Abstract
Mass rearing of insects, used both as biological control agents and for food and feed, is receiving increasing attention. Efforts are being made to improve diets that are currently in use, and to identify alternative diets, as is the case with the predatory flower bug (Orius majusculus) and other heteropteran predators, due to the high costs of their current diet, the eggs of the Mediterranean flour moth (E. kuehniella). The assessment of alternative diets may include measurements of the predator’s fitness-related traits (development time, weight, etc.), and biochemical analyses such as lipid and protein content in the diet and the insects. However, assessing diet quality via the predator’s fitness-related traits is laborious, and biochemical composition is often difficult to relate to the measured traits. Isotope analysis, previously used for diet reconstruction studies, can also serve as a tool for the assessment of diet quality. Here, the variation in discrimination factors or isotope enrichment (Δ15N and Δ13C) indicates the difference in isotopic ratio between the insect and its diet. In this study, we investigated the link between Δ15N and diet quality in the predatory bug Orius majusculus. Three groups of bugs were fed different diets: Ephestia kuehniella eggs, protein-rich Drosophila melanogaster and lipid-rich D. melanogaster. The isotopic enrichment and fitness-related measurements were assessed for each group. Results show a relation between Δ15N and fitness-related measurements, which conform to the idea that lower Δ15N indicates a higher diet quality.
Keywords:
nitrogen; carbon; isotope; isotope enrichment; isotope discrimination; diet quality; Heteroptera; rearing 1. Introduction
There is a growing body of studies looking into alternative diets for the mass-rearing of predators used in biological control [1,2,3,4,5,6,7]. The fitness-related measurements used as reference for diet quality (development time, weight, etc.) are often complemented by biochemical analyses of the body of the predator and/or the prey [1,2,3,4,5,6,7,8,9]. The information on basic components (nitrogen, lipids and carbohydrates) of a given diet can indicate how nutritionally balanced the diet is [10]. Likewise, the nutritional composition of the entomophagous insects can give an indication of the lack or excess of a particular nutrient [3,4,11,12,13]. However, such analyses do not always show clear patterns which relate to diet quality [2,5,6,7,14]. Often, further complex analyses, that assess minerals, fatty acids, etc., are required [1,2,3,4,8].
Stable Isotope Analysis has been widely used in trophic studies for diet reconstruction, and also has potential as a tool for assessing diet quality [15,16,17,18,19]. In diet quality assessments, the quality is inferred from isotope enrichment (also referred to as isotope discrimination or fractionation). Isotope enrichment (Δ15N and Δ13C) refers to the difference in carbon and nitrogen isotopic ratios (δ15N and δ13C) between an animal and its diet [19]. For carbon, the enrichment between the diet and consumer is assumed to be low (~1‰); the carbon isotopic ratio is therefore indicative of the source of carbon in the diet (C3, C4 or marine plants) [15,20]. In contrast, δ15N is known to increase with the trophic level by ~3–4‰, and thus predators are typically enriched relative to their diets [15,20]. The enrichment is caused by the discrimination of heavy isotopes, derived from a combination of biochemical and physiological processes, leading to a faster excretion of the lighter isotopes [17,21]. In relation to diet quality, Webb et al. (1998) reported higher Δ15N values linked to the consumption of lower quality diets in locusts (Locusta migratoria) [22], which conforms to the idea that a high-quality diet is metabolized with fewer physiological processes than a low-quality diet. However, varying allocation patterns between age and sex may affect enrichment patterns [23]. Thus, Oelbermann and Scheu (2002) attribute increased enrichment as associated with increased diet quality in the spider Pardosa lugubris (Walckenaer) (Aranae: Lycosidae) to the differences in age and size of the spiderlings [24].
The aim of this study was to investigate whether isotope enrichment, and Δ15N in particular, is a good indicator of diet quality in the predatory bug Orius majusculus (Reuter) (Heteroptera: Anthocoridae), which is currently mass-reared for its use in biological control. For that purpose, we performed stable isotope analysis on one-day-old adults that had been reared on three different diets: Ephestia kuehniella (Zeller) (Lepidoptera: Pyralidae) eggs, protein-rich Drosophila melanogaster (Meigen) (Diptera: Drosophilidae) and lipid-rich D. melanogaster. The three diets were of different quality according to fitness-related traits measured in Montoro et al. (2020a) [6]. We expected to find a negative relationship between Δ15N and diet quality. We furthermore provide the results regarding Δ13C. In principle, we would expect Δ13C to follow the trends of Δ15N. However, a diet-induced change in energy metabolism substrates (protein, carbohydrates and fat), and/or related differences in fat and carbohydrate deposition, could lead to differences in Δ13C, which would then differ from those observed for Δ15N.
