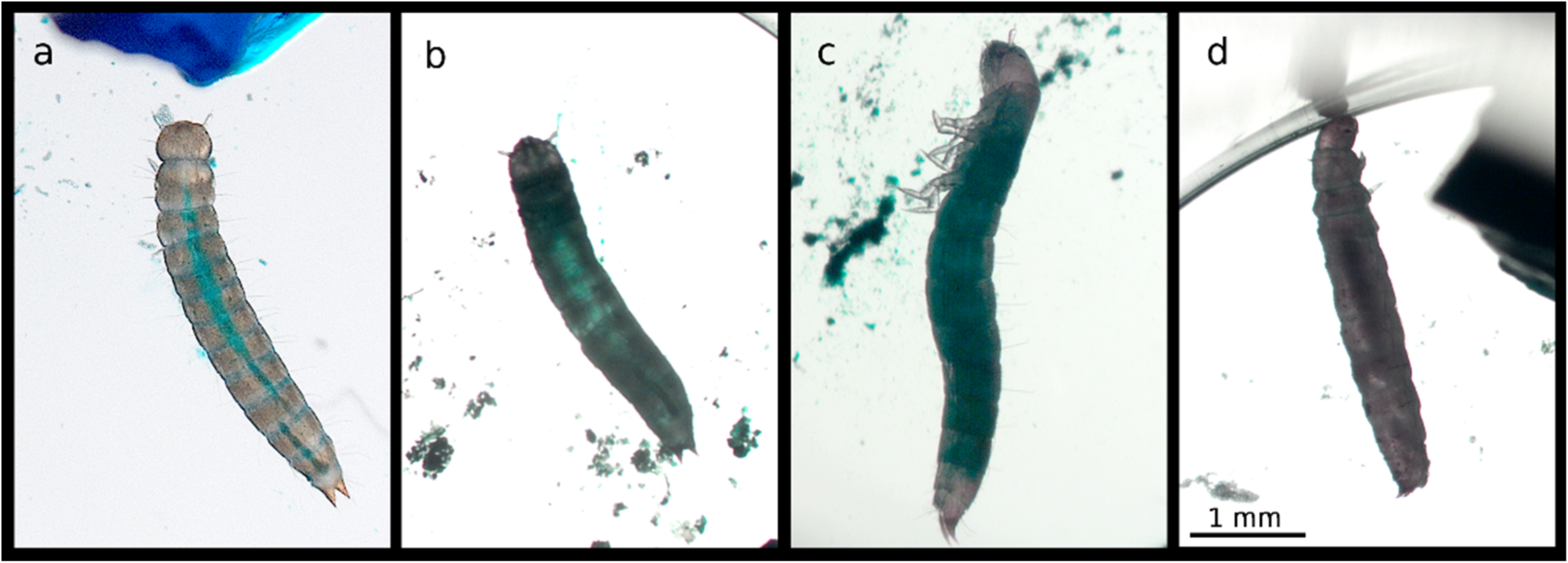
3.1. Chemical Induction of a Smurf Phenotype in T. castaneum and T. molitor
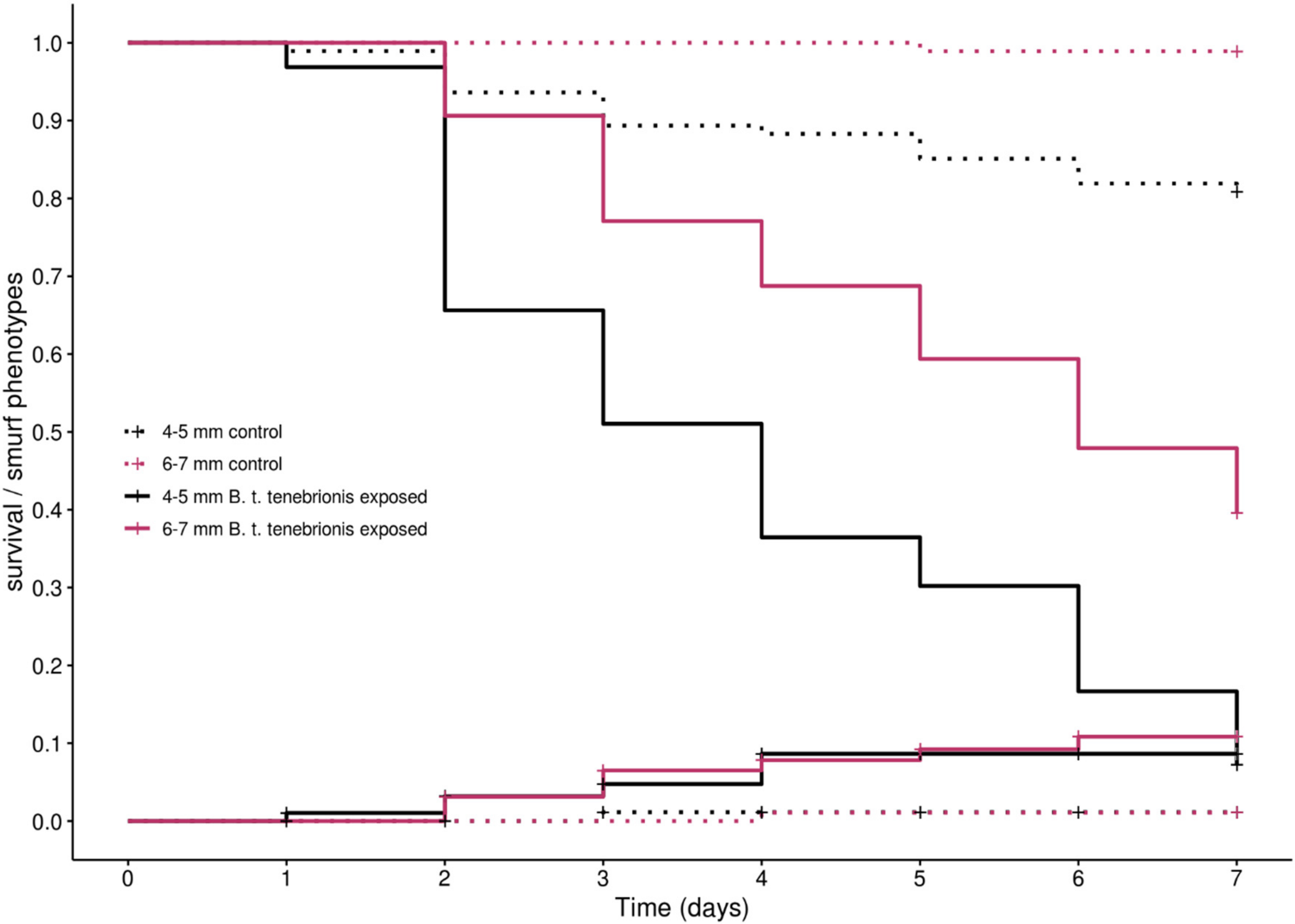
3.2. B. thuringiensis tenebrionis Exposure in Two Age/Size Classes of T. molitor Larvae
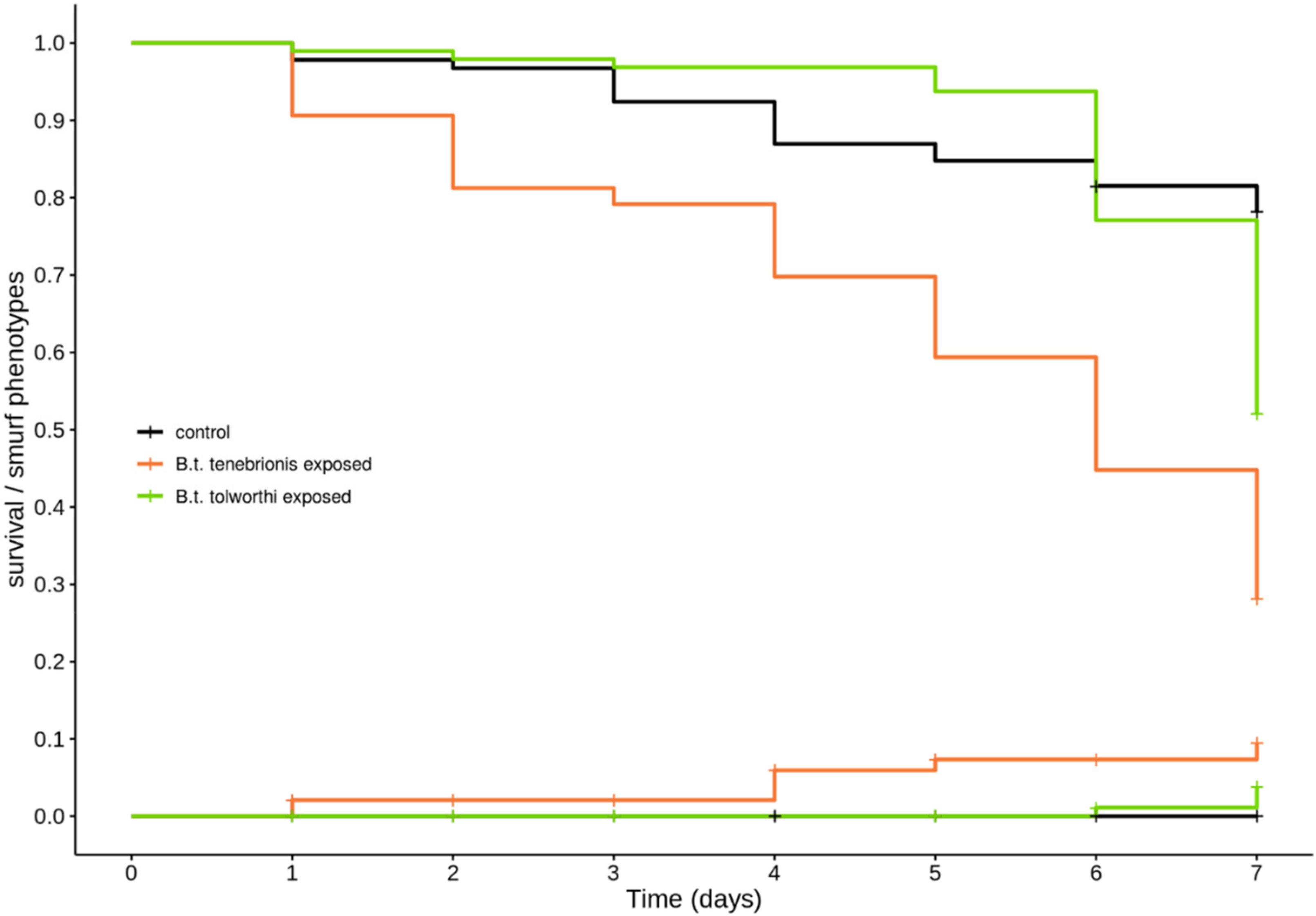
3.3. B. thuringiensis tenebrionis and B. thuringiensis tolworthi Exposure of T. castaneum Larvae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Glare, T.R.; O’Callaghan, M. Bacillus Thuringiensis: Biology, Ecology and Safety, 1st ed.; Wiley: New York, NY, USA, 2000; ISBN 978-0-471-49630-4. [Google Scholar]
- Jurat-Fuentes, J.L.; Crickmore, N. Specificity determinants for Cry insecticidal proteins: Insights from their mode of action. J. Invertebr. Pathol. 2017, 142, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Malovichko, Y.V.; Nizhnikov, A.A.; Antonets, K.S. Repertoire of the Bacillus thuringiensis virulence factors unrelated to major classes of protein toxins and its role in specificity of host-pathogen interactions. Toxins 2019, 11, 347. [Google Scholar] [CrossRef] [PubMed]
- Verplaetse, E.; Slamti, L.; Gohar, M.; Lereclus, D. Cell differentiation in a Bacillus thuringiensis population during planktonic growth, biofilm formation, and host infection. mBio 2015, 6, e00138-15. [Google Scholar] [CrossRef]
- Tetreau, G. Interaction between insects, toxins, and bacteria: Have we been wrong so far? Toxins 2018, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Hilbeck, A.; Defarge, N.; Bøhn, T.; Krautter, M.; Conradin, C.; Amiel, C.; Panoff, J.-M.; Trtikova, M. Impact of antibiotics on efficacy of Cry toxins produced in two different genetically modified Bt maize varieties in two lepidopteran herbivore species, Ostrinia nubilalis and Spodoptera littoralis. Toxins 2018, 10, 489. [Google Scholar] [CrossRef]
