The Effect of Increasing Topsoil Disturbance on Surface-Active Invertebrate Composition and Abundance under Grazing and Cropping Regimes on Vertisols in North-West New South Wales, Australia
Abstract
1. Introduction
- (1)
- Do invertebrate taxa and their levels of surface-active abundance vary seasonally (winter, spring, summer and autumn)?
- (2)
- Do invertebrate taxa and their seasonal abundance vary under grazing and cropping land use types?
- (3)
- Were there fewer invertebrate taxa and lower abundance where fallow length is shorter and tillage more intense?
2. Materials and Methods
- (1)
- Seasonality (winter, spring, summer and autumn) within year of collection,
- (2)
- Land use type (either cropped or grazed) and
- (3)
- Agricultural management regimes (NP, IP, LFZ, SFZ, LFC, SFC) across a topsoil disturbance typology (Table 1).
3. Results
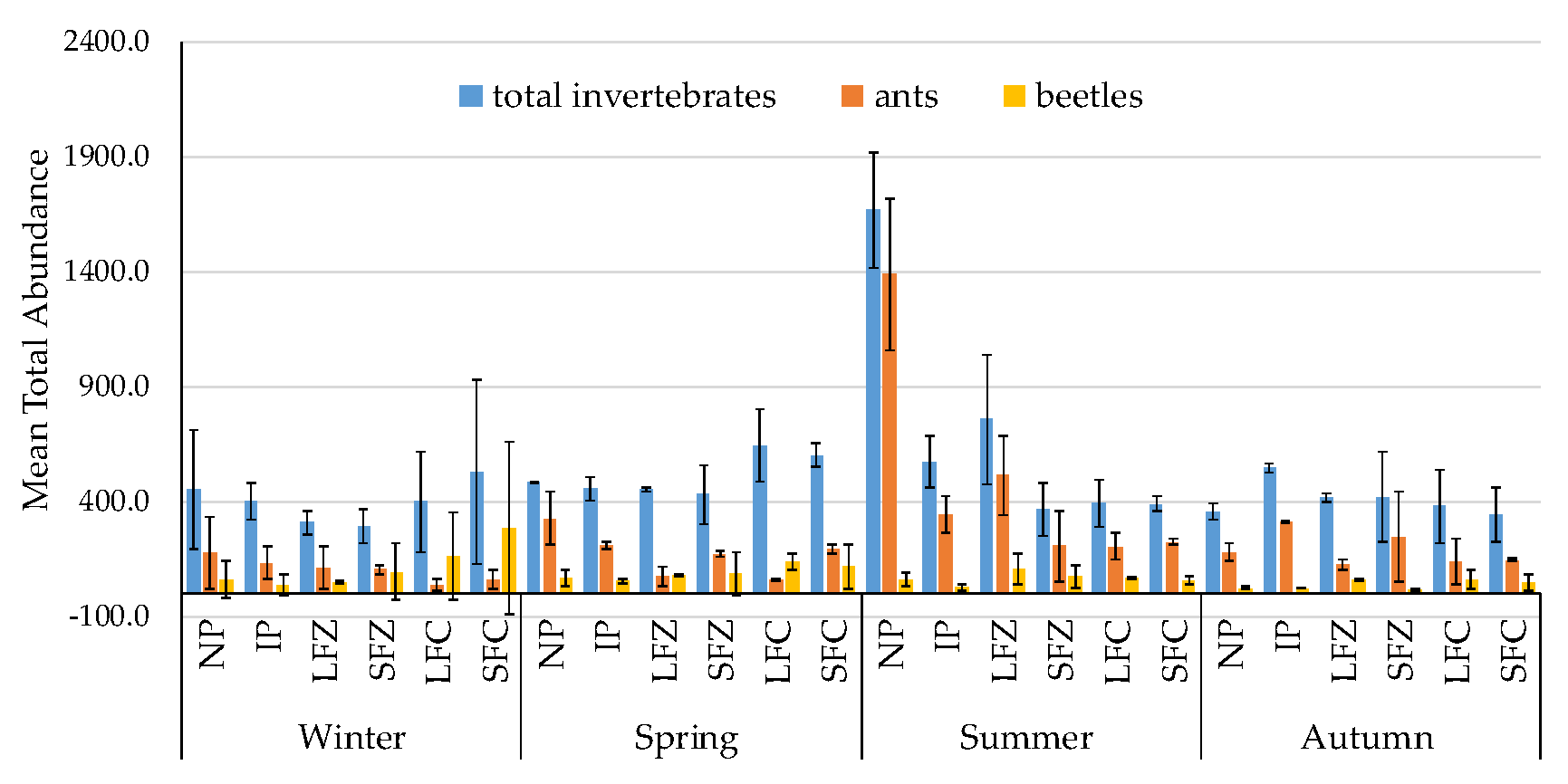
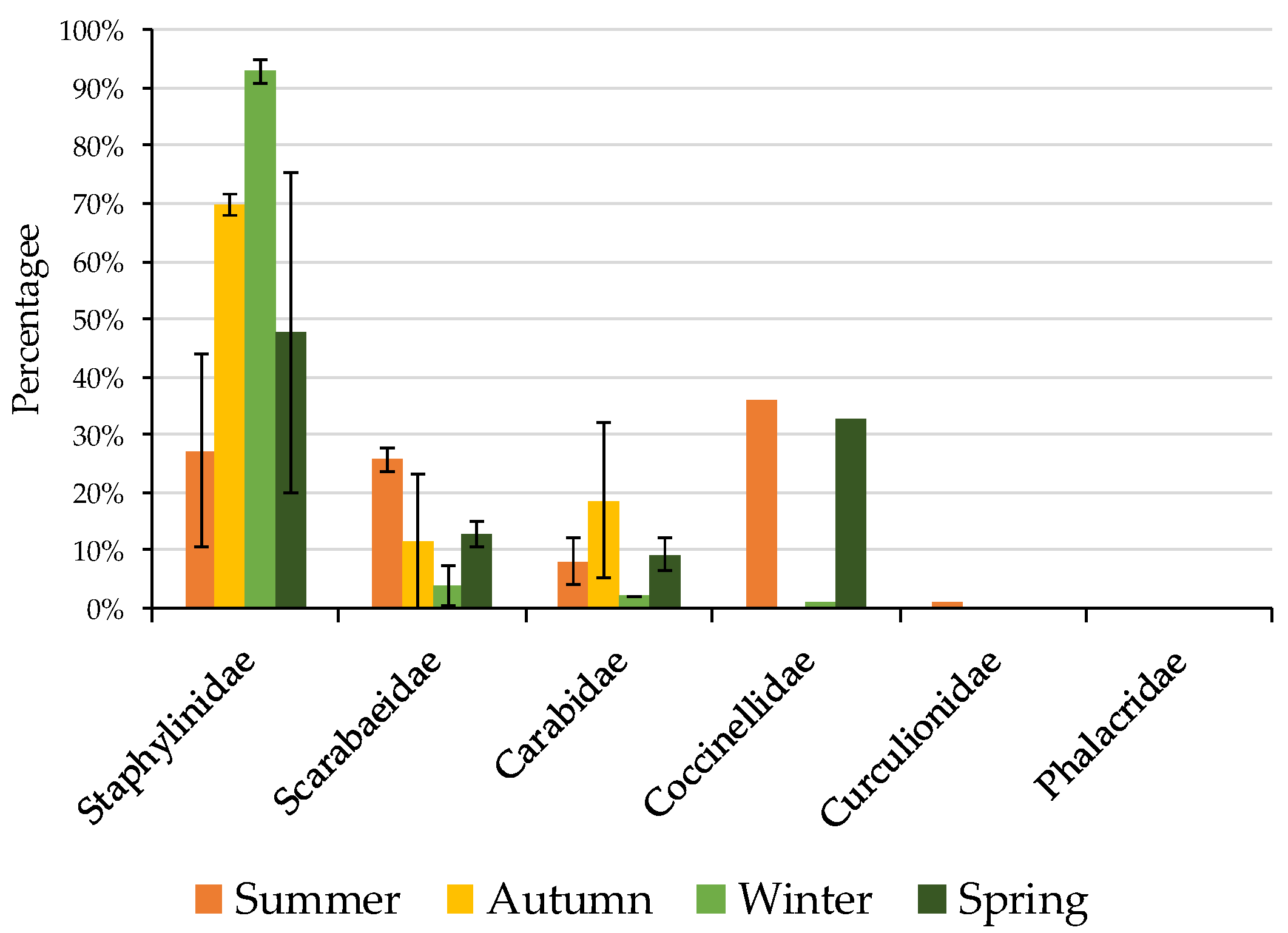
3.1. Non-Formicidae (Other Invertebrates excluding Ants) Abundance
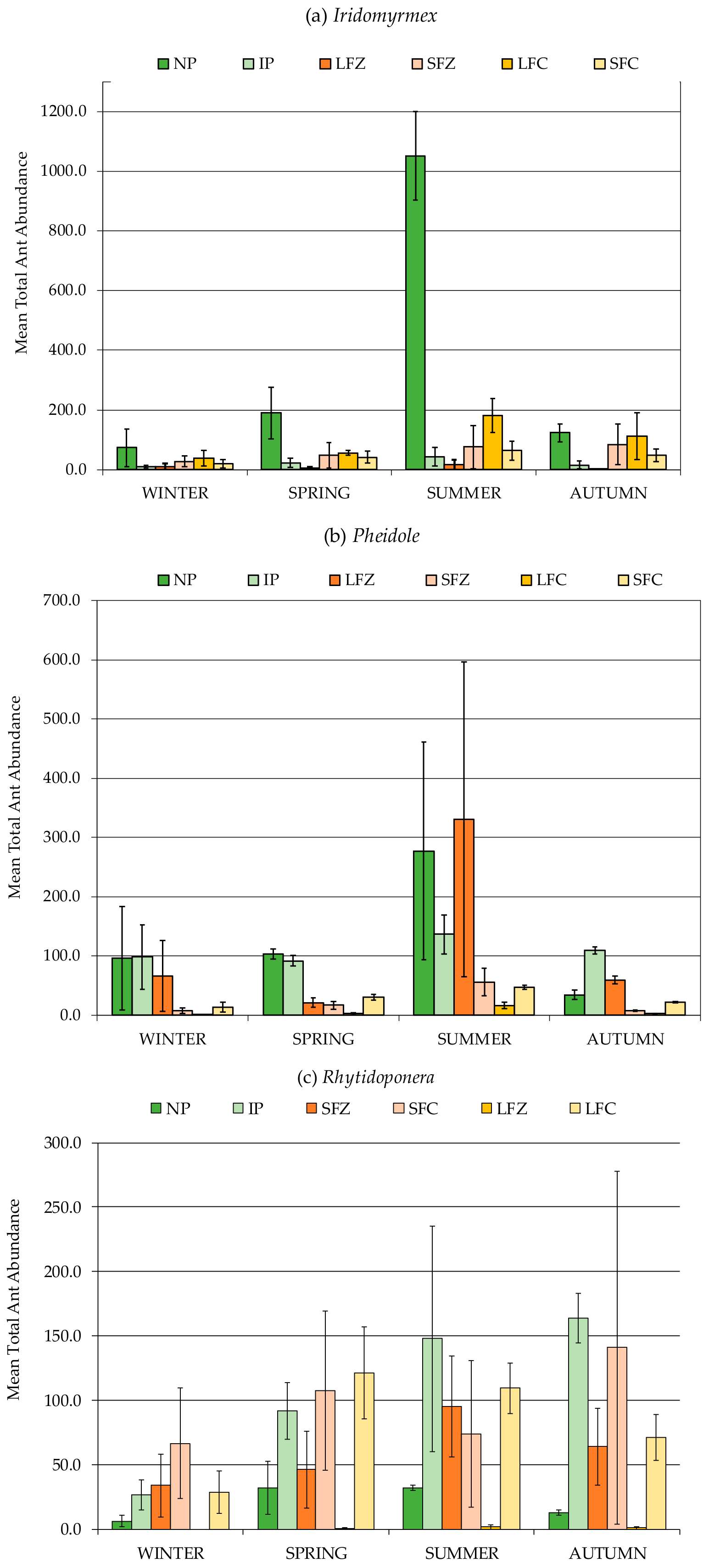
3.2. Formicidae Composition and Abundance (Ants)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thrupp, L.A. Cultivating Diversity: Agrobiodiversity and Food Security; World Resources Institute: Washington, DC, USA, 1998. [Google Scholar]
- Rana, N.; Rana, S.A.; Khan, H.A.; Sohail, A. Assessment of possible threats to soil macro-invertebrates diversity in wheat fields from high input farming. Int. J. Agric. Biol. 2010, 12, 801–808. [Google Scholar]
- Roger-Estrade, R.; Anger, C.; Bertrand, M.; Richard, G. Tillage and soil ecology: Partners for sustainable agriculture. Soil Tillage Res. 2010, 111, 33–40. [Google Scholar] [CrossRef]
- Evans, L.T. Agricultural intensification and sustainability. Outlook Agric. 2003, 32, 83–89. [Google Scholar] [CrossRef]
- Hole, D.G.; Perkins, A.J.; Wilson, J.D.; Alexander, I.H.; Grice, P.V.; Evans, A.D. Does organic farming benefit biodiversity? Biol. Conserv. 2005, 122, 113–130. [Google Scholar] [CrossRef]
- Kibblewhite, M.G.; Ritz, K.; Swift, M.J. Soil health in agricultural systems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 36, 685–701. [Google Scholar] [CrossRef]
- Wickramasinghe, L.P.; Harris, S.; Jones, G.; Vaughan, N. Bat activity and species richness on organic and conventional farms: Impact of agricultural intensification. Appl. Soil Ecol. 2003, 40, 984–993. [Google Scholar] [CrossRef]
