Abstract
African citrus psyllid (Trioza erytreae (Del Guercio)) is a vector insect of the bacterium Candidatus Liberibacter africanus, the putative causal agent of Huanglongbing, the most devastating citrus disease in the world. The insect was found on the island of Madeira in 1994 and in mainland Portugal in 2015. Present in the north and center of the country, it is a threat to Algarve, the main citrus-producing region. Trioza erytreae eggs and first instar nymphs are sensitive to the combination of high temperatures and low relative humidity. Daily maximum air temperature and minimum relative humidity data from 18 weather stations were used to calculate the water vapor pressure deficit (vpd) from 2004 to 2018 at various locations. Based on the mean vpd and the number of unfavorable days (vpd < 34.5 and vpd < 56 mbar) of two time periods (February to May and June to September), less favorable zones for T. erytreae were identified. The zones with thermal and water conditions like those observed in the Castelo Branco and Portalegre (Center), Beja (Alentejo), Alte, and Norinha (Algarve) stations showed climatic restrictions to the development of eggs and first instar nymphs of African citrus psyllid. Effective control measures, such as the introduction and mass release of Tamarixia dryi (Waterson), a specific parasitoid, and chemical control are necessary in favorable periods for T. erytreae development, such as in spring and in areas with limited or no climate restrictions.
1. Introduction
African citrus psyllid (Trioza erytreae (Del Guercio)) is a vector insect of the bacterium Candidatus Liberibacter africanus, the putative causal agent of Huanglongbing (HLB), also known as Greening, which is the most devastating citrus disease in the world. This species was discovered on the island of Madeira in 1994 and on the Canary Islands in 2002, and was found in continental Europe in Spain in 2014 and mainland Portugal in 2015 [1,2,3]. So far, the bacteria associated with HLB have not been found in Europe, but the presence of the insect vector makes an eventual accidental introduction of the bacteria a looming disaster for European citrus production. To prevent the arrival of the insect at the main citrus-producing areas of these countries, efforts to contain the insect by establishing containment plans including restrictions on the transit of plant material have been conducted [3]. Despite all the preventive measures included in these containment plans, T. erytreae spread widely in the coastal region of Portugal [4].
The African HLB pathosystem (HLBaf) associated with the bacterium Candidatus Liberibacter africanus (CLaf) transmitted by T. erytreae is climate-dependent and is intolerant to high temperatures [5,6]. In South Africa, the insect and CLaf find the best conditions at high altitudes in cold and humid areas [6,7,8,9]. Catling [10] showed that hot and dry days with high water vapor pressure deficits (vpd) were lethal to T. erytreae eggs and first instar nymphs. These adverse conditions determined the seasonal abundance and geographical distribution of the insect [11,12,13]. There was a significant inverse correlation between the occurrence of T. erytreae and days with vpd greater than 25.9 mmHg (34.5 mbar), a condition causing 70% mortality of T. erytreae eggs and nymphs [11,12,13,14]. Green and Catling [8] related vpd to survival of T. erytreae early life stages and found that no survival was observed when the vpd was above 56 mbar.
Thus, areas with prolonged periods of high vpd may be unsuitable for the development of the early stages of T. erytreae, thereby limiting population growth. The southern region of Portugal, the country’s main citrus-producing zone, has a temperate climate with a dry, hot summer (Csa) [14] and low altitudes. Using historical climatic data from 2004 to 2018 from 12 national weather stations distributed throughout mainland Portugal and from six regional weather stations from southern Portugal (Algarve), we determined which periods in what regions the weather conditions would be unfavorable or even lethal for the early stages of T. erytreae. Water vapor pressure deficit (vpd) was assessed alongside two important abiotic factors, temperature and air humidity. Using vpd values obtained from the network of stations included in this study, less favorable areas and periods for the development of T. erytreae were able to be predicted. This information is important in the building of forecasting models, the establishment of risk zones, and the adaptation of monitoring procedures and pest control tactics to distinct zones.
2. Materials and Methods
Daily data on maximum temperature (tmax in °C) and minimum relative humidity (RHmin in %) were obtained from 12 automatic Portuguese Institute of Sea and Atmosphere (IPMA) weather stations and six Regional Directorate for Agriculture and Fisheries of Algarve (DRAP Algarve) weather stations from 2004 to 2018 (Table 1, Figure 1). Using these climate variables, the daily vapor pressure deficit (vpd) was calculated according to the formula , where saturation pressure was [15] and the current pressure was .