- Kho, M.F.; Bellier, A.; Balasubramani, V.; Hu, Y.; Hsu, W.; Nielsen-LeRoux, C.; McGillivray, S.M.; Nizet, V.; Aroian, R.V. The pore-forming protein Cry5B elicits the pathogenicity of Bacillus sp. against Caenorhabditis elegans. PLoS ONE 2011, 6, e29122. [Google Scholar] [CrossRef]
- Wan, L.; Lin, J.; Du, H.; Zhang, Y.; Bravo, A.; Soberón, M.; Sun, M.; Peng, D. Bacillus thuringiensis targets the host intestinal epithelial junctions for successful infection of Caenorhabditis elegans. Environ. Microbiol. 2019, 21, 1086–1098. [Google Scholar] [CrossRef]
- Sutter, G.R.; Raun, E.S. Histopathology of the European-corn borer larvae treated with Bacillus thuringiensis. J. Invertebr. Pathol. 1966, 9, 90–103. [Google Scholar] [CrossRef]
- Caccia, S.; Di Lelio, I.; La Storia, A.; Marinelli, A.; Varricchio, P.; Franzetti, E.; Banyuls, N.; Tettamanti, G.; Casartelli, M.; Giordana, B.; et al. Midgut microbiota and host immunocompetence underlie Bacillus thuringiensis killing mechanism. Proc. Natl. Acad. Sci. USA 2016, 113, 9486–9491. [Google Scholar] [CrossRef]
- Miyasono, M.; Inagaki, S.; Yamamoto, M.; Ohba, K.; Ishiguro, T.; Takeda, R.; Hayashi, Y. Enhancement of delta-endotoxin activity by toxin-free spore of Bacillus thuringiensis against the diamonback moth, Plutella xylostella. J. Invertebr. Pathol. 1994, 63, 111–112. [Google Scholar] [CrossRef]