- Kremen, C.; Williams, N.M.; Thorp, R.W. Crop pollination from native bees at risk. Proc. Nat. Acad. Sci. USA 2002, 99, 16812–16816. [Google Scholar] [CrossRef]
- Wardle, D.A. Impacts of disturbance on detritus food webs in agro-ecosystems of contrasting tillage and weed management practices. Adv. Ecol. Res. 1994, 26, 105–185. [Google Scholar]
- Freckman, D.W.; Blackburn, T.H.; Brussaard, L.; Hutchings, P.; Palmer, M.A.; Snelgrove, P.V.R. Linking biodiversity and ecosystem functioning of soils and sediments. Ambio 1997, 26, 556–562. [Google Scholar]
- Tilman, D.; Knops, J.; Wedin, D.; Reich, P.; Ritchie, M.; Sieman, E. The influence of functional diversity and composition on ecosystem processes. Science 1997, 277, 1300–1305. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G.A. An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C. Biodiversity, conservation and inventory—Why insects matter. Biodivers. Conserv. 1993, 2, 191–214. [Google Scholar] [CrossRef]
- Vanhook, T. Conservation challenge in agriculture and the role of entomologists. Fla Entomlogist 1994, 77, 42–73. [Google Scholar]
- Kuperman, R.G. Relationships between soil properties and community structure of soil macroinvertebrates in oak-hickory forests along an acidic deposition gradient. Appl. Soil Ecol. 1996, 4, 125–137. [Google Scholar] [CrossRef]
- Whitford, W.G. The importance of the biodiversity of soil biota in arid ecosystems. Biol. Conserv. 1996, 5, 185–195. [Google Scholar] [CrossRef]
- Wolters, V. Functional aspects of animal diversity in soil: Introduction and overview. Appl. Soil Ecol. 1998, 10, 185–190. [Google Scholar]
- Ekschmitt, K.; Griffiths, B.S. Soil biodiversity and its implications for ecosystem functioning in a heterogeneous and variable environment. Appl. Soil Ecol. 1998, 10, 201–215. [Google Scholar] [CrossRef]
- Waid, J.S. Does soil biodiversity depend upon metabolic activity and influence? Appl. Soil Ecol. 1999, 13, 151–158. [Google Scholar] [CrossRef]
- Hemerik, L.; Brussaard, L. Diversity of soil macro-invertebrates in grasslands under restoration succession. Eur. J. Soil Biol. 2002, 38, 145–150. [Google Scholar] [CrossRef]
- Andre, H.M.; Ducarme, X.; Lebrun, P. Soil biodiversity: Myth, reality or conning? Oikos 2002, 97, 3–24. [Google Scholar] [CrossRef]
