Comparison of Survival and Development of Gypsy Moth Lymantria dispar L. (Lepidoptera: Erebidae) Populations from Different Geographic Areas on North American Conifers
Abstract
1. Introduction
2. Materials and Methods
2.1. Gypsy Moth Populations
2.2. Foliage Setup and Rearing
2.3. Artificial Diet Rearing
2.4. Pupae and Adults
2.5. Data Analysis
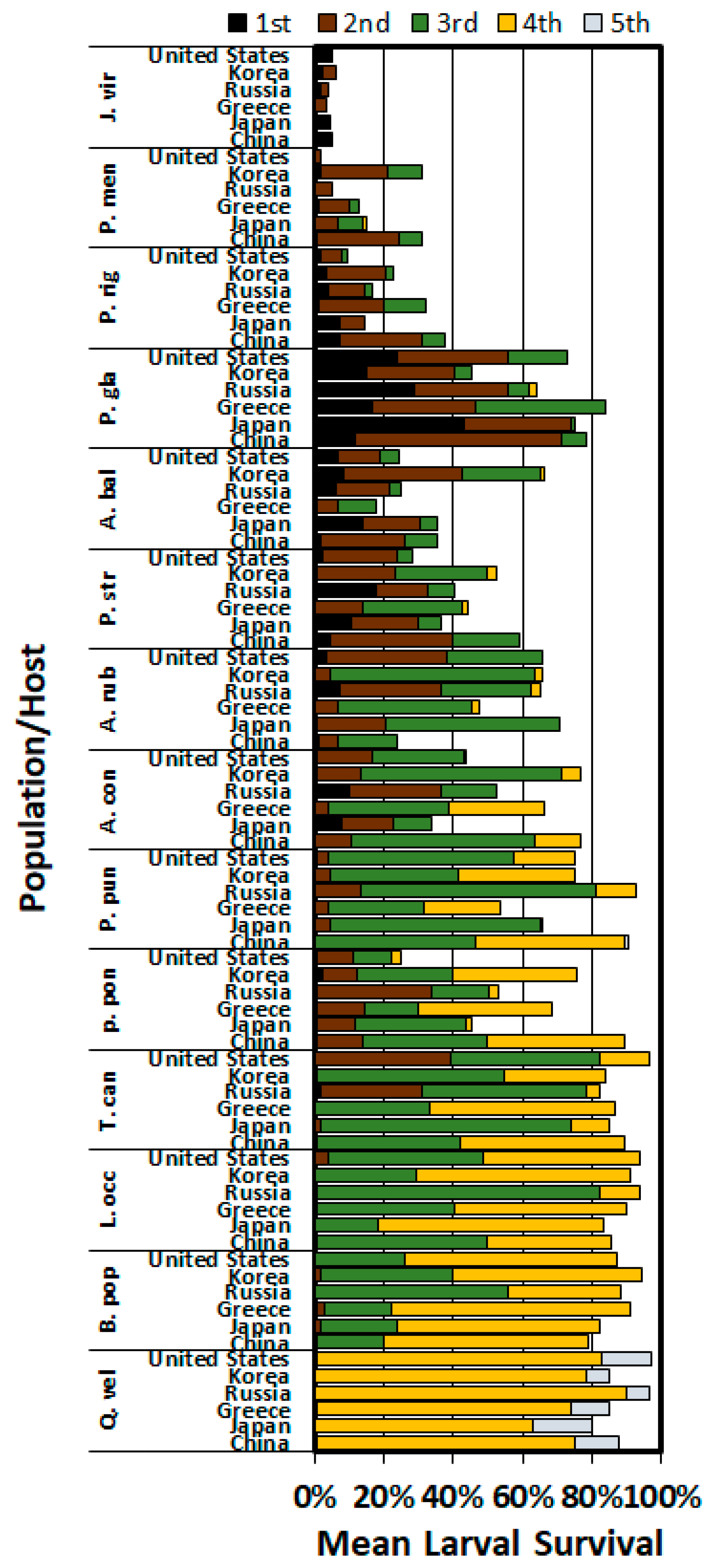
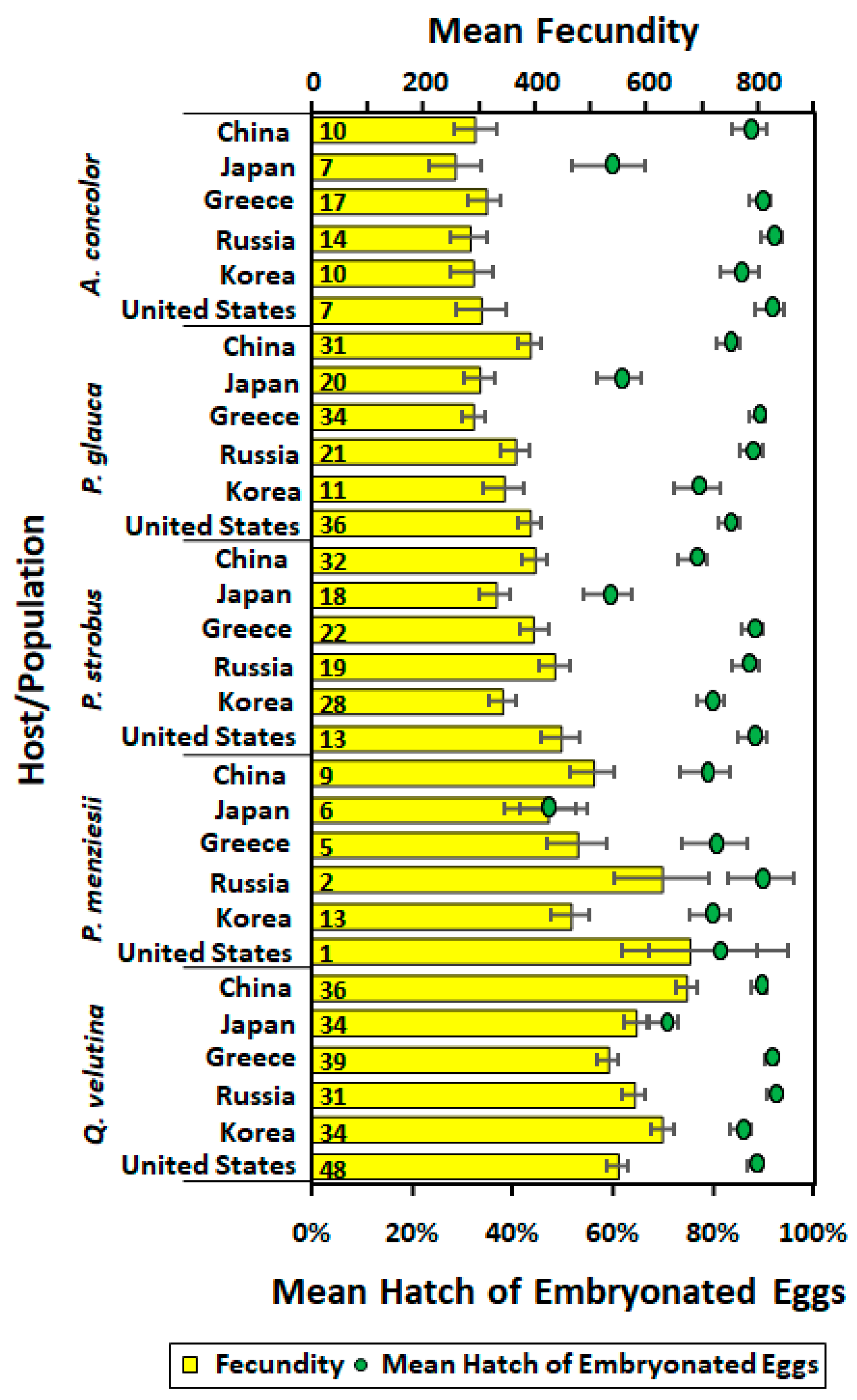
3. Results
3.1. Week-of-Setup Controls
3.2. Development and Survival on Hosts at 14 Days
3.3. Development and Survival to Adult on Six Hosts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Endara, M.J.; Coley, P.D.; Ghabash, G.; Nicholls, J.A.; Dexter, K.G.; Donoso, D.A.; Stone, G.N.; Pennington, R.T.; Kursar, T.A. Coevolutionary arms race versus host defense chase in a tropical herbivore-plant system. Proc. Natl. Acad. Sci. USA 2017, 114, E7499–E7505. [Google Scholar] [CrossRef]
- Seebacher, F.; White, C.R.; Franklin, C.E. Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Chang. 2015, 5, 61–66. [Google Scholar] [CrossRef]