- Chiang, A.S.; Yen, D.F.; Peng, W.K. Germination and proliferation of Bacillus thuringiensis in the gut of rice moth larva, Corcyra cephalonica. J. Invertebr. Pathol. 1986, 48, 96–99. [Google Scholar] [CrossRef]
- Bauer, L.S.; Pankratz, H.S. Ultrastructural effects of Bacillus thuringiensis var. san diego on midgut cells of the cottonwood leaf beetle. J. Invertebr. Pathol. 1992, 60, 15–25. [Google Scholar] [CrossRef]
- Rera, M.; Bahadorani, S.; Cho, J.; Koehler, C.L.; Ulgherait, M.; Hur, J.H.; Ansari, W.S.; Lo, T., Jr.; Jones, D.L.; Walker, D.W. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell. Metab. 2011, 14, 623–634. [Google Scholar] [CrossRef]
- Rera, M.; Clark, R.I.; Walker, D.W. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc. Natl. Acad. Sci. USA 2012, 109, 21528–21533. [Google Scholar] [CrossRef] [PubMed]
- Dambroise, E.; Monnier, L.; Ruisheng, L.; Aguilaniu, H.; Joly, J.-S.; Tricoire, H.; Rera, M. Two phases of aging separated by the Smurf transition as a public path to death. Sci. Rep. 2016, 6, 23523. [Google Scholar] [CrossRef]
- Vijendravarma, R.K.; Narasimha, S.; Chakrabarti, S.; Babin, A.; Kolly, S.; Lemaitre, B.; Kawecki, T.J. Gut physiology mediates a trade-off between adaptation to malnutrition and susceptibility to food-borne pathogens. Ecol. Lett. 2015, 10, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.; Huger, A.M.; Langenbruch, G.A.; Schnetter, W. Bacillus thuringiensis var. tenebrionis: Ein neuer, gegenüber Larven von Coleopteren wirksamer Pathotyp. J. Appl. Entomol. 1983, 96, 500–508. [Google Scholar] [CrossRef]