Table 1.
Location of the Portuguese Institute of the Sea and Atmosphere (IPMA) and Regional Directorate for Agriculture and Fisheries of Algarve (DRAP Algarve) weather stations and presence of Trioza erytreae in the region.
Figure 1.
Portuguese Institute of the Sea and Atmosphere (black circles, IPMA) and Regional Directorate for Agriculture and Fisheries of Algarve (stars, DRAP Algarve) weather stations.
The monthly vpd was calculated for each season from February to September of each year using daily vpd data. The data were organized into two four-month periods. The first period, from February to May, covered the beginning of citrus flush and the months of the spring season, including bloom and the development of the leaves that accompany fruiting. The second period, from June to October, included the summer months and September, covering the later flush. These two periods covered the full span of when the trees had the young leaves necessary for egg laying and the development of immature T. erytreae.
For both periods, the number of days with vpd higher than 34.5 mbar (= 25.9 mm Hg) and the number of days with vpd higher than 56 were calculated, with the conditions leading to greater than 70% mortality of T. erytreae eggs and first instar nymphs, respectively, [10] and the total mortality of these developmental stages [10]. Green and Catling [8] proposed the following equation to estimate T. erytreae survival (s) according to the variable vpd, i.e., .
Daily data regarding the maximum temperature and minimum relative humidity of 12 national IPMA stations and six DRAP Algarve stations were statistically analyzed considering the season as the treatment and the year as the replicate (n = 15). Data that met the parametric analysis assumptions were subjected to analysis of variance; the means were clustered using the Scott–Knott method (p < 0.05). The other data were submitted to deviance analysis using generalized linear models (Poisson distribution) and generalized Tukey’s test (p < 0.05). Analyses were performed using R (version 3.5.3) software [16]. Box-plot graphs were generated for the vpd data. The numbers of days with vpds higher than 34.5 or 56 mbar were tabulated.
Daily vapor pressure deficit data and the number of days spent above 34.5 and above 56 mbar were determined for all stations. IPMA stations were organized according to the agrarian regions, i.e., North (Bragança, Montalegre, Porto and Vila Real), Center (Aveiro, Castelo Branco, Coimbra, Guarda and Viseu), Lisbon and Tagus Valley (Portalegre), Alentejo (Beja), and Algarve (Faro). DRAP Algarve stations followed the regional division of the Algarve: Atlantic Coast (Aljezur), Barlavento (Arrochela, Norinha, and Alte), and Sotavento (Patacão and Cacela).
3. Results
From February to May, the lowest mean vpds were observed in Aveiro, Guarda, and Montalegre, and the highest vpds were observed in Beja and Portalegre. The other zones showed intermediate vpds. From June to September, Aveiro and Porto exhibited the lowest vpds, with the highest vpds found in Beja, Castelo Branco, and Portalegre (Figure 2).
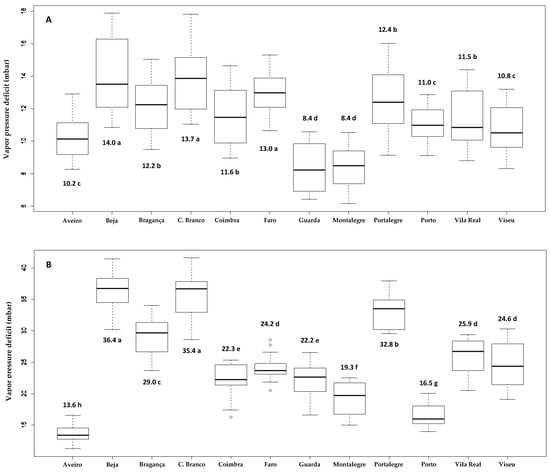
Figure 2.
Box-plot graphs of mean water vapor pressure deficits in twelve areas of mainland Portugal in the spring (A) (February to May) and summer (B) (June to September) from 2004 to 2018 (ANOVA: February to May, F = 17.04, p < 0.001; June to September, F = 91.00, p < 0.001). Highlighted means with the same letters were not different by Scott–Knott grouping test (p < 0.05).