- Matsuki, M.; Kay, N.; Serin, J.; Scott, J.K. Variation in the ability of larvae of phytophagous insects to develop on evolutionarily unfamiliar plants: A study with gypsy moth Lymantria dispar and Eucalyptus. Agric. For. Entomol. 2011, 13, 1–13. [Google Scholar] [CrossRef]
- Lechowicz, M.J.; Mauffette, Y. Host preference of the gypsy moth in eastern North America versus European forests. Rev. D Entomol. Du Que. 1986, 31, 43–51. [Google Scholar]
- Keena, M.A.; Cote, M.J.; Grinberg, P.S.; Wallner, W.E. World distribution of female flight and genetic variation in Lymantria dispar (Lepidoptera: Lymantriidae). Environ. Entomol. 2008, 37, 636–649. [Google Scholar] [CrossRef]
- Wallner, W.E.; Humble, L.M.; Levin, R.E.; Baranchikov, Y.N.; Carde, R.T. Response of adult lymantriid moths to illumination devices in the Russian Far-East. J. Econ. Entomol. 1995, 88, 337–342. [Google Scholar] [CrossRef]
- Pogue, M.G.; Schaefer, P.W. A Review of Selected Species of Lymantria hübner (1819) Including Three New Species (Lepidoptera: Noctuidae: Lymantriinae) from Subtropical and Temperate Regions of Asia, Some Potentially Invasive to North America. FHTET-2006-07; U.S. Department of Agriculture, Forest Health Technology Enterprise Team: Washington, DC, USA, 2007.