2. Materials and Methods
2.1. Experimental Design
Predator and prey organisms used for the stable isotope analysis were re-sampled from Montoro et al. (2020a) [6]. In the study, first instars of the predator O. majusculus (<24 h old) were reared throughout their development on one of three different diets (prey organisms): Ephestia kuehniella eggs, protein-rich D. melanogaster and lipid-rich D. melanogaster, all provided in surplus. Frozen E. kuehniella eggs were supplied by EWH BioProduction (Tappernøje, Denmark). Drosophila melanogaster were reared on artificial diets enriched with either proteins or lipids as described in Jensen et al. 2010 to produce flies of different composition [25]. Protein-rich D. melanogaster cultures were reared with a medium containing a ratio of 3:2 casein (Sigma C-5890, Sigma-Aldrich, Steinheim, Germany) and basic medium (Carolina Instant Drosophila Medium Formula 4-24, Burlington, NC, USA). To produce lipid-rich D. melanogaster, the medium contained a ratio of 1:4 sucrose (Sigma 84097, Sigma-Aldrich, Steinheim, Germany) and basic medium (Carolina Instant Drosophila Medium Formula 4-24, Burlington, NC, USA). All D. melanogaster cultures were reared at the University of Copenhagen laboratory in plastic bottles of 6 cm diameter at 21 ± 0.5 °C with a photoperiod of 12:12 (L:D). Each plastic bottle contained 15 g of the designated medium mixed with water and 13 grains of yeast.
The diets offered were of different quality for the predator according to several parameters analyzed in Montoro et al. (2020a) [6]. In the current study, we present as quality parameters the proportion of females laying eggs and the developmental speed of the predators. Developmental speed was calculated as: 1/Total development time. We randomly selected 10 male and 10 female O. majusculus per treatment for isotope analysis. They were freeze killed at −20 °C, dried at 50 °C for 48 h and then weighed on a Mettler Toledo XP6 balance (Mettler Toledo, Glostrup, Denmark) one day after becoming adults. Due to minimum weight restrictions for the isotope analysis, two O. majusculus were pooled per sample leading to a total of five samples per sex and treatment (n = 30). For the diet analysis, six samples of E. kuehniella eggs, seven of protein-rich D. melanogaster and eight of lipid-rich D. melanogaster were selected (n = 21). Drosophila melanogaster samples consisted of a mix of 50:50 males and females, mirroring the mix given to the predators as diet [6]. Ephestia kuehniella egg samples were a clutch of eggs weighing approximately 1.16 ± 0.04 mg per sample. (Supplementary Tables S1 and S2).
2.2. Stable Isotope Analysis
The 15N/14N and 13C/12C in the samples was analyzed at the University of California-Davis Stable Isotope facility on a PDZ Europa ANCA GSL elemental analyzer (Elementar Analysensysteme GmbH, Hanau, Germany) interfaced with a PDZ Europa 20-20 isotope ratio mass spectrometer (IRMS) (Sercon Ltd., Cheshire, United Kingdom). Briefly, the samples are combusted in the elemental analyzer and the produced gases (first N2, then CO2) are sent to the IRMS in a helium carrier. During analysis, samples are interspersed with different replicates of reference materials. Values are reported as δ15N and δ13C, and expressed in parts per thousand (‰) according to the relationship:
where R is the ratio of heavy-to-light isotopes in the sample (Rsample) and in the relevant international standard (Rstandard). The international standard for carbon is VPDB (Vienna Pee Dee Belemnite) and Air for nitrogen. The standard deviation of the measurements is 0.2‰ for δ13C and 0.3‰ for δ15N [26]. Δ15N and Δ13C were calculated as the difference in δ15N and δ13C between the diet and the predator [19,24].
δ = [(Rsample − Rstandard)/Rstandard] × 103,
2.3. Statistical Analysis
All statistical analyses were performed in R v3.2.5 [27]. Model residuals were explored graphically to adhere to the criteria of normality and homoscedasticity for linear model analysis. The effect of diet and sex on δ15N, δ13C, Δ15N and Δ13C was analyzed within the linear models. P-values are provided for multiple comparison analyses with Bonferroni adjustment.