In the Algarve, the lowest mean vpd was found in Aljezur in both periods analyzed. The highest vpds were observed in Alte and Norinha, the inland stations. No differences were observed between the stations from February to May, but the highest vpd occurred in Alte from June to September (Figure 3).
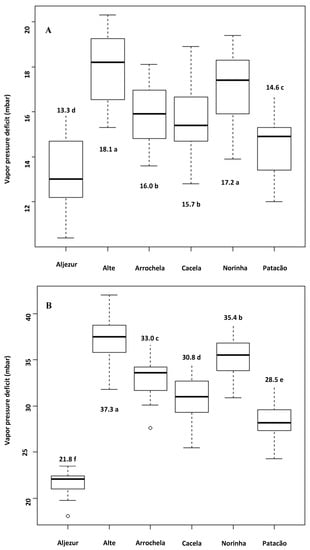
Figure 3.
Box-plot graphs of mean water vapor pressure deficits in six zones of Algarve in the spring (A) (February to May) and summer (B) (June to September) from 2004 to 2018 (ANOVA: February to May, F = 17.91, p < 0.001; June to September. F = 84.00, p < 0.001). Highlighted means with the same letters were not different by Scott–Knott grouping test (p < 0.05).
There were few days with vpd above 34.5 mbar in the period from February to May at the IPMA stations. The highest mean was obtained at Beja station, with 4.2 days after 4 months. In the four-month period from June to September, more days were observed in this condition in the Castelo Branco (65.7 days), Beja (58.6 days), and Portalegre regions (54.4 days), amounting to almost half of the analyzed period (Table 2). No observations were made of vpd above 56 mbar from February to May at the national stations. From June to September, Beja showed 9.5 days, Castelo Branco showed 6.1 days, and Portalegre showed 4.5 days at these extreme conditions.

Table 2.
Mean number of days with water vapor pressure deficits (vpd) above 34.5 and 56.0 mbar in areas of mainland Portugal in the spring and summer from 2004 to 2018.
In the Algarve, Alte and Norinha spent the most days with vpd greater than 34.5 mbar during the February–May period. From June to September, the Alte station showed 69.4 days with vpd above 34.5 mbar. The second station with the most days with vpd above 34.5 mbar was Norinha. Readings over 56 mbar were observed on 9.3 days in Alte, 6.2 days in Norinha, and 5.3 days in Arrochela (Table 2).
Stations were classified into three categories according to their vpd conditions for T. erytreae survival: 1) favorable conditions; 2) unfavorable vpd conditions (vpd values: >20 days above 34.5 or one or more days above 56); and 3) very unfavorable vpd conditions (vpd values: >20 days above 34.5 and one or more days above 56). These stations were marked on the map (Figure 4) with green and white and blue and red circles, respectively. As yet, the expansion of T. erytreae as monitored by the Directorate General for Food and Veterinary of the Portuguese Ministry of Agriculture [4], marked in orange in Figure 4, has not reached any of the zones classified as very unfavorable; only one case has come close to an unfavorable zone so far.
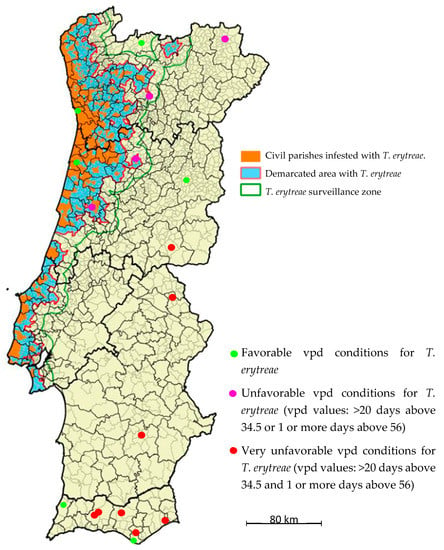
Figure 4.
Distribution map of Trioza erytreae in mainland Portugal. The delimitation of the “Infested Zone” is based on the civil parishes where T. erytreae was detected. A “buffer zone” was added to this zone, surrounding a 3 km radius considering the flight capacity of the insect. A “Surveillance Zone” of 10 km radius was also defined around the Demarcated Zone (Infested Area + Buffer Zone). Available online by the General Directorate of Food and Veterinary of the Portuguese Ministry of Agriculture [4] (http://www.dgv.min-agricultura.pt/portal/page/portal/DGV/genericos?generico=221911&cboui=221911).