- Bardgett, R.D. Causes and consequences of biological diversity in soil. Zoology 2002, 105, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Usher, M.B.; Hopkins, D.W. (Eds.) Biological Diversity and Function in Soils; University Press: Cambridge, UK, 2005. [Google Scholar]
- de Bello, F.; Lavorel, S.; Díaz, S.; Harrington, R.; Cornelissen, J.H.C.; Bardgett, R.D.; Berg, M.P.; Cipriotti, P.; Feld, C.K.; Hering, D.; et al. Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers. Conserv. 2010, 19, 2873–2893. [Google Scholar] [CrossRef]
- Srivastava, J.P.; Smith, N.J.H.; Forno, D.A. Agriculture as Friend and Foe of Biodiversity. In Biodiversity and Agricultural Intensification: Partners for Development and Conservation; Srivastava, J.P., Smith, N.J.H., Forno, D.A., Eds.; The World Bank: Washington, DC, USA, 1996; pp. 1–10. [Google Scholar]
- Vietmeyer, N. Harmonizing biodiversity conservation and agricultural development. In Environmentally Sustainable Development Studies and Monographs; Series No. 11 Biodiversity and Agricultural Intensification; Srivastava, J.P., Smith, N.J.H., Forno, D.A., Eds.; The World Bank: Washington, DC, USA, 1996; pp. 11–30. [Google Scholar]
- Giller, K.E.; Beare, M.H.; Lavelle, P.M.; Izac, A.M.N.; Swift, M.J. Agricultural intensification, soil biodiversity and agroecosystem function. Appl. Soil Ecol. 1997, 6, 3–16. [Google Scholar] [CrossRef]
- Swift, M.J. Special Issue: Agricultural intensification, soil biodiversity and agroecosystem function in the tropics. Appl. Soil Ecol. 1997, 6, 1–2. [Google Scholar] [CrossRef]
- Wood, D.; Lenné, J.M. Agrobiodiversity and natural biodiversity: Some parallels. In Agrobiodiversity Characterization, Utilization and Management; Wood, D., Lenné, J.M., Eds.; CABI: Wallingford, UK, 1999; pp. 425–445. [Google Scholar]
- Jackson, W. Natural systems agriculture: A truly radical alternative. Agric. Ecosyst. Environ. 2002, 88, 111–117. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S.; Bloem, J.; de Vries, F.T.; Kalbitz, K.; Wagg, C. Linking soil biodiversity and agricultural soil management. Curr. Opin. Environ. Sustain. 2012, 4, 523–528. [Google Scholar] [CrossRef]