- Andreev, I.M.; Bulushova, N.V.; Zalunin, A.; Chestukhina, G.G. Effect of entomocidal proteins from Bacillus thuringiensis on ion permeability of apical membranes of Tenebrio molitor larvae gut epithelium. Biochemistry 2009, 74, 1096–1103. [Google Scholar] [CrossRef]
- Fabrick, J.; Oppert, C.; Lorenzen, M.D.; Morris, K.; Oppert, B.; Jurat-Fuentes, J.L. A novel Tenebrio molitor cadherin is a functional receptor for Bacillus thuringiensis Cry3Aa toxin. J. Biol. Chem. 2009, 284, 18401–18410. [Google Scholar] [CrossRef]
- Perez, C.J.; Tang, J.D.; Shelton, A.M. Comparison of leaf-dip and diet bioassays for monitoring Bacillus thuringiensis resistance in field populations of diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 1997, 90, 94–101. [Google Scholar] [CrossRef]
- Rausell, C.; Martínez-Ramírez, A.C.; García-Robles, I.; Real, M.D. A binding site for Bacillus thuringiensis Cry1Ab toxin is lost during larval development in two forest pests. Appl. Environ. Microbiol. 2000, 66, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalek, N.; Sellami, S.; Kallassy-Awad, M.; Tounsi, M.F.; Mebarkia, A.; Tounsi, S.; Rouis, S. Influence of Ephestia kuehniella stage larvae on the potency of Bacillus thuringiensis Cry1Aa delta-endotoxin. Pestic. Biochem. Physiol. 2017, 137, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.; Rausell, C.; Real, M.D. Proteome response of Tribolium castaneum larvae to Bacillus thuringiensis toxin producing strains. PLoS ONE 2013, 8, e55330. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.; Schoppmeier, M.; Real, M.D.; Rausell, C. Sodium solute symporter and cadherin proteins act as Bacillus thuringiensis Cry3Ba toxin functional receptors in Tribolium castaneum. J. Biol. Chem. 2013, 288, 18013–18021. [Google Scholar] [CrossRef]