4. Discussion
The most adverse zones for insect development are Beja, Castelo Branco, Portalegre, Alte, and Norinha based on the variables we evaluated that indicated lethal days for early stages of T. erytreae. The five zones where the insect was present, i.e., Montalegre and Porto and Vila Real in the north and Aveiro and Coimbra in central Portugal [4], were favorable to T. erytreae due to low vpd and few lethal days. In the Algarve, Aljezur was the most suitable zone for the insect because there was practically no limitation of the development of eggs and early nymphs. Extremely adverse days to psyllid development during June to September with vpd above 56 mbar occurred in Beja (9.5 days) and Alte (9.3 days).
Very hot and dry days, represented by the highest vpd levels (i.e., >34.5 mbar) may not control the African HLB vector, but they could be an abiotic factor that limits population outbreaks from June to September. The number of lethal days found in Beja Alte and Norinha were similar to those in Letaba (South Africa) in 1965/67, where T. erytreae populations were rarely found in the summer months [10]. Further, the occurrence of lethal days and irregular flushing in South Africa were responsible for low T. erytreae densities in 1968–1970 [17]. However, adults are not susceptible to high vpd [11]. The succession of eight lethal days caused the mortality of susceptible life stages (eggs and first instar nymphs) in Nelspruit in 2012, but not in adults [18]. Thus, the absence of adverse spring days may allow adults to develop and transmit the disease during this season, as well as in summer. The high longevity of adults from 17 to 50 days [19] with availability of summer flush allows T. erytreae to stay in citrus [20].
In southern Portugal, citrus flush begins in February with bloom, allowing plenty of feeding and oviposition sites for T. erytreae. During this period there are no lethal days and the insect population may increase. In summer, despite the availability of flush, the survival of eggs and early instars may be difficult. The density of T. erytreae is higher in the highlands [20] but Portugal’s commercial citrus industry is in the lowlands with hot and dry summers, which can be aggravated by increasing climatic extremes [21]. The insect might be more of a pest in climatically favorable areas if host plants are available.
Citrus trees are widely distributed in noncommercial and urban areas in Portugal. Sour orange (Citrus aurantium L.) and lemon trees (Citrus limon (L.) Burm. f.) in backyards and along roadways are very common in Portugal [22]. Elimination of Rutaceae host plants, such as Clausena anisata (Willd.), near citrus has been recommended [23]. Murraya koenigii (L.) was the best breeding host of T. erytreae among those tested by Aidoo et al. [24], thus. it may also be a candidate for regulatory action.
In the colder months of the year from November to February, it is plausible that the insect will find the low temperature conditions too adverse. The minimum base temperature for T. erytreae is 10–12 °C [19]. Therefore, control measures, such as classical biological control with the introduction of Tamarixia dryi, an efficient and specialist parasitoid [24,25,26], and chemical control (mainly with neonicotinoid insecticides), may be focused on spring flush when the weather is favorable to insect survival and development. With warmer summers, smaller populations of T. erytreae are expected; however the insect should be sampled regularly for the presence of CLaf and controlled in an area-wide manner [27]. Warm and dry areas are less favorable to T. erytreae [28] and may be more suitable for citrus production in Portugal in the long run. If HLB is introduced in Portugal, citrus-production should move to inland areas of the Algarve, which are less favorable to the HLBaf pathosystem (CLAf and T. erytreae), thereby potentially facilitating the management of the disease.
5. Conclusions
Based on the water vapor pressure deficit (vpd) monthly means and number of days above 34.5 and 56 mbar, the period from February to May (spring) is more suitable to T. erytreae than the period from June to September (summer) in Portugal. In the zones of Castelo Branco, Portalegre (Center), Beja (Alentejo), Alte, and Norinha (Algarve), the early developmental stages of T. erytreae may be affected negatively by climatic limitations and their development on warmer and drier days may be limited.