- Lehman, R.M.; Cambardella, C.A.; Stott, D.E.; Acosta-Martinez, V.; Manter, D.K.; Buyer, J.S.; Maul, J.E.; Smith, J.L.; Harold, P.; Collins, H.P.; et al. Understanding and Enhancing Soil Biological Health: The Solution for Reversing Soil Degradation. Sustainability 2015, 7, 988–1027. [Google Scholar] [CrossRef]
- Lobry de Bruyn, L.A. Ant composition and activity in naturally-vegetated and farmland environments on contrasting soils at Kellerberrin, Western Australia. Soil Biol. Biochem. 1993, 25, 1043–1056. [Google Scholar] [CrossRef]
- Decaëns, T.; Dutoit, T.; Alard, D.; Lavelle, P. Factors influencing soil macrofaunal communities in post-pastoral successions of western France. Appl. Soil Ecol. 1998, 9, 361–367. [Google Scholar] [CrossRef]
- Folgarait, P.J.; Thomas, F.; Desjardins, T.; Grimaldi, M.; Tayasu, I.; Curmi, P.; Lavelle, P.M. Soil properties and the macrofauna community in abandoned rice fields of northeastern Argentina. Biol. Fert. Soils 2003, 38, 349–357. [Google Scholar] [CrossRef]
- Decaëns, T. Macroecological patterns in soil communities. Global Ecol. Biogeogr. 2010, 19, 287–302. [Google Scholar] [CrossRef]
- Commonwealth of Australia. Australian Catchment, River and Estuary Assessment; National Land and Water Resources Audit: Canberra, Australia, 2002. [Google Scholar]
- Lobry de Bruyn, L.A.; Abbey, J.A. Characterisation of farmers’ soil sense and the implications for on-farm monitoring of soil health. Aust. J. Exp. Agric. 2003, 43, 285–305. [Google Scholar] [CrossRef]
- Shaw, G. Soil Health Issues for Australian Cotton Production: Growers’ Perspective; CRDC: Narrabri, Australia, 2005. [Google Scholar]
- Nielsen, U.N.; Wall, D.H.; Six, J. Soil biodiversity and the environment. Ann. Rev. Environ. Resour. 2015, 40, 63–90. [Google Scholar] [CrossRef]
- Nkem, J.N.; de Bruyn, L.A.L.; Grant, C.; Hulugalle, N.R. Changes in invertebrate populations over the growing cycle of a N-fertilised wheat crop in rotations with cotton in a grey Vertosol. Appl. Soil Ecol. 2002, 20, 67–74. [Google Scholar] [CrossRef]
- Bell, M.; Seymour, N.; Stirling, G.R.; Stirling, A.M.; Van Zwieten, L.; Vancov, T.; Sutton, G.; Moody, P. Impacts of management on soil biota in Vertosols supporting the broadacre grains industry in northern Australia. Aust. J. Soil Res. 2006, 44, 433–452. [Google Scholar] [CrossRef][Green Version]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014; p. 886.