- Milutinović, B.; Stolpe, C.; Peuβ, R.; Armitage, S.A.O.; Kurtz, J. The red flour beetle as a model for bacterial oral infections. PLoS ONE 2013, 8, e64638. [Google Scholar] [CrossRef]
- Wang, P.; Granados, R.R. Calcofluor disrupts the midgut defense system in insects. Insect Biochem. Mol. Biol. 2000, 30, 135–143. [Google Scholar] [CrossRef]
- Amcheslavsky, A.; Jiang, J.; Ip, Y.T. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell 2009, 4, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Leclerq, J. Sur les besoins nutritifs de la larve de Tenebrio molitor L. Biochim. Biophys. Acta 1948, 2, 329–332. [Google Scholar] [CrossRef]
- Ben-Dov, E.; Saxena, D.; Wang, Q.; Manasherob, R.; Boussiba, S.; Zaritsky, A. Ingested particles reduce susceptibility of insect larvae to Bacillus thuringiensis. J. Appl. Entomol. 2003, 127, 146–152. [Google Scholar] [CrossRef]
- Oppert, B. Rapid bioassay to screen potential biopesticides in Tenebrio molitor larvae. Biopestic. Int. 2010, 6, 67–73. [Google Scholar]
- Wong, R.; Piper, M.D.W.; Wertheim, B.; Partridge, L. Quantification of food intake in Drosophila. PLoS ONE 2009, 4, e6063. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.R.; McCracken, A.A.; Simons, M.J.; Henriques, C.M.; Rera, M. How to catch a smurf?—Ageing and beyond … In Vivo assessment of intestinal permeability in multiple model organisms. Bio Protoc. 2018, 8, e2722. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2013. Available online: http://www.R-project.org/ (accessed on 15 March 2018).