- U. S. Department of Agriculture, Animal Plant Health Inspection Service, Plant Protection and Quarantine. Asian Gypsy Moth Response Guidelines. Available online: http://www.aphis.usda.gov/plant_health/plant_pest_info/gypsy_moth/downloads/AGMSurveyResponseGuidelines.pdf (accessed on 12 March 2020).
- Rozhkov, A.S.; Vasil’eva, T.G. Gypsy moth in eastern Siberia. In Neparnyy Shelkopryad v Sredney i Vostochnoy Sibiri; Nauka: Novosibirsk, Russia, 1982; pp. 4–19. [Google Scholar]
- Keena, M.A. Inheritance and world variation in thermal requirements for egg hatch in Lymantria dispar (Lepidoptera: Erebidae). Environ. Entomol. 2016, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Baranchikov, Y.N. Ecological basis of the evolution of host relationships in Eurasian gypsy moth populations. In Proceedings, Lymantriidae: A Comparison of Features of New and Old World Tussock Moths. GTR-NE-123; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Broomall, PA, USA, 1989; pp. 319–338. [Google Scholar]
- Turova, G.I. Gypsy Moth of the Far East Forests (Distribution, Biology, Economic Significance, Management); Institute of Forest Siberian Branch Russian Academy of Science: Krasnoyarsk, Russia, 1992. [Google Scholar]
- Liebhold, A.; Gottschalk, K.; Muzika, R.M.; Montgomery, M.E.; Young, R.; O’Day, K.; Kelley, B. Suitability of North American Tree Species to the Gypsy Moth: A Summary of Field and Laboratory Tests. GTR-NE-211; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1995; pp. 1–34.
- Grijpma, P. Overview of research on lymantrids in eastern and western Europe. In Proceedings Lymantriidae: A Comparison of Features of New and Old World Tussock Moth. In Proceedings, Lymantriidae: A comparison of Features of New and Old World Tussock Moth, GTR-NE-123; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Broomall, PA, USA, 1989; pp. 21–49. [Google Scholar]
- Miller, J.C.; Hanson, P.E. Laboratory studies on development of gypsy moth, Lymantria dispar (L.) (Lepidoptera: Lymantriidae), larvae on foliage of gymnosperms. Can. Entomol. 1989, 121, 425–429. [Google Scholar] [CrossRef]
- Castedo-Dorado, F.; Lago-Parra, G.; Lombardero, M.J.; Liebhold, A.M.; Álvarez-Taboada, M.F. European gypsy moth (lymantria dispar dispar l.) completes development and defoliates exotic radiata pine plantations in spain. New Zealand J. For. Sci. 2016, 46, 18. [Google Scholar] [CrossRef]
- Schaefer, P.W.; Weseloh, R.M.; Sun, X.L.; Wallner, W.E.; Yan, J.J. Gypsy-moth, Lymantria (=Ocneria) dispar (L.) (Lepidoptera: Lymantriidae), in the People’s Republic of China. Environ. Entomol. 1984, 13, 1535–1541. [Google Scholar] [CrossRef]
- Schaefer, P.W.; Ikebe, K.; Higashiura, Y. Gypsy moth, Lymantria dispar (L.) and its natural enemies in the Far East (especially Japan). In Annotated Bibliography and Guide to the Literature Through 1986 and Host Plant List for Japan; University of Delaware, Agricultural Experiment Station: Newark, DE, USA, 1988; p. 160. [Google Scholar]
- Gninenko, Y.I.; Orlinskii, A.D. Outbreaks of Lymantria dispar in Russian forests during the 1990s. EPPO Bull. 2003, 33, 325–329. [Google Scholar] [CrossRef]
- Kozhanchikov, I.V. Gypsy moth. In Fauna Sssr. Nasekomye Cheshuekrylyye. Volnyanki Orgyidae; Izd.AN SSSR: Moscow/Leningrad, Russia, 1950; Volume 12, p. 582. [Google Scholar]
- Kim, C.H.; Nam, S.H.; Lee, S.M. Insecta. (vlll); Ministry of Education: Seoul, Korea, 1982.