- Samways, M.J. Species temporal variability: Epigaeic ant assemblages and management for abundance and scarcity. Oecologia 1990, 84, 482–490. [Google Scholar] [CrossRef]
- Anderson, A.N. Sampling communities of ground-foraging ants: Pitfall catches compared with quadrat counts in an Australian tropical savanna. Aust. J. Ecol. 1991, 16, 237–279. [Google Scholar] [CrossRef]
- Clive, E.A. The assessment of populations of soil-inhabiting invertebrates. Agric. Ecosyst. Environ. 1991, 34, 145–176. [Google Scholar]
- Dangerfield, J.M. Abundance and diversity of soil macrofauna in northern Botswana. J. Trop. Ecol. 1997, 13, 527–538. [Google Scholar] [CrossRef]
- Abensperg-Traun, M.; Steven, D.E. The effects of pitfall trap-diameter on ant species richness (hymenoptera: Formicidae) and species composition of the catch in a semi-arid eucalypt woodland. Aust. J. Ecol. 1995, 20, 282–287. [Google Scholar] [CrossRef]
- Skvarla, M.J.; Larson, J.L.; Dowling, A.P.G. Pitfalls and Preservatives: A Review. J. Entomol. Soc. Ont. 2014, 145, 15–43. [Google Scholar]
- CSIRO. Insects of Australia; CSIRO: Canberra, Australia, 1991. [Google Scholar]
- StatView. SAS Institute Incorporated; SAS Institute: Cary, NC, USA, 1999. [Google Scholar]
- Abbott, I.; Parker, C.A.; Sills, I.D. Changes in the abundance of large soil animals and physical properties of soils following cultivation. Aust. J. Soil Res. 1979, 17, 343–353. [Google Scholar] [CrossRef]
- Baker, G.H. Recognising and responding to the influences of agriculture and other land-use practices on soil fauna in Australia. Appl. Soil Ecol. 1998, 9, 301–310. [Google Scholar] [CrossRef]
- Marchao, R.L.; Lavelle, P.; Celini, L.; Balbino, L.C.; Vilela, L.; Becquer, T. Soil macrofauna under integrated crop-livestock systems in a Brazilian Cerrado Ferralsol. Pesquisa Agropecuaria Brasileira 2009, 44, 1011–1020. [Google Scholar] [CrossRef]
- King, K.L.; Hutchinson, K.J. The effects of sheep stocking intensity on the abundance and distribution of mesofauna in pastures. J. Appl. Ecol. 1976, 13, 41–55. [Google Scholar] [CrossRef]
- Hutchinson, K.J.; King, K.L. The effects of sheep stocking level on invertebrate abundance, biomass and energy utilisation in a temperate, sown grassland. J. Appl. Ecol. 1980, 17, 369–387. [Google Scholar] [CrossRef]
- Plantureux, S.; Peeters, A.; McCracken, D. Biodiversity in intensive grasslands: Effect of improvement and challenges. Agron. Res. 2005, 3, 153–164. [Google Scholar]
- Schon, N.L.; Mackay, A.D.; Minor, M.A. Effects of dairy cow treading pressures and food resources on invertebrates in two contrasting and co-occurring soils. Soil Res. 2011, 49, 703–714. [Google Scholar] [CrossRef]
- Anderson, A.N. The use of ant communities to evaluate change in Australian terrestrial ecosystems: A review and recipe. Proc. Ecol. Soc. Aust. 1990, 16, 347–357. [Google Scholar]
- Andrew, N.R.; Hart, R.A.; Jung, M.P.; Hemmings, Z.; Terblanche, J.S. Can temperate insects take the heat? A case study of the physiological and behavioural responses in a common ant, Iridomyrmex purpureus (Formicidae), with potential climate change. J. Insect Physiol. 2013, 59, 870–880. [Google Scholar] [CrossRef]
- Fox, B.J.; Fox, M.D.; Archer, E. Experimental confirmation of competition between two dominant species of Iridomyrmex (Hymenoptera, Formicidae). Aust. J. Ecol. 1985, 10, 105–110. [Google Scholar] [CrossRef]