- Therneau, T.M. Coxme: Mixed Effects Cox Models. R package version 2.2-10. 2018. Available online: https://CRAN.R-project.org/package=coxme (accessed on 20 March 2020).
- Therneau, T.M. A Package for Survival Analysis in S. Package Version 2.38. 2015. Available online: https://CRAN.R-project.org/package=survival (accessed on 10 April 2020).
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000; ISBN 0-387-98784-3. [Google Scholar]
- Akaike, H. Canonical correlation analysis of time series and the use of an information criterion. In System Identification Advances and Case Studies; Mehra, R.K., Lainiotis, D.G., Eds.; Academic Press Inc.: New York, NY, USA, 1976. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A.; Kosinski, M. Survminer: Drawing Survival Curves using ‘ggplot2’. R Package Version 0.4.2. 2018. Available online: https://CRAN.R-project.org/package=survminer (accessed on 3 September 2019).
- Buchon, N.; Broderick, N.A.; Poidevin, M.; Pradervand, S.; Lemaitre, B. Drosophila intestinal response to bacterial infection: Activation of host defense and stem cell proliferation. Cell Host Microbe 2009, 5, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, A.; Jurat-Fuentes, J.L. Intestinal regeneration as an insect resistance mechanism to entomopathogenic bacteria. Curr. Opin. Insect Sci. 2016, 15, 104–110. [Google Scholar] [CrossRef]
- Herrnstadt, C.; Soares, G.G.; Wilcox, E.R.; Edwards, D.L. A new strain of Bacillus thuringiensis with activity against coleoptertan insects. Nature 1986, 4, 305–308. [Google Scholar]
- Belfiore, C.J.; Vadlamudi, R.K.; Osman, Y.A.; Bulla, L.A. A specific binding protein from Tenebrio molitor for the insecticidal toxin of Bacillus thuringiensis subsp. tenebrionis. Biochem. Biophys. Res. Commun. 1994, 200, 359–364. [Google Scholar] [CrossRef]
- Somerville, H.J.; Tanada, Y.; Omi, E.M. Lethal effect of purified spore and crystalline endotoxin preparations of Bacillus thuringiensis on several lepidopterous insects. J. Invertebr. Pathol. 1970, 16, 241–248. [Google Scholar] [CrossRef]
- Broderick, N.A.; Raffa, K.F.; Handelsman, J. Chemical modulators of the innate immune response alter gypsy moth larval susceptibility to Bacillus thuringiensis. BMC Microbiol. 2010, 10, 129. [Google Scholar] [CrossRef]
- Jing, T.; Wang, F.; Qi, F.; Wang, Z. Insect anal droplets contain diverse proteins related to gut homeostasis. BMC Genom. 2018, 19, 784. [Google Scholar] [CrossRef]
- Moreira, N.R.; Cardoso, C.; Dias, R.O.; Ferreira, C.; Terra, W.R. A physiologically-oriented transcriptomic analysis of the midgut of Tenebrio molitor. J. Insect Physiol. 2017, 99, 58–66. [Google Scholar] [CrossRef]
- Morris, K.; Lorenzen, M.D.; Hiromasa, Y.; Tomich, J.M.; Oppert, C.; Elpidina, E.N.; Vinokurov, K.; Jurat-Fuentes, J.L.; Fabrick, J.; Oppert, B. Tribolium castaneum larval gut transcriptome and proteome: A resource for the study of the coleopteran gut. J. Proteome Res. 2009, 8, 3889–3898. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.M.; Oh, C.T.; Bae, Y.S.; Lee, W.J. A direct role for dual oxidase in Drosophila gut immunity. Science 2005, 310, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Daffre, S.; Kylsten, P.; Samakovlis, C.; Hultmark, D. The lysozyme locus in Drosophila melanogaster: An expanded gene family adapted for expression in the digestive tract. Mol. Gen. Genet. 