- Baranchikov, Y.; Montgomery, M.E. Comparison of the polyphagy of gypsy moths from different continents. Trans. St. Petersburg For. Eng. Acad. 2009, 183, 40–46. [Google Scholar]
- Matsuki, M.; Kay, M.; Serin, J.; Floyd, R.; Scott, J.K. Potential risk of accidental introduction of Asian gypsy moth (Lymantria dispar) to Australasia: Effects of climatic conditions and suitability of native plants. Agric. For. Entomol. 2001, 3, 305–320. [Google Scholar] [CrossRef]
- Kensuke, O.; Hideho, H. Suitability of plant species as food for Asian gypsy moth larvae of the Hokkaido population. Bull. Hokkaido For. Res. Inst. 2011, 48, 47–54. [Google Scholar]
- Wei, J.; Luo, Y.; Shi, J.; Wang, D.; Shen, S. Larval instar impact on host selection suitability of Asian gypsy moth (Lymantria dispar asiatica, AGM). Plant Quar. 2012, 26, 6–10. [Google Scholar]
- Keena, M.A.; Shi, J. Effects of temperature on first instar Lymantria (lepidoptera: Erebidae) survival and development with and without food. Environ. Entomol. 2019, 48, 655–666. [Google Scholar] [CrossRef]
- Keena, M.A. Comparison of the hatch of Lymantria dispar (Lepidoptera: Lymantriidae) eggs from Russia and the United States after exposure to different temperatures and durations of low temperature. Ann. Entomol. Soc. Am. 1996, 89, 564–572. [Google Scholar] [CrossRef]
- Bell, R.A.; Owens, D.C.; Shapiro, M.; Tardif, J.R. Development of mass rearing technology. In The Gypsy Moth: Research Toward Integrated Pest Management, Technical Bulletin 1584; Doane, C.C., McManus, M.L., Eds.; United States Department of Agriculture: Washington, DC, USA, 1981; pp. 599–633. [Google Scholar]
- SAS_Institute, Sas/stat User’s Guide, version 9.4.; SAS Institute: Cary, NC, USA, 2015.
- Limbu, S.; Keena, M.; Chen, F.; Cook, G.; Nadel, H.; Hoover, K. Effects of temperature on development of Lymantria dispar asiatica and Lymantria dispar japonica (Lepidoptera: Erebidae). Environ. Entomol. 2017, 46, 1012–1023. [Google Scholar] [CrossRef]
- Mosher, F.H. Food Plants of the Gipsy Moth in America. Bulletin No. 250; United States Department of Agriculture: Washington, DC, USA, 1915; p. 39.
- Barbosa, P.; Greenblatt, J. Suitability, digestibility and assimilation of various host plants of the gypsy-moth Lymantria-dispar L.l (Lepidoptera: Lymantriidae). Oecologia 1979, 43, 111–119. [Google Scholar] [CrossRef]
- Hough, J.A.; Pimentel, D. Influence of host foliage on development, survival, and fecundity of gypsy moth. Environ. Entomol. 1978, 7, 97–102. [Google Scholar] [CrossRef]
- Joseph, G.; Kelsey, R.G. Acceptability and suitability of douglas-fir as a secondary host for gypsy-moth (Lepidoptera, Lymantriidae). Environ. Entomol. 1994, 23, 396–405. [Google Scholar] [CrossRef][Green Version]
- McManus, M. The Role of Behavior in the Dispersal of Newly Hatched Gyspy Moth Larvae Research Paper NE-267; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Upper Darby, PA, USA, 1973; pp. 1–10.
- McCormick, A.C.; Arrigo, L.; Eggenberger, H.; Mescher, M.C.; De Moraes, C.M. Divergent behavioural responses of gypsy moth (Lymantria dispar) caterpillars from three different subspecies to potential host trees. Sci. Rep. 2019, 9, 1–12. [Google Scholar]
- Powell, J.S.; Raffa, K.F. Effects of selected Larix laricina terpenoids on Lymantria dispar (Lepidoptera: Lymantriidae) development and behavior. Environ. Entomol. 1999, 28, 148–154. [Google Scholar] [CrossRef]
- Barbosa, P.; Krischik, V.A. Influence of alkaloids on feeding preference of eastern deciduous forest trees by the gypsy-moth, Lymantria-dispar. Am. Nat. 1987, 130, 53–69. [Google Scholar] [CrossRef]
- Beninger, C.W.; AbouZaid, M.M. Flavonol glycosides from four pine species that inhibit early instar gypsy moth (Lepidoptera: Lymantriidae) development. Biochem. Syst. Ecol. 1997, 25, 505–512. [Google Scholar] [CrossRef]
- Lindroth, R.L.; Hemming, J.D.C. Responses of the gypsy-moth (Lepidoptera: Lymantriidae) to tremulacin, an aspen phenolic glycoside. Environ. Entomol. 1990, 19, 842–847. [Google Scholar] [CrossRef]
- Joseph, G.; Kelsey, R.G.; Moldenke, A.F.; Miller, J.C.; Berry, R.E.; Wernz, J.G. Effects of nitrogen and Douglas-fir allelochemicals on development of the gypsy moth, Lymantria dispar. J. Chem. Ecol. 1993, 19, 1245–1263. [Google Scholar] [CrossRef]
- Stermitz, F.; Kamm, C.; Tawara, J. Piperidine alkaloids of spruce (Picea) and fir (Abies) species. Biochem. Syst. Ecol. 2000, 28, 177–181. [Google Scholar] [CrossRef]
- Stermitz, F.; Tawara, J.; Boeckl, M.; Pomeroy, M.; Foderaro, T.; Todd, F. Piperidine alkaloid content of Picea (spruce) and Pinus (pine). Phytochemistry 1994, 35, 951–953. [Google Scholar] [CrossRef]
- Montgomery, M.E. Variation in the susceptibility of tree species for gypsy moth. In Proceedings of the U.S. Department of Agriculture Interagency Gypsy Moth Review 1990, GTR-NE-146, East Windsor, CT, USA, 22–25 January 1990; United States Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1991; pp. 1–13. [Google Scholar]
- Mediterranean Basin. Available online: https://en.wikipedia.org/wiki/Mediterranean_Basin (accessed on 19 March 2020).