- Parr, C.L. Dominant ants can control assemblage species richness in a South African savanna. J. Anim. Ecol. 2008, 77, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Samways, M.J.; Osborn, R.; Carliel, F. Effect of a highway on ant (Hymenoptera: Formicidae) species composition and abundance, with recommendations for roadside verge width. Biodivers. Conserv. 1997, 6, 903–913. [Google Scholar] [CrossRef]
- Roth, D.S.; Perfecto, I.; Rathcke, B. The effects of management-systems on ground-foraging ant diversity in Costa Rica. Ecol. Appl. 1994, 4, 423–436. [Google Scholar] [CrossRef]
- Radford, B.J.; Key, A.J.; Robertson, L.N.; Thomas, G.A. Conservation tillage increases soil water storage, soil animal populations, grain yield, and response to fertiliser in the semi-arid subtropics. Aust. J. Exp. Agric. 1995, 35, 223–232. [Google Scholar] [CrossRef]
- Holt, J.A.; Bristow, K.L.; McIvor, J.G. The effects of grazing pressure on soil animals and hydraulic properties of two soils in semi-arid tropical Queensland. Aust. J. Soil Res. 1996, 34, 69–79. [Google Scholar] [CrossRef]
- Benton, T.G.; Vickery, J.A.; Wilson, J.D. Farmland biodiversity: Is habitat heterogeneity the key? Trends Ecol. Evol. 2003, 18, 182–188. [Google Scholar] [CrossRef]
- Sáenz-Romo, M.G.; Veas-Bernal, A.; Martínez-García, H.; Campos-Herrera, R.; Ibáñez-Pascual, S.; Martínez-Villar, E.; Pérez-Moreno, I.; Marco-Mancebón, V.S. Ground cover management in a Mediterranean vineyard: Impact on insect abundance and diversity. Agric. Ecosyst. Environ. 2019, 283, 106571. [Google Scholar] [CrossRef]
- Mashavakure, N.; Mashingaidze, A.B.; Musundire, R.; Nhamo, N.; Gandiwa, E.; Thierfelder, C.; Muposhi, V.K. Soil dwelling beetle community response to tillage, fertilizer and weeding intensity in a sub-humid environment in Zimbabwe. Appl. Soil Ecol. 2019, 135, 120–128. [Google Scholar] [CrossRef]
- Mashavakure, N.; Mashingaidze, A.B.; Musundire, R.; Nhamo, N.; Gandiwa, E.; Thierfelder, C.; Muposhi, V.K. Spider community shift in response to farming practices in a sub-humid agroecosystem of Southern Africa. Agric. Ecosyst. Environ. 2019, 272, 237–245. [Google Scholar] [CrossRef]
- Gibb, H.; Parr, C.L. How does habitat complexity affect ant foraging success? A test using functional measures on three continents. Oecologia 2010, 164, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Fromm, H.; Winter, K.; Filser, J.; Hantschel, R.; Beese, F. The influence of soil type and cultivation system on the spatial distributions of the soil fauna and microorganisms and their interaction. Geoderma 1993, 60, 109–118. [Google Scholar] [CrossRef]
- McLaughlin, A.; Mineau, P. The impact of agricultural practices on biodiversity. Agric. Ecosyst. Environ. 1995, 55, 201–212. [Google Scholar] [CrossRef]
- Cortet, J.; Ronce, D.; Poinsot-Balaguer, N.; Beaufreton, C.; Chabert, A.; Viaux, P.; de Fonseca, J.P.C. Impacts of different agricultural practices on the biodiversity of microarthropod communities in arable crop systems. Eur. J. Soil Biol. 2002, 38, 239–244. [Google Scholar] [CrossRef]
- Bedano, J.C.; Domínguez, A.; Arolfo, R.; Wall, L.G. Effect of Good Agricultural Practices under no-till on litter and soil invertebrates in areas with different soil types. Soil Tillage Res. 2016, 158, 100–109. [Google Scholar] [CrossRef]