Author Contributions
Conceptualization and methodology, P.E.B.P. and A.D.; data curation, P.E.B.P. and T.C.; funding acquisition, L.N. and A.D.; investigation, P.E.B.P.; project administration, A.D.; resources, T.C., J.C.T., C.S., and A.D.; supervision, A.D.; writing—original draft P.E.B.P.; writing—review and editing P.E.B.P., T.C., J.C.T., L.N., C.S., and A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was supported by European Union’s Horizon 2020 research and innovation program under grant agreement No 817526 project PRE-HLB - “Preventing HLB epidemics for ensuring citrus survival in Europe”.
Acknowledgments
The first author is grateful to Instituto Federal de Educação, Ciência e Tecnologia do Triângulo Mineiro, Uberaba, Brazil, for the opportunity to be his postdoctoral trainee and the University of Algarve, Gambelas, for accepting him as a visiting professor.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Perez-Otero, R.; Mansilla, J.P.; Estal, P. Detección de la psila africana de los cítricos, Trioza erytreae (Del Guercio, 1918) (Hemiptera: Psylloidea: Triozidae), en la Península Ibérica. Arq. Entomol. Galegos 2015, 13, 119–122. [Google Scholar]
- Siverio, F.; Marco-Noales, E.; Bertolini, E.; Teresani, G.R.; Peñalver, J.; Mansilla, P.; Aguín, O.; Pérez-Otero, R.; Abelleira, A.; Guerra-García, J.A.; et al. Survey of huanglongbing associated with ‘Candidatus Liberibacter’ species in Spain: Analyses of citrus plants and Trioza erytreae. Phytopathol. Mediterr. 2017, 56, 98–110. [Google Scholar]
- Direção Geral de Alimentação e Veterinária de Portugal Ofício Circular n.3/2015; Governo de Portugal: Lisboa, Portugal, 2015.
- Direção Geral de Alimentação e Veterinária de Portugal Zona Demarcada de Trioza Erytreae; Governo de Portugal: Lisboa, Portugal, 2019.
- Pietersen, G.; Arrebola, E.; Breytenbach, J.H.J.; Korsten, L.; le Roux, H.F.; la Grange, H.; Lopes, S.A.; Meyer, J.B.; Pretorius, M.C.; Schwerdtfeger, M.; et al. A Survey for ‘Candidatus Liberibacter’ Species in South Africa Confirms the Presence of Only ‘Ca. L. africanus’ in Commercial Citrus. Plant Dis. 2010, 94, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Bové, J.M. Heat-tolerant Asian HLB meets heat-sensitive African HLB in the Arabian Peninsula! Why? J. Citrus Pathol. 2014, 1, 1–78. [Google Scholar]
- Moran, V.; Blowers, J. On the biology of the South African citrus psylla, Trioza erytreae (Del Guercio) (Homoptera: Psyllidae). J. Entomol. Soc. South. Afr. 1967, 30, 96–106. [Google Scholar]
- Green, G.C.; Catling, H.D. Weather-induced mortality of the citrus psylla, Trioza erytreae (Del Guercio) (Homoptera: Psyllidae), a vector of greening virus, in some citrus producing areas of Southern Africa. Agric. Meteorol. 1971, 8, 305–317. [Google Scholar] [CrossRef]
- da Graça, J.V.; Douhan, G.W.; Halbert, S.E.; Keremane, M.L.; Lee, R.F.; Vidalakis, G.; Zhao, H. Huanglongbing: An overview of a complex pathosystem ravaging the world’s citrus. J. Integr. Plant Biol. 2016, 58, 373–387. [Google Scholar] [CrossRef]
- Catling, H. The bionomics of the South African citrus psylla, Trioza erytreae (Del Guercio) (Homoptera: Psyllidae). 3. The influence of extremes of weather on survival. J. Entomol. Soc. South. Afr. 1969, 32, 273–290. [Google Scholar]
- Samways, M. Weather and monitoring the abundance of the adult citrus psylla, Trioza erytreae (Del Guercio) (Hom., Triozidae). J. Appl. Entomol. 1987, 103, 502–508. [Google Scholar] [CrossRef]
- Samways, M.J. Biogeography and monitoring outbreaks of the african citrus psylla, Trioza erytreae (Del Guercio). In Proceedings of the 4th International Asia Pacific Conference on Citrus Rehabilitation, Chiang Mai, Thailand, 4–10 February 1990; pp. 188–197. [Google Scholar]
- Catling, H.D. The bionomics of the South African citrus psylla, Trioza erytreae (Del Guercio) (Homoptera: Psyllidae). 6. Final population studies and discussion of population dynamics. J. Entomol. Soc. South. Afr. 1972, 35, 235–251. [Google Scholar]
- Barceló, A.M.; Nunes, L.F. Atlas Climático Ibérico; Agência Estatal de Meteorologia de Espanha e Instituto de Meteorologia de Portugal, 2009; ISBN 9788478370795. [Google Scholar]
- Murray, F.W. On the computation of saturation vapor pressure. J. Appl. Meteorol. 1967, 6, 203–204. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.org/ (accessed on 11 March 2019).