| Subspecies | Country | Closest City, Region | Collection Date a | Egg Masses Received | Latitude | Longitude |
|---|---|---|---|---|---|---|
| Lymantria dispar asiatica | China | Harbin, Heilongjiang | Aug-2012 (Oct-2012) | 6 individual | 45.78° N | 126.61° E |
| Lymantria dispar asiatica | Russia | Mineralni, Primorski | Aug-1992 | 20 individual | 44.10° N | 133.15° E |
| Lymantria dispar asiatica | Korea | Samhwa-Dong, Gangovon-Do | Aug-2009 (Nov-2014) | 16 individual | 37.49° N | 129.06° E |
| Lymantria dispar japonica | Japan | Takizawa, Morika, Nishine Hachimantai City, Northern Iwate District, Honshu | Oct-2005 (Nov-2014) | 15 individual | 39.73° N | 141.08° E |
| Lymantria dispar dispar | Greece | Kavála, Macedonia | Feb-1997 | 58 individual | 41.00° N | 24.25° E |
| Lymantria dispar dispar | United States | Bethany, New Haven County, CT | Mar-1994 | 12 individual | 41.25° N | 73.00° W |
| Duration on Host | Julian Day Setup | Host Family | Host Genus | Host Species | Authority | Host Common Name | Host Region | Foliage Source Location a | Host Height (n) |
|---|---|---|---|---|---|---|---|---|---|
| 14 Days | 124 | Aceraceae | Acer | rubrum | Linnaeus | Red Maple | E | Hamden, CT | 25 m trees (5) |
| 14 Days | 116 | Betulaceae | Betula | populifolia | Marshall | Gray Birch | E | Ansonia, CT | 6 m trees (10) |
| 14 Days | 123 | Cupressaceae | Juniperus | virginiana | Linnaeus | Eastern Red Cedar | E | Ansonia, CT | 10 m trees (5) |
| Pupation | 130 | Fagaceae | Quercus | velutina | Lamarck | Black Oak | E | Ansonia, CT | 25 m trees (5) |
| 14 Days | 118 | Pinaceae | Abies | balsamea | (L.) Miller | Balsam Fir (Canada) | N | Vans Pines Nursery | 0.3 m plugs (32) |
| Pupation | 117 | Pinaceae | Abies | concolor | (Gordon) Lindley | White Fir (Santa Fe) | W | Vans Pines Nursery | 0.6 m potted (51) |
| 14 Days | 120 | Pinaceae | Larix | occidentalis | Nuttall | Western Larch | W | Ansonia, CT | 2 m trees (4) |
| Pupation | 125 | Pinaceae | Picea | glauca | (Moench) Voss | White Spruce | N | Vans Pines Nursery & Derby, CT | 0.6 m potted (25) & 30 m trees (10) |
| 14 Days | 131 | Pinaceae | Picea | pungens | Engelmann | Colorado Blue Spruce | W | Hamden, CT | 30 m tree (1) |
| 14 Days | 127 | Pinaceae | Pinus | palustris | Miller | Longleaf Pine | S | Nature Hills Nursery | 1.2 m potted (3) |
| 14 Days | 126 | Pinaceae | Pinus | ponderosa | Douglas | Ponderosa Pine | W | Vans Pines Nursery | 0.6 m potted (25) |
| 14 Days | 132 | Pinaceae | Pinus | rigida | Miller | Pitch Pine | NE | Van Pines Nursery | 0.6 m potted (27) |
| Pupation | 116, 124, 133 | Pinaceae | Pinus | strobus | Linnaeus | Eastern White Pine | E | Ansonia, CT | 25 m trees (5) |
| Pupation | 119 | Pinaceae | Pinus | taeda | Linnaeus | Loblolly Pine | S | Pineville, LA | 30 m trees (4) |
| Pupation | 134 | Pinaceae | Pseudotsuga | menziesii | (Mirbel) Franco | Douglas Fir (Blue) | W | Vans Pines Nursery & Hamden, CT | 0.4 m potted (42) & 25 m trees (2) |