- Neave, P.; Fox, C.A. Response of soil invertebrates to reduced tillage systems established on a clay loam soil. Appl. Soil Ecol. 1998, 9, 423–428. [Google Scholar] [CrossRef]
- Wilson-Rummenie, A.C.; Radford, B.C.; Robertson, L.N.; Simpson, G.D.; Bell, K.L. Reduced tillage increases population density of soil macrofauna in semiarid environment in Central Queensland. Environ. Entomol. 1999, 28, 163–172. [Google Scholar] [CrossRef]
- Kladivko, E.J. Tillage systems and soil ecology. Soil Tillage Res. 2001, 61, 61–67. [Google Scholar] [CrossRef]
- van Straalen, N.M. Evaluation of bioindicator systems derived from soil arthropod communities. Appl. Soil Ecol. 1998, 9, 429–437. [Google Scholar] [CrossRef]
- Gerlach, J.; Samways, M.; Pryke, J. Terrestrial invertebrates as bioindicators: An overview of available taxonomic groups. J. Insect Conserv. 2013, 17, 831–850. [Google Scholar] [CrossRef]
- Lobry de Bruyn, L. Ants as bioindicators of soil function in rural environments. Agric. Ecosyst. Environ. 1999, 74, 425–441. [Google Scholar] [CrossRef]
- Babin-Fenske, J.; Anand, M. Terrestrial insect communities and the restoration of an industrially perturbed landscape: Assessing success and surrogacy. Rest. Ecol. 2010, 18, 73–84. [Google Scholar] [CrossRef]
- Hulugalle, N.R.; Lobry de Bruyn, L.A.; Entwistle, P. Residual effects of tillage and crop rotation on soil properties, soil invertebrate numbers and nutrient uptake in an irrigated Vertisol sown to cotton. Appl. Soil Ecol. 1997, 7, 11–30. [Google Scholar] [CrossRef]
- de Bruyn, L.A.L.; Conacher, A.J. The bioturbation activity of ants in agriculturally and naturally vegetated habitats of semi-arid environments. Aust. J. Soil Res. 1994, 32, 555–570. [Google Scholar] [CrossRef]
- de Bruyn, L.A.L.; Conacher, A.J. The effect of ant biopores on water infiltration in soils in undisturbed bushland and in farmland in a semi-arid environment. Pedobiolgia 1994, 38, 193–207. [Google Scholar]
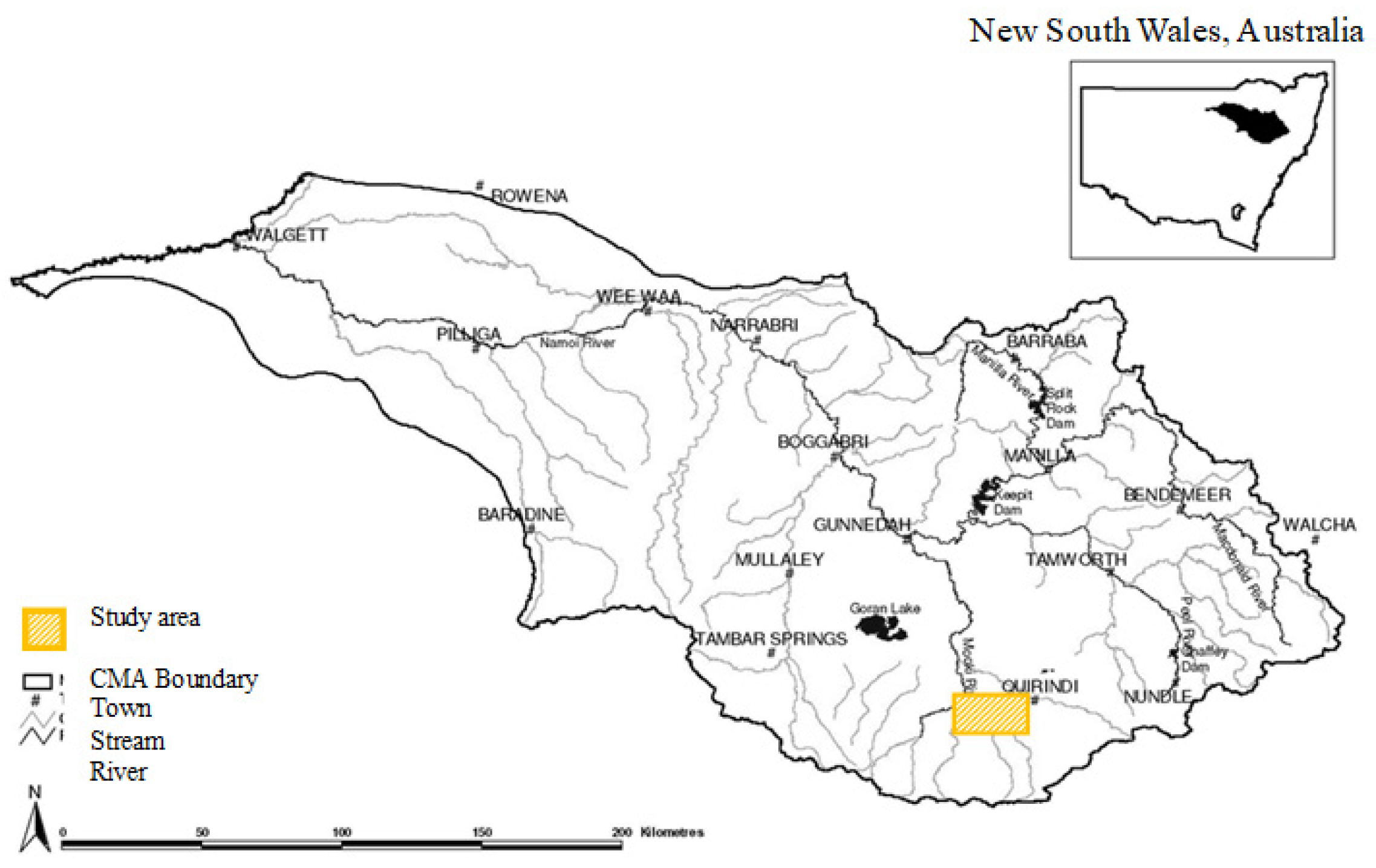
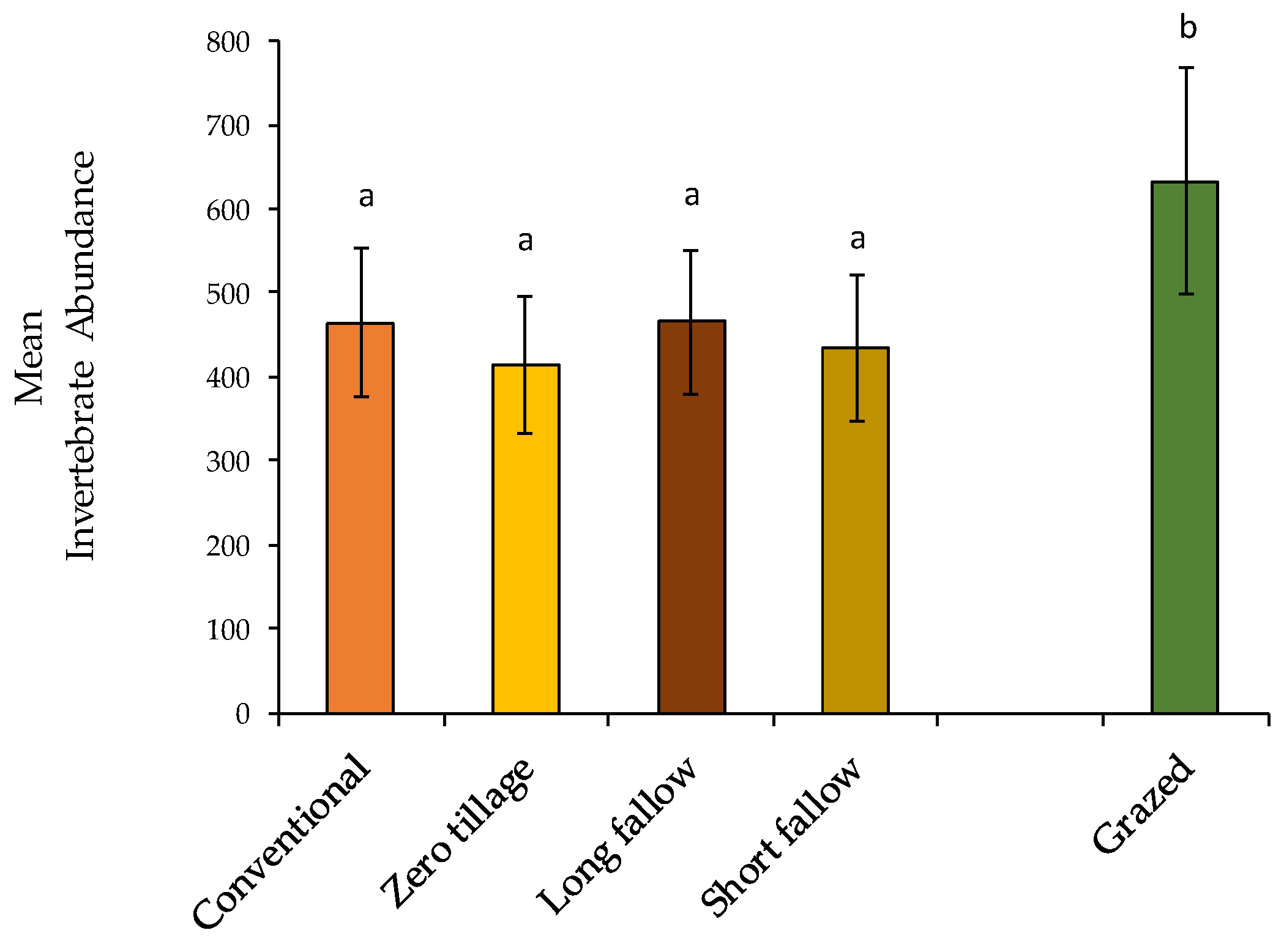

| Land Use Types | Agricultural Management Regime | Tillage Intensity (Number of Passes) | Length of Fallow (Months) | Stocking Density (dse/ha/yr) | Pesticide/Herbicide Spraying (Number of Passes) | Overall Level of Topsoil Disturbance |
|---|---|---|---|---|---|---|
| Grazing | NP | None | None | 3 | None | Low |
| IP | None | None | 9 | None | Low | |
| Cropping | LFZ | 0 | 12–18 | None | 0–1 | Low–Moderate |
| SFZ | 0 | 6–9 | None | 1–3 | Moderate | |
| LFC | 3 | 12–18 | None | 0–1 | Moderate–High | |
| SFC | 6 | 6–9 | None | 1–2 | High |
| Taxa | No | % | Taxa | No | % |
|---|---|---|---|---|---|
| Hymenoptera (Ants only) | 44,658 | 47.2 | Diplopoda | 855 | 0.9 |
| Diptera | 15,402 | 16.3 | Dermaptera | 812 | 0.9 |
| Coleoptera | 15,231 | 16.1 | Blattodea | 543 | 0.6 |
| Orthoptera | 4750 | 5.0 | Thysanoptera | 411 | 0.4 |
| Hemiptera | 3472 | 3.7 | Chilopoda | 166 | 0.2 |
| Araneae | 3467 | 3.7 | Lepidoptera | 129 | 0.1 |
| Acarina | 2960 | 3.1 | Isopoda | 117 | 0.1 |
| Hymenoptera (no Ants) | 1552 | 1.6 | Mecoptera | 34 | 0.04 |
| Grand total | 94,557 | ||||
| Taxa | Season | |||
|---|---|---|---|---|
| Autumn | Winter | Spring | Summer | |
| Formicidae (Ants) | 192.9 b | 100.3 b | 180.5 b | 455.8 a |
| (23.2) | (21.3) | (24.3) | (93.8) | |
| Iridomyrmex | 68.2 b | 30.6 b | 61.3 b | 248 a |
| (14.8) | (9) | (12.7) | (70.9) | |