3. Results and Discussion
There were significant differences in δ15N and δ13C between O. majusculus fed the three different diets (δ15N: F(2,26) = 1804.05, p < 0.001; δ13C: F(2,26) = 1820.96, p < 0.001) (Table 1). In the case of δ15N, sex also had an effect (F(1,26) = 17.31, p < 0.001), and males had higher δ15N level than females across treatments (t-value = 4.16, p < 0.001, n = 30).

Table 1.
δ15N, δ13C, Δ15N and Δ13C values (mean ± SE) of O. majusculus reared on three different diets: E. kuehniella eggs (E. kuehniella), protein-rich D. melanogaster (P-rich flies) and lipid-rich D. melanogaster (L-rich flies).
Diet also had an impact on isotope enrichment for both Δ15N and Δ13C (Δ15N: F(2,26) = 60.00, p < 0.001; Δ13C: F(2,26) = 34.69, p < 0.001). The Δ15N value was higher in the predators fed a lipid-rich D. melanogaster diet, followed by those fed the protein-rich D. melanogaster and E. kuehniella eggs (Table 1). Furthermore, Δ15N was higher in males than in females (t-value = 4.16, p < 0.001, n = 30). The Δ13C variations among diets followed a different pattern than Δ15N. The lowest Δ13C was found in the predators fed the protein-rich D. melanogaster, and there were no significant differences between predators fed E. kuehniella eggs and lipid-rich D. melanogaster (Table 1). Furthermore, there was no difference in Δ13C between sexes.
This study shows that stable isotope values (δ15N, δ13C) and isotope enrichment (Δ15N, Δ13C) change significantly with diet. The values of isotope enrichment corresponded to those observed in previous studies on invertebrates, i.e., ~1‰ for Δ13C and ~3–4‰ for Δ15N [15,20,22,24,28]. Importantly, the differences in δ15N between the diets (prey organisms) and the predator (O. majusculus) related directly to group differences in fitness parameters, such as those given by the proportion of females laying eggs and developmental speed (See Montoro et al. 2020a [6]) (Figure 1). Lower quality diets (characterized by lower developmental speed and a lower number of females laying eggs) were associated with higher Δ15N, compared to higher quality diets. These findings are in line with the results from Webb et al. (1998) and Adams and Sterner (2000) [22,29], confirming that a high-quality diet is metabolized with fewer physiological processes than a low-quality diet. The nitrogen enrichment was thus most pronounced for the O. majusculus reared on lipid-rich D. melanogaster, followed by those fed on protein-rich D. melanogaster and finally those fed on E. kuehniella eggs (the highest quality diet).
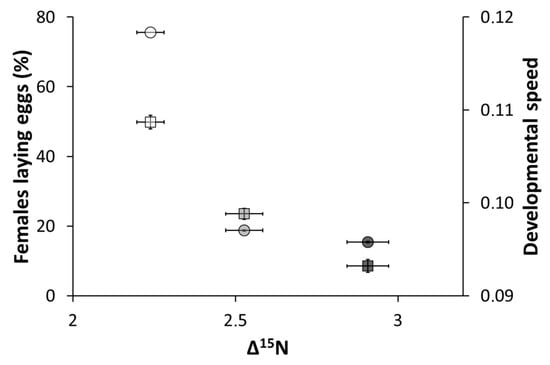
Figure 1.
Proportion of females laying eggs (±SE) and developmental speed (±SE) of O. majusculus according to the nitrogen isotope enrichment (Δ15N ± SE). Each Δ15N value corresponds to a different diet: in white, E. kuehniella eggs; in grey, protein-rich D. melanogaster; and in black, lipid-rich D. melanogaster. The circles show the percentage of females laying eggs and the squares the developmental speed. Data on females laying eggs and developmental speed come from Montoro et al. (2020) [6].
Webb et al. (1998) and Oelbermann and Scheu (2002) reported increased Δ13C values in locusts (L. migratoria) and spiderlings of P. lugubris, respectively, when fed low-quality food [22,24], however they found no clear overall trend relating to prey quality. Similarly, in our study Δ13C also varied with diet but did not relate to food quality as Δ15N did. The highest Δ13C values were recorded for predators fed on E. kuehniella eggs and on lipid-rich D. melanogaster, representing the highest and lowest quality diets, respectively. We therefore note that Δ13C results may also in this case involve ambiguities, as have been pointed out in a previous study [30]. The contrast between Δ15N and Δ13C, however, would also allow the speculation that differences in the quality of the three diets could result from differences in amino acid composition and protein quality [31].