| 14 Days | 133 | Pinaceae | Tsuga | canadensis | (L.) Carrière | Eastern Hemlock | E | Ansonia, CT | 2 m trees (5) |
| Host | China | Japan | Greece | Russia | Korea | United States | Statistics |
|---|---|---|---|---|---|---|---|
| A. balsamea | 8.0 ± 2.0 ab (34) | 5.0 ± 1.0 b (36) | 8.0 ± 2.0 ab (20) | 4.0 ± 1.0 b (29) | 13.0 ± 3.0 a (68) | 7.0 ± 2.0 ab (25) | F = 2.96; df = 5, 182; p = 0.0136 |
| A. concolor | 26.0 ± 4.0 ab (80) | 10.0 ± 2.0 cd (37) | 44.0 ± 7.0 a (68) | 8.0 ± 1.0 d (53) | 32.0 ± 5.0 ab (81) | 18.0 ± 3.0 bc (45) | F = 14.68; df = 5, 333; p <0.0001 |
| A. rubrum | 28.0 ± 4.0 ab (48) | 35.0 ± 4.0 a (75) | 32.0 ± 5.0 a (47) | 16.0 ± 2.0 b (68) | 35.0 ± 5.0 a (68) | 17.0 ± 2.0 b (69) | F = 7.17; df = 5, 346; p <0.0001 |
| B. populifolia | 121.0 ± 11.0 a (79) | 118.0 ± 10.0 a (82) | 126.0 ± 11.0 a (93) | 82.0 ± 7.0 b (90) | 128.0 ± 11.0 a (90) | 110.0 ± 9.0 ab (89) | F = 3.67; df = 5, 493; p = 0.0028 |
| J. virginiana | 0.4 ± 0.3 a (3) | 1.3 ± 0.8 a (7) | 2.1 ± 2.0 a (4) | 1.3 ± 0.9 a (2) | 1.7 ± 0.9 a (11) | 0.4 ± 0.3 a (3) | F = 0.89; df = 5, 15; p = 0.5095 |
| L. occidentalis | 56.0 ± 3.0 c (87) | 104.0 ± 5.0 a (87) | 63.0 ± 3.0 bc (91) | 43.0 ± 2.0 d (88) | 100.0 ± 5.0 a (93) | 73.0 ± 4.0 b (89) | F = 51.00; df = 5, 505; p <.0001 |
| P. glauca | 8.0 ± 1.0 ab (80) | 5.0 ± 1.0 b (75) | 11.0 ± 2.0 a (85) | 7.0 ± 1.0 ab (62) | 9.0 ± 1.0 ab (45) | 6.0 ± 1.0 ab (73) | F = 3.84; df = 5, 387; p = 0.0021 |
| P. menziesii | 11.0 ± 2.0 a (29) | 11.0 ± 3.0 a (17) | 8.0 ± 2.0 a (11) | 9.0 ± 3.0 a (4) | 11.0 ± 3.0 a (32) | 14.0 ± 8.0 a (3) | F = 0.20; df = 5, 71; p = 0.9627 |
| P. ponderosa | 39.0 ± 9.0 ab (95) | 19.0 ± 5.0 b (47) | 57.0 ± 13.0 a (76) | 23.0 ± 5.0 ab (55) | 51.0 ± 12.0 ab (66) | 20.0 ± 5.0 b (38) | F = 4.04; df = 5, 348; p = 0.0014 |
| P. pungens | 72.0 ± 10.0 a (84) | 62.0 ± 9.0 a (67) | 64.0 ± 10.0 a (54) | 51.0 ± 7.0 a (91) | 68.0 ± 10.0 a (78) | 59.0 ± 8.0 a (76) | F = 0.69; df = 5, 420; p = 0.6347 |
| P. rigida | 6.0 ± 2.0 a (40) | 2.0 ± 1.0 a (10) | 3.0 ± 1.0 a (26) | 2.0 ± 1.0 a (21) | 2.0 ± 1.0 a (12) | 4.0 ± 2.0 a (22) | F = 1.87; df = 5, 106; p = 0.1062 |
| P. strobus | 4.0 ± 1.0 a (73) | 4.0 ± 1.0 a (47) | 5.0 ± 2.0 a (53) | 2.0 ± 1.0 a (50) | 6.0 ± 2.0 a (65) | 3.0 ± 1.0 a (33) | F = 2.10; df = 5, 275; p = 0.0655 |
| Q. velutina | 337.0 ± 13.0 bc (90) | 293.0 ± 11 c (81) | 419.0 ± 16.0 a (86) | 378.0 ± 14.0 ab (90) | 347.0 ± 13.0 b (87) | 385.0 ± 14.0 ab (95) | F = 10.55; df = 5, 499; p <.0001 |
| T. canadensis | 45.0 ± 3 a (91) | 39.0 ± 3 a (86) | 47.0 ± 3.0 a (88) | 17.0 ± 1.0 b (82) | 42.0 ± 3.0 a (86) | 40.0 ± 3.0 a (90) | F = 28.78; df = 5, 493; p <.0001 |
| Gypsy Moth Population | |||||||
|---|---|---|---|---|---|---|---|
| Host | China | Japan | Greece | Russia | Korea | United States | Statistics |
