Within-Tree Distribution and Survival of the Eucalyptus Longhorned Borer Phoracantha semipunctata (Coleoptera: Cerambycidae) in a Mediterranean-Type Ecosystem
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Tree Selection
2.2. Tree Characteristics and Borer Life Stages
2.3. Statistical Analyses
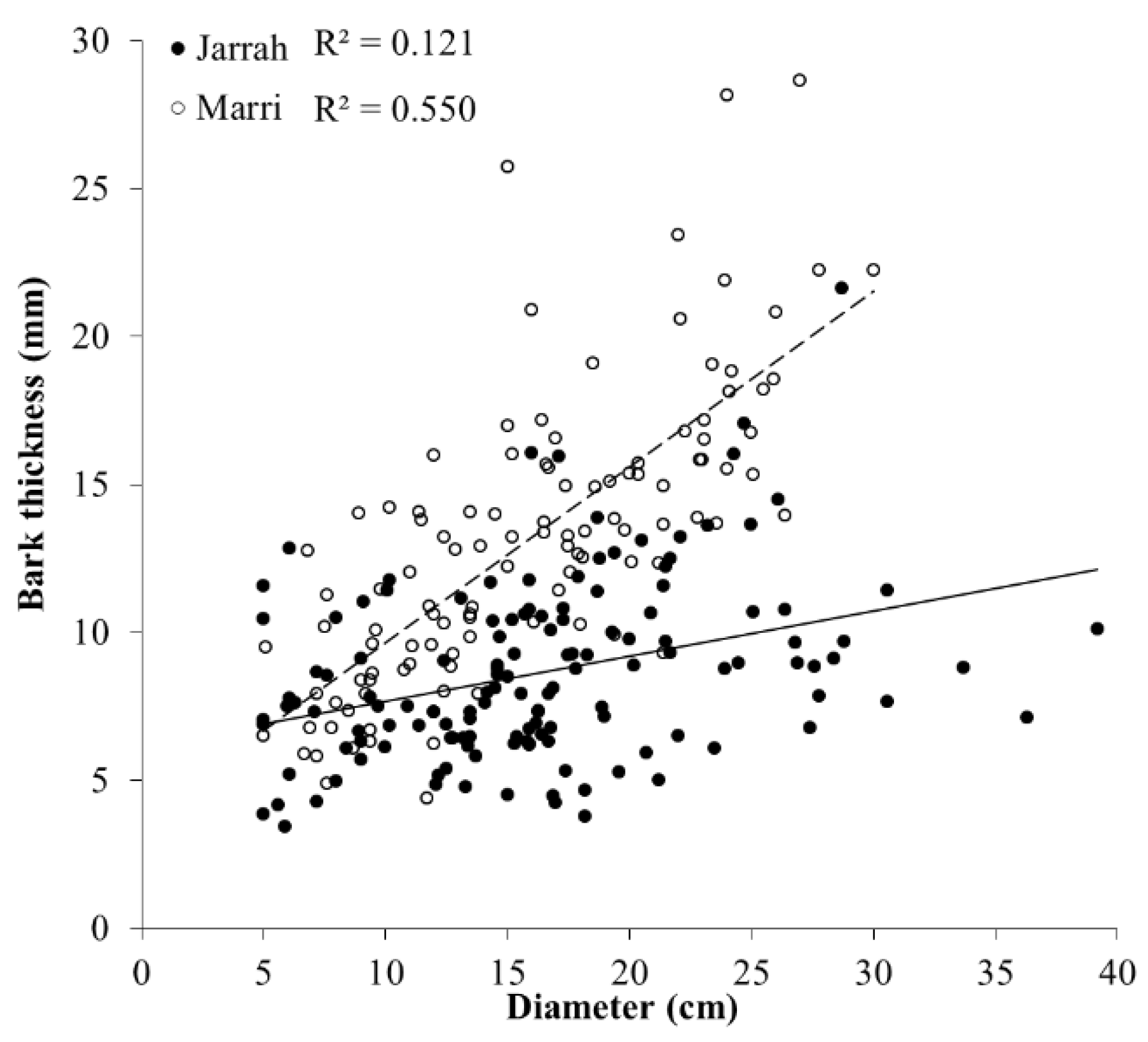
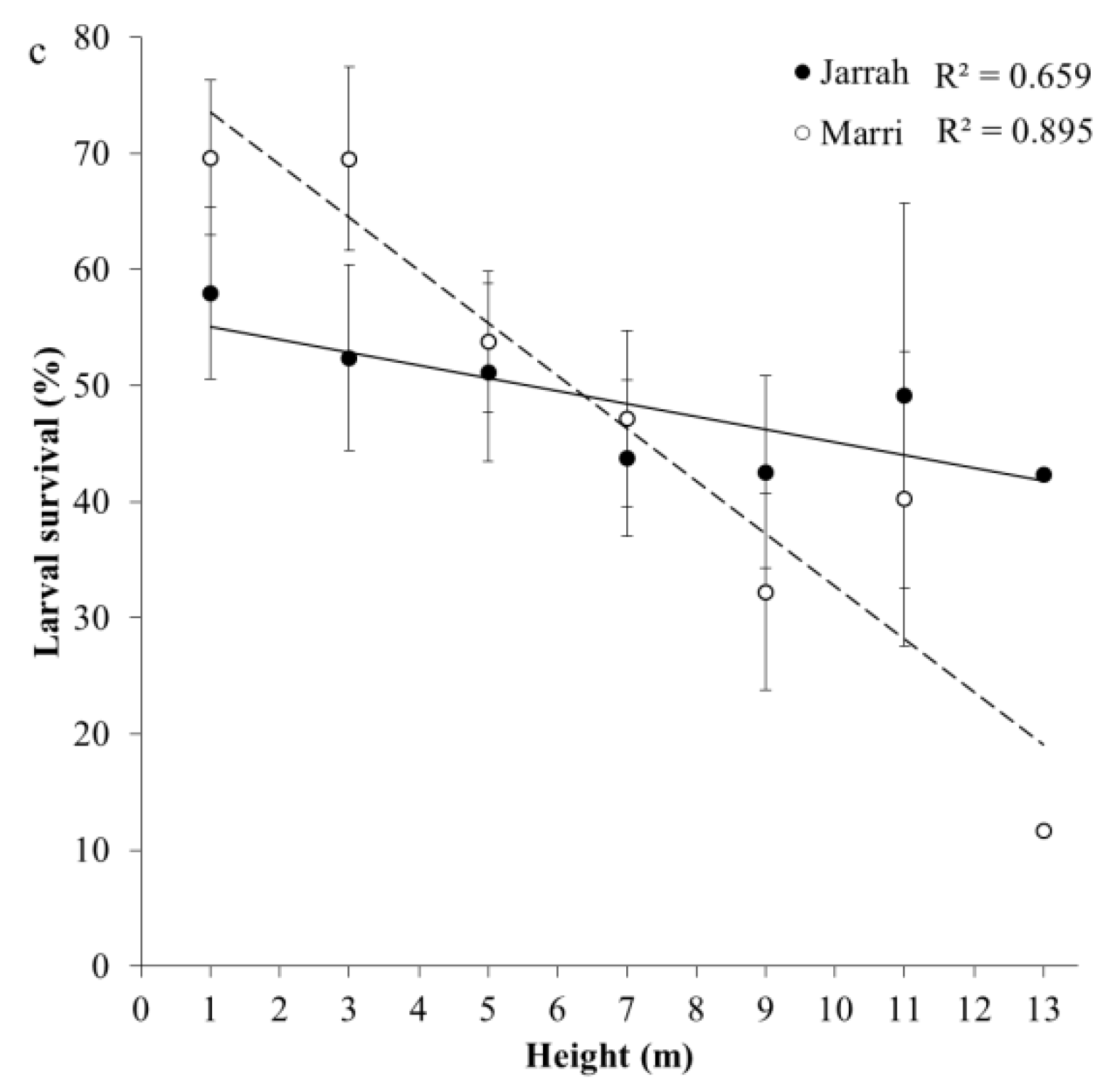
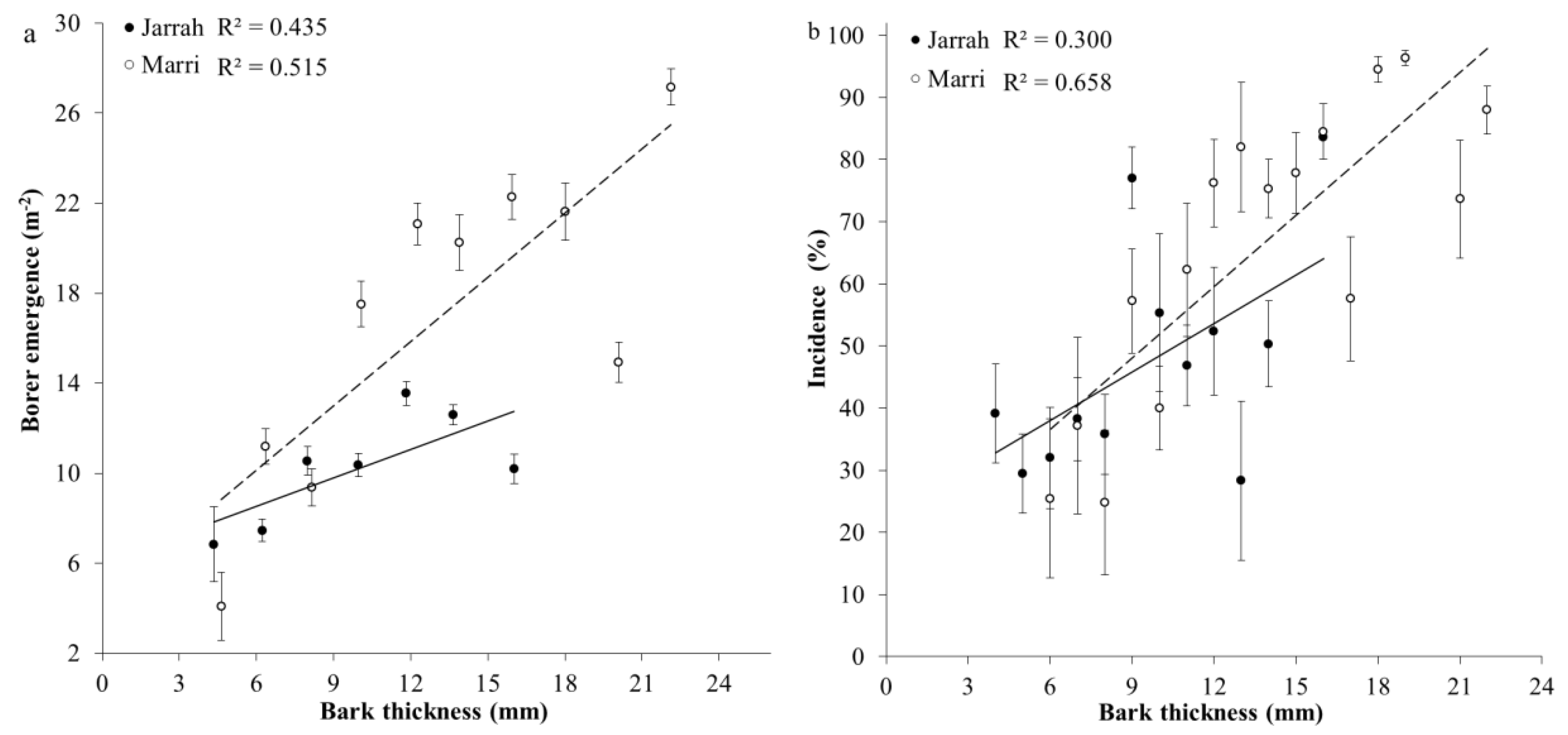
3. Results
3.1. Tree Characteristics and Borer Emergence