4. Conclusions
Our results demonstrate that conducting an analysis of the 15N stable isotope can be particularly useful for studies investigating diet quality. In the case of O. majusculus, the Δ15N values change significantly with diet quality, as higher Δ15N values were recorded in lower quality diets when compared to higher quality diets. These changes relate to the fitness-traits measured in Montoro et al. (2020a) more clearly than the measurements of total carbon, nitrogen and lipid, and the carbon-to-nitrogen ratio, did [6]. The simplicity and the low sample size required for the method suggests that this would be an uncomplicated alternative to assess diet quality. The Δ13C values also varied significantly with diet, but the changes did not relate to food quality as the Δ15N did.
Supplementary Materials
The research data is found in supplementary materials. The following are available online at https://www.mdpi.com/2075-4450/11/4/255/s1, Table S1: Isotope Data; Table S2: Enrichment Data.
Author Contributions
Conceptualization, P.M.J., L.S. and M.M.; Investigation, M.M.; Writing-Original draft preparation, M.M.; Writing-Review and editing, M.M., P.M.J. and L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Danish Council for Independent Research (DFF), grant number DFF - 4184-00248.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Ferkovich, S.M.; Shapiro, J.P. Increased egg-laying in Orius insidiosus (Hemiptera: Anthocoridae) fed artificial diet supplemented with an embryonic cell line. Biol. Control 2004, 31, 11–15. [Google Scholar] [CrossRef]
- De Clercq, P.; Arijs, Y.; Van Meir, T.; Van Stappen, G.; Sorgeloos, P.; Dewettinck, K.; Rey, M.; Grenier, S.; Febvay, G. Nutritional value of brine shrimp cysts as a factitious food for Orius laevigatus (Heteroptera: Anthocoridae). Biocontrol Sci. Techn. 2005, 15, 467–479. [Google Scholar] [CrossRef]
- Zapata, R.; Specty, O.; Grenier, S.; Febvay, G.; Pageaux, J.F.; Delobel, B.; Castañé, C. Carcass analysis to improve a meat-based diet for the artificial rearing of the predatory mirid bug Dicyphus tamaninii. Arch. Insect Biochem. 2005, 60, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Sighinolfi, L.; Febvay, G.; Dindo, M.L.; Rey, M.; Pageaux, J.; Baronio, P.; Grenier, S. Biological and biochemical characteristics for quality control of Harmonia axyridis (Pallas) (Coleoptera, Coccinellidae) reared on a liver-based diet. Arch. Insect Biochem. 2008, 68, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Schultz, H.; da Silva, E.; de Lima Aguiar-Menezes, E.; Santos Resende, A.L.; Ribeiro Costa Rouws, J.; Moreira da Silva, A.R. Adequacy of Drosophila melanogaster as prey for the development and reproduction of Coleomegilla maculata. BioControl 2019, 64, 43–54. [Google Scholar] [CrossRef]
- Montoro, M.; De Fine Licht, H.H.; Sigsgaard, L. Nutritional quality of Drosophila melanogaster as factitious prey for rearing the predatory bug Orius Majusculus. Insect Sci. 2020. [Google Scholar] [CrossRef]
- Montoro, M.; De Clercq, P.; Overgaard, J.; Sigsgaard, L. Fitness consequences of artificial diets with different macronutrient composition for the predatory bug, Orius majusculus. Entomol. Exp. Appl. 2020. [Google Scholar] [CrossRef]
- Specty, O.; Febvay, G.; Grenier, S.; Delobel, B.; Piotte, C.; Pageaux, J.F.; Ferran, A.; Guillaud, J. Nutritional plasticity of the predatory ladybeetle Harmonia axyridis (Coleoptera: Coccinellidae): Comparison between natural and substitution prey. Arch. Insect Biochem. 2003, 52, 81–91. [Google Scholar] [CrossRef]
- He, X.; Sigsgaard, L. A floral diet increases the longevity of the coccinellid Adalia bipunctata but does not allow molting or reproduction. Front. Ecol. Evol. 2019, 7, 1–11. [Google Scholar] [CrossRef]
- Cohen, A.C. Function of Insect Diet Components. In Insect diets, Science and Technology; Cohen, A.C., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 29–56. [Google Scholar]