| A. balsamea | 35.4 ± 7.4 ab | 35.7 ± 7.4 ab | 17.8 ± 5.5 b | 25.1 ± 6.5 b | 66.2 ± 7.3 a | 24.7 ± 6.5 b | F = 4.92; df = 5, 24; p = 0.0031 |
| A. concolor | 76.9 ± 6.1 a | 33.8 ± 7.1 b | 66.3 ± 7.1 ab | 52.8 ± 7.6 ab | 77.0 ± 6.1 a | 44.0 ± 7.5 b | F = 5.45; df = 5, 24; p = 0.0017 |
| A. rubrum | 24.2 ± 7.5 b | 70.7 ± 8.2 a | 47.5 ± 9.4 ab | 65.2 ± 8.8 a | 65.7 ± 8.7 a | 66.0 ± 8.7 a | F = 3.47; df = 5, 24; p = 0.0168 |
| B. populifolia | 79.2 ± 4.2 b | 82.1 ± 3.9 ab | 91.0 ± 2.7 ab | 88.2 ± 3.2 ab | 94.4 ± 2 a | 87.0 ± 3.3 ab | F = 2.9; df = 5, 24; p = 0.0347 |
| J. virginiana | 5.3 ± 2.7 a | 4.6 ± 2.4 a | 3.5 ± 1.9 a | 4.1 ± 2.1 a | 6.4 ± 3.1 a | 5.3 ± 2.7 a | F = 0.26; df = 5, 24; p = 0.9317 |
| L. occidentalis | 85.3 ± 4.0 a | 83.2 ± 4.2 a | 89.8 ± 3.2 a | 94.0 ± 2.3 a | 91.2 ± 3.0 a | 94.1 ± 2.3 a | F = 1.92; df = 5, 24; p = 0.1281 |
| P. glauca | 78.2 ± 4.8 a | 74.9 ± 5.1 a | 84.0 ± 4.2 a | 64.2 ± 5.8 ab | 45.3 ± 6.0 b | 73.0 ± 5.3 a | F = 5.83; df = 5, 24; p = 0.0012 |
| P. menziesii | 30.9 ± 6.5 a | 15.0 ± 4.7 ab | 12.8 ± 4.3 ab | 5.4 ± 2.3 bc | 30.9 ± 6.5 a | 2.2 ± 1.1 c | F = 7.77; df = 5, 24; p = 0.0002 |
| P. palustris* | 0.2 ± 0.1 a | 0.2 ± 0.1 a | 0.2 ± 0.1 a | 0.2 ± 0.1 a | 0.2 ± 0.1 a | 0.2 ± 0.1 a | F = 0.11; df = 5, 24; p = 0.9901 |
| P. ponderosa | 89.6 ± 4.8 a | 45.7 ± 12.0 b | 68.7 ± 10.6 ab | 53.2 ± 12.1 ab | 75.7 ± 9.2 ab | 24.8 ± 9.3 b | F = 4.43; df = 5, 24; p = 0.0053 |
| P. pungens | 90.5 ± 3.7 ab | 66.0 ± 7.2 bc | 53.5 ± 7.6 c | 92.6 ± 3.1 a | 75.2 ± 6.4 abc | 75.1 ± 6.4 abc | F = 5.62; df = 5, 24; p = 0.0014 |
| P. rigida | 37.7 ± 11.5 a | 14.8 ± 6.5 a | 32.3 ± 10.8 a | 17 ± 7.2 a | 22.6 ± 8.8 a | 9.8 ± 4.6 a | F = 1.68; df = 5, 24; p = 0.1778 |
| P. strobus | 59.4 ± 7.5 a | 36.4 ± 7.3 a | 44.4 ± 7.6 a | 40.6 ± 7.5 a | 52.5 ± 7.7 a | 28.2 ± 6.7 a | F = 2.15; df = 5, 30; p = 0.0866 |
| P. taeda * | 0.2 ± 0.1 a | 0.2 ± 0.1 a | 0.2 ± 0.1 a | 0.2 ± 0.1 a | 0.2 ± 0.1 a | 0.2 ± 0.1 a | F = 0.11; df = 5, 24; p = 0.9901 |
| Q. velutina | 87.5 ± 3.7 abc | 79.8 ± 4.7 c | 84.9 ± 4.1 bc | 96.4 ± 1.6 ab | 85.3 ± 4.0 bc | 97.0 ± 1.4 a | F = 4.79; df = 5, 24; p = 0.0036 |
| T. canadensis | 89.2 ± 3.4 ab | 85.0 ± 4.1 ab | 86.6 ± 3.9 ab | 82.2 ± 4.5 b | 83.9 ± 4.3 ab | 96.3 ± 1.6 a | F = 2.51; df = 5, 24; p = 0.0579 |
| Host | Population | Time to Adult (d) | % Mating Success | |||
|---|---|---|---|---|---|---|
| Male | n | Female | n | |||
| P. taeda * | Korea | 62 | 1 | NA | 0 | NA |
| A. concolor | China | 50.8 ± 1.2 abcd | 10 | 51.2 ± 1 abcd | 13 | 83.3 |
| A. concolor | Japan | 55.6 ± 1.4 abcd | 7 | 59.3 ± 1.1 abcd | 12 | 77.8 |
| A. concolor | Greece | 44.5 ± 1.1 cde | 11 | 48.3 ± 0.9 bcde | 19 | 89.5 |
| A. concolor | Russia | 56 ± 1 ab | 13 | 56.6 ± 1 ab | 14 | 100.0 |
| A. concolor | Korea | 49.2 ± 1 bcde | 15 | 54.5 ± 1 ab | 13 | 100.0 |
| A. concolor | United States | 49.6 ± 0.8 bcde | 20 | 52.1 ± 1.3 abc | 8 | 100.0 |
| P. glauca | China | 53.4 ± 0.6 ab | 38 | 55.9 ± 0.6 ab | 34 | 100.0 |