| Pheidole | 59.3 b | 42.8 b | 45.2 b | 120 a |
| (8.6) | (16.8) | (8.1) | (33.8) | |
| Rhytidoponera | 76.2 a | 26.6 b | 72.9 a | 77.8 a |
| (19.4) | (7.6) | (19.2) | (19.9) | |
| Coleoptera | 39.2 b | 134.8 a | 93.6 ab | 61.1 ab |
| (5) | (42.6) | (13.3) | (9.9) | |
| Hemiptera | 25.1 | 8.3 | 30 | 9 |
| (10.7) | (2.1) | (20.5) | (2.4) | |
| Araneae | 21.8 a | 13.3 b | 27.7 a | 29.4 a |
| (4.2) | (4) | (4) | (4) | |
| Hymenoptera (excluding ants) | 9.1 ab | 3.0 c | 13.6 a | 6.7 bc |
| (0.9) | (0.7) | (2.8) | (1) | |
| Acarina | 4.7 b | 24.4 a | 5.5 b | 10.9 ab |
| (0.9) | (9.7) | (1.2) | (2.3) | |
| Taxa | Land Use Type | Management Regimes | ||||||
|---|---|---|---|---|---|---|---|---|
| Grazing | Cropping | NP | IP | LFZ | SFZ | LFC | SFC | |
| Formicidae (Ants) | 383.2 a | 157.0 b | 517.8 a | 248.6 ab | 206.6 ab | 181.9 b | 109.7 b | 155.3 b |
| (70.6) | (16.7) | (133) | (36.3) | (72.3) | (35) | (36.3) | (24.4) | |
| Iridomyrmex | 191.0 a | 57.5 b | 359.3 a | 22.8 b | 24.5 b | 59.2 b | 96.5 b | 41.3 b |
| (54.4) | (8.1) | (101) | (5.6) | (15.7) | (17.4) | (22.1) | (8.1) | |
| Pheidole | 118.6 a | 32.5 b | 128 a | 109 ab | 120 ab | 22.2 bc | 5.4 c | 28.2 bc |
| (22.3) | (9.4) | (38.8) | (22.5) | (71.4) | (4.9) | (2.9) | (3.8) | |
| Rhytidoponera | 64.1 a | 63.0 a | 20.8 bc | 107.5 a | 59.8 ab | 97.3 ab | 0.84 c | 82.6 ab |
| (13.5) | (11.3) | (5.3) | (24.3) | (16.5) | (28.7) | (0.3) | (21) | |
| Coleoptera | 45.1 b | 100.7 a | 53.7 b | 36.5 b | 74.5 ab | 67.7 ab | 106.7 a | 127.5 a |
| (5.5) | (17.1) | (9.8) | (4.8) | (13.7) | (18.3) | (18.5) | (42) | |
| Araneae | 29.0 a | 20.0 b | 23.8 ab | 34.2 a | 20.5 b | 16.4 b | 23.7 ab | 19.9 b |
| (3.1) | (1.7) | (3.4) | (5) | (4.4) | (1.9) | (4.4) | (2.8) | |
| Acarina | 18.2 a | 7.9 b | 8.7 b | 27.8 a | 2.3 b | 5.3 b | 8.3 b | 11.4 b |
| (4.3) | (3.2) | (1.7) | (8.1) | (0.9) | (1.8) | (2.8) | (8.2) | |
| Hymenoptera (excluding ants) | 9.5 a | 7.4 a | 9.7 ab | 9.3 ab | 10.4 ab | 3.8 b | 13.3 a | 5.0 b |
| (1) | (1.2) | (1.4) | (1.4) | (4.1) | (0.8) | (3.8) | (0.7) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkem, J.N.; Lobry de Bruyn, L.; King, K. The Effect of Increasing Topsoil Disturbance on Surface-Active Invertebrate Composition and Abundance under Grazing and Cropping Regimes on Vertisols in North-West New South Wales, Australia. Insects 2020, 11, 237. https://doi.org/10.3390/insects11040237
Nkem JN, Lobry de Bruyn L, King K. The Effect of Increasing Topsoil Disturbance on Surface-Active Invertebrate Composition and Abundance under Grazing and Cropping Regimes on Vertisols in North-West New South Wales, Australia. Insects. 2020; 11(4):237. https://doi.org/10.3390/insects11040237
Chicago/Turabian StyleNkem, Johnson N., Lisa Lobry de Bruyn, and Kathleen King. 2020. "The Effect of Increasing Topsoil Disturbance on Surface-Active Invertebrate Composition and Abundance under Grazing and Cropping Regimes on Vertisols in North-West New South Wales, Australia" Insects 11, no. 4: 237. https://doi.org/10.3390/insects11040237
APA StyleNkem, J. N., Lobry de Bruyn, L., & King, K. (2020). The Effect of Increasing Topsoil Disturbance on Surface-Active Invertebrate Composition and Abundance under Grazing and Cropping Regimes on Vertisols in North-West New South Wales, Australia. Insects, 11(4), 237. https://doi.org/10.3390/insects11040237