- Grenier, S.; De Clercq, P. Comparison of artificially vs. naturally reared natural enemies and their potential for use in biological control. In Quality Control and Production of Biological Control Agents –Theory and Testing Procedures; van Lenteren, J.C., Ed.; CABI Publishing: Cambridge, UK, 2003; pp. 115–131. [Google Scholar]
- Dindo, M.L.; Grenier, S.; Sighinolfi, L.; Baronio, P. Biological and biochemical differences between in vitro- and in vivo-reared Exorista larvarum. Entomol. Exp. Appl. 2006, 120, 167–174. [Google Scholar] [CrossRef]
- Grenier, S. Artificial rearing of entomophagous insects, with emphasis on nutrition and parasitoids-General outlines from personal experience. Karaelmas Sci. Eng. J. 2012, 2, 1–12. [Google Scholar] [CrossRef][Green Version]
- Ferkovich, S.M.; Shapiro, J.P. Enhanced oviposition in the insidious flower bug, Orius majusculus (Hemiptera: Anthocoridae) with a partially purified nutritional factor from prey eggs. Fla. Entomol. 2005, 88, 253–257. [Google Scholar] [CrossRef]
- DeNiro, M.; Epstein, S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Ac. 1981, 45, 341–351. [Google Scholar] [CrossRef]
- Kling, G.W.; Fry, B.; O’Brien, W.J. Stable isotopes and planktonic trophic structure in arctic lakes. Ecology 1992, 73, 561–566. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Gratton, C.; Forbes, A.E. Changes in δ13C stable isotopes in multiple tissues of insect predators fed isotopically distinct prey. Oecologia 2006, 147, 615–624. [Google Scholar] [CrossRef]
- Caut, S.; Angulo, E.; Courchamp, F. Variation in discrimination factors (Δ15N and Δ13C): The effect of diet isotopic values and applications for diet reconstruction. J. Appl. Ecol. 2009, 46, 443–453. [Google Scholar] [CrossRef]
- Peterson, B.; Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Jensen, P.M.; Madsen, P.; Jensen, L.S.; Pipper, C.B. Differences in carbon and nitrogen stable isotope signatures amongst wild and released pheasant populations. Eur. J. Wildlife Res. 2012, 58, 755–760. [Google Scholar] [CrossRef]
- Webb, S.C.; Hedges, R.E.M.; Simpson, S.J. Diet quality influences the δ13C and δ15N of locusts and their biochemical components. J. Exp. Biol. 1998, 201, 2903–2911. [Google Scholar]
- Ben-David, M.; Flaherty, E.A. Stable isotopes in mammalian research: A beginner’s guide. J. Mammal. 2012, 93, 312–328. [Google Scholar] [CrossRef]
- Oelbermann, K.; Scheu, S. Stable isotope enrichment (δ15N and δ13C) in a generalist predator (Pardosa lugubris, Araneae: Lycosidae): Effects of prey quality. Oecologia 2002, 130, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Mayntz, D.; Wang, T.; Simpson, S.J.; Overgaard, J. Metabolic consequences of feeding and fasting on nutritionally different diets in the wolf spider Pardosa prativaga. J. Insect Physiol. 2010, 56, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- UCDavis Stable Isotope Facility. Available online: https://stableisotopefacility.ucdavis.edu/ (accessed on 6 April 2020).
- R Core Team. R: A Language and Environment for Statistical Computing. Version 3.2.5 [Software]. Available online: http://www.R-project.org/ (accessed on 15 January 2020).
- Markow, T.A.; Anwar, S.; Pfeiler, E. Stable isotope ratios of carbon and nitrogen in natural populations of Drosophila species and their hosts. Funct. Ecol. 2000, 14, 261–266. [Google Scholar] [CrossRef]
- Adams, T.S.; Sterner, R.W. The effect of dietary nitrogen content on trophic level 15N enrichment. Limnol. Oceanogr. 2000, 45, 601–607. [Google Scholar] [CrossRef]
- Arostegui, M.C.; Schindler, D.E.; Holtgrieve, G.W. Does lipid-correction introduce biases into isotopic mixing models? Implications for diet reconstruction studies. Oecologia 2019, 191, 745–755. [Google Scholar] [CrossRef]
- Robbins, C.T.; Felicetti, L.A.; Florin, S.T. The impact of protein quality on stable nitrogen isotope ratio discrimination and assimilated diet estimation. Oecologia 2010, 162, 571–579. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).