| P. glauca | Japan | 58.6 ± 0.7 a | 30 | 63 ± 0.8 a | 22 | 90.6 |
| P. glauca | Greece | 48.3 ± 0.6 bcde | 44 | 50.8 ± 0.6 abcd | 35 | 97.1 |
| P. glauca | Russia | 52.5 ± 0.9 abc | 17 | 56.9 ± 0.7 ab | 30 | 95.5 |
| P. glauca | Korea | 53.9 ± 0.8 ab | 21 | 61.3 ± 1.1 a | 12 | 91.7 |
| P. glauca | United States | 52.3 ± 0.7 abc | 30 | 55 ± 0.6 ab | 36 | 100.0 |
| P. strobus | China | 54 ± 0.6 ab | 38 | 56.6 ± 0.6 ab | 34 | 97.0 |
| P. strobus | Japan | 58 ± 0.8 ab | 23 | 59.9 ± 0.9 a | 19 | 100.0 |
| P. strobus | Greece | 47.8 ± 0.8 bcde | 24 | 50.5 ± 0.8 abcd | 24 | 100.0 |
| P. strobus | Russia | 55.1 ± 1 ab | 15 | 55.8 ± 0.8 ab | 24 | 79.2 |
| P. strobus | Korea | 52.2 ± 0.6 abc | 33 | 58.1 ± 0.7 ab | 30 | 96.6 |
| P. strobus | United States | 51.4 ± 0.9 abcd | 19 | 52.3 ± 1.1 abc | 12 | 100.0 |
| P. menziesii | China | 51.2 ± 0.9 abcd | 17 | 53.4 ± 1.2 ab | 10 | 100.0 |
| P. menziesii | Japan | 51.9 ± 1.1 abcd | 11 | 56.2 ± 1.5 ab | 6 | 100.0 |
| P. menziesii | Greece | 47.8 ± 1.7 bcde | 5 | 50 ± 1.7 bcde | 5 | 100.0 |
| P. menziesii | Russia | 52 ± 3.7 abcd | 1 | 53 ± 2.1 abc | 3 | 100.0 |
| P. menziesii | Korea | 52.7 ± 1 abc | 14 | 56.1 ± 0.9 ab | 16 | 100.0 |
| P. menziesii | United States | 47.5 ± 2.6 bcde | 2 | 50 ± 3.7 abcde | 1 | 100.0 |
| Q. velutina | China | 37.3 ± 0.5 de | 49 | 41 ± 0.6 de | 39 | 92.3 |
| Q. velutina | Japan | 38.8 ± 0.6 de | 42 | 41.3 ± 0.6 de | 37 | 94.4 |
| Q. velutina | Greece | 34.8 ± 0.6 e | 39 | 35.7 ± 0.6 e | 41 | 100.0 |
| Q. velutina | Russia | 35.9 ± 0.5 e | 50 | 37.6 ± 0.6 de | 38 | 86.5 |
| Q. velutina | Korea | 39.3 ± 0.5 de | 46 | 42.4 ± 0.6 de | 38 | 91.9 |
| Q. velutina | United States | 34.9 ± 0.6 e | 41 | 36.8 ± 0.5 de | 53 | 94.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keena, M.A.; Richards, J.Y. Comparison of Survival and Development of Gypsy Moth Lymantria dispar L. (Lepidoptera: Erebidae) Populations from Different Geographic Areas on North American Conifers. Insects 2020, 11, 260. https://doi.org/10.3390/insects11040260
Keena MA, Richards JY. Comparison of Survival and Development of Gypsy Moth Lymantria dispar L. (Lepidoptera: Erebidae) Populations from Different Geographic Areas on North American Conifers. Insects. 2020; 11(4):260. https://doi.org/10.3390/insects11040260
Chicago/Turabian StyleKeena, Melody A., and Jessica Y. Richards. 2020. "Comparison of Survival and Development of Gypsy Moth Lymantria dispar L. (Lepidoptera: Erebidae) Populations from Different Geographic Areas on North American Conifers" Insects 11, no. 4: 260. https://doi.org/10.3390/insects11040260
APA StyleKeena, M. A., & Richards, J. Y. (2020). Comparison of Survival and Development of Gypsy Moth Lymantria dispar L. (Lepidoptera: Erebidae) Populations from Different Geographic Areas on North American Conifers. Insects, 11(4), 260. https://doi.org/10.3390/insects11040260