1994, 242, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Van Frankenhuyzen, K.; Gringorten, L.; Dedes, J.; Gauthier, D. Susceptibility of different instars of the Spruce Budworm (Lepidoptera: Tortricidae) to Bacillus thuringiensis var. kurstaki estimated with a droplet-feeding method. J. Econ. Entomol. 1997, 90, 560–565. [Google Scholar] [CrossRef]
- Keller, M.; Sneh, B.; Strizhov, N.; Prudovsky, E.; Regev, A.; Koncz, C.; Schell, J.; Zilberstein, A. Digestion of delta-endotoxin by gut proteases may explain reduced sensitivity of advanced instar larvae of Spodoptera littoralis to Cry1C. Insect Biochem. Mol. Biol. 1996, 26, 365–373. [Google Scholar] [CrossRef]
- Gilliland, A.; Chambers, C.E.; Bone, E.J.; Ellar, D.J. Role of Bacillus thuringiensis Cry1 delta endotoxin binding in determining potency during lepidopteran larval development. Appl. Environ. Microbiol. 2002, 68, 1509–1515. [Google Scholar] [CrossRef]
- Da Silva, I.H.S.; Goméz, I.; Sánchez, J.; Martínez de Castro, D.L.; Valicente, F.H.; Soberón, M.; Polanczyk, R.A.; Bravo, A. Identification of midgut membrane proteins from different instars of Helicoverpa armigera (Lepidoptera: Noctuidae) that bind to Cry1Ac toxin. PLoS ONE 2018, 13, e0207789. [Google Scholar] [CrossRef]
- Moustafa, M.A.; Vlasák, J.; Sehnal, F. Fragments of Tenebrio molitor cadherin enhance Cry3Aa toxicity for the red flour beetle, Tribolium castaneum (Herbst). J. Appl. Entomol. 2016, 140, 277–286. [Google Scholar] [CrossRef]
- Sutherland, P.W.; Harris, M.O.; Marwick, N.P. Effects of starvation and the Bacillus thuringiensis endotoxin CryIAc on the midgut cells, feeding behaviour, and growth of lightbrown apple moth larvae. Ann. Entomol. Soc. Am. 2003, 96, 250–264. [Google Scholar] [CrossRef]
- Rombach, M.C.; Aguda, R.M.; Picard, L.; Roberts, D.W. Arrested feeding of the asiatic Rice Borer (Lepidoptera: Pyralidae) by Bacillus thuringiensis. J. Econ. Entomol. 1989, 82, 416–419. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanchi, C.; Lindeza, A.S.; Kurtz, J. Comparative Mortality and Adaptation of a Smurf Assay in Two Species of Tenebrionid Beetles Exposed to Bacillus thuringiensis. Insects 2020, 11, 261. https://doi.org/10.3390/insects11040261
Zanchi C, Lindeza AS, Kurtz J. Comparative Mortality and Adaptation of a Smurf Assay in Two Species of Tenebrionid Beetles Exposed to Bacillus thuringiensis. Insects. 2020; 11(4):261. https://doi.org/10.3390/insects11040261
Chicago/Turabian StyleZanchi, Caroline, Ana Sofia Lindeza, and Joachim Kurtz. 2020. "Comparative Mortality and Adaptation of a Smurf Assay in Two Species of Tenebrionid Beetles Exposed to Bacillus thuringiensis" Insects 11, no. 4: 261. https://doi.org/10.3390/insects11040261
APA StyleZanchi, C., Lindeza, A. S., & Kurtz, J. (2020). Comparative Mortality and Adaptation of a Smurf Assay in Two Species of Tenebrionid Beetles Exposed to Bacillus thuringiensis. Insects, 11(4), 261. https://doi.org/10.3390/insects11040261