- Catling, H.D.; Green, G.C. The influence of weaher on the survival and population fluctuations of Trioza erytreae (Del Guercio)—A vector of greening. In Int. Organ. Citrus Virol. Conf. Proc.; University of California: Riverside, CA, USA, 1972; Volume 5, pp. 58–64. [Google Scholar]
- Cook, G.; Maqutu, V.Z.; Van Vuuren, S.P. Population Dynamics and Seasonal Fluctuation in the Percentage Infection of Trioza erytreae with ‘Candidatus’ Liberibacter Africanus, the African Citrus Greening Pathogen, in an Orchard Severely Infected with African Greening and Transmission by field-collected Trioza erytreae. Afr. Entomol. 2014, 22, 127–135. [Google Scholar]
- Catling, H.D. Notes on the biology of the South African citrus psylla, Trioza erytreae (Del Guercio) (Homoptera: Psyllidae). J. Entomol. Soc. South. Afr. 1973, 36, 299–306. [Google Scholar]
- Samways, M.J. Prediction of upsurges in populations of the insect vector (Trioza erytreae, Hemiptera: Triozidae) of citrus greening disease using low-cost trapping. J. Appl. Ecol. 1987, 24, 881–891. [Google Scholar] [CrossRef]
- Miranda, P.M.A.; Coelho, F.E.S.; Tomé, A.R.; Valente, M.A.; Carvalho, A.; Pires, C.; Pires, H.O.; Pires, V.C.; Ramalho, C. 20 th Century Portuguese Climate and Climate Scenarios. In Climate Change in Portugal: Scenarios, Impacts and Adaptation Measures; (SIAM Project); Santos, F.D., Forbes, K., Moite, R., Eds.; 2002; pp. 23–83, Gradiva; 454p. [Google Scholar]
- Duarte, A.M. Breves notas sobre a citricultura portuguesa. Agrotec 2012, 3, 40–44. [Google Scholar]
- Van den Berg, M.A.; Deacon, V.E. Developmental biology and population studies on the citrus psylla Trioza erytreae (Del Guercio) (Hemiptera: Triozidae). Fruits 1992, 47, 583–589. [Google Scholar]
- Aidoo, O.F.; Tanga, C.M.; Khamis, F.M.; Rasowo, B.A.; Mohamed, S.A.; Badii, B.K.; Salifu, D.; Sétamou, M.; Ekesi, S.; Borgemeister, C. Host suitability and feeding preference of the African citrus triozid Trioza erytreae Del Guercio (Hemiptera: Triozidae), natural vector of “Candidatus Liberibacter africanus.”. J. Appl. Entomol. 2018, 1–9. [Google Scholar] [CrossRef]
- Aubert, B. Trioza erytreae Del Guercio and Diaphorina citri Kuwayama (Homoptera:Psylloidea), the two vectors of Citrus Greening Disease: Biological aspects and possible control strategies. Fruits 1987, 42, 149–162. [Google Scholar]
- Pérez-Rodríguez, J.; Krüger, K.; Pérez-Hedo, M.; Ruíz-Rivero, O.; Urbaneja, A.; Tena, A. Classical biological control of the African citrus psyllid Trioza erytreae, a major threat to the European citrus industry. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Paiva, P.; Neto, L.; Duarte, A. Áreas de gestão fitossanitária para a Ceratitis capitata e Trioza erytreae. Nova abordagem para a citricultura portuguesa. Agrotec 2019, 33, 61–63. [Google Scholar]
- Van den Berg, M.A. Synopsis of strategies to reduce populations of citrus psylla, Trioza erytreae, and the spread of greening. Fruits 1994, 49, 229–234. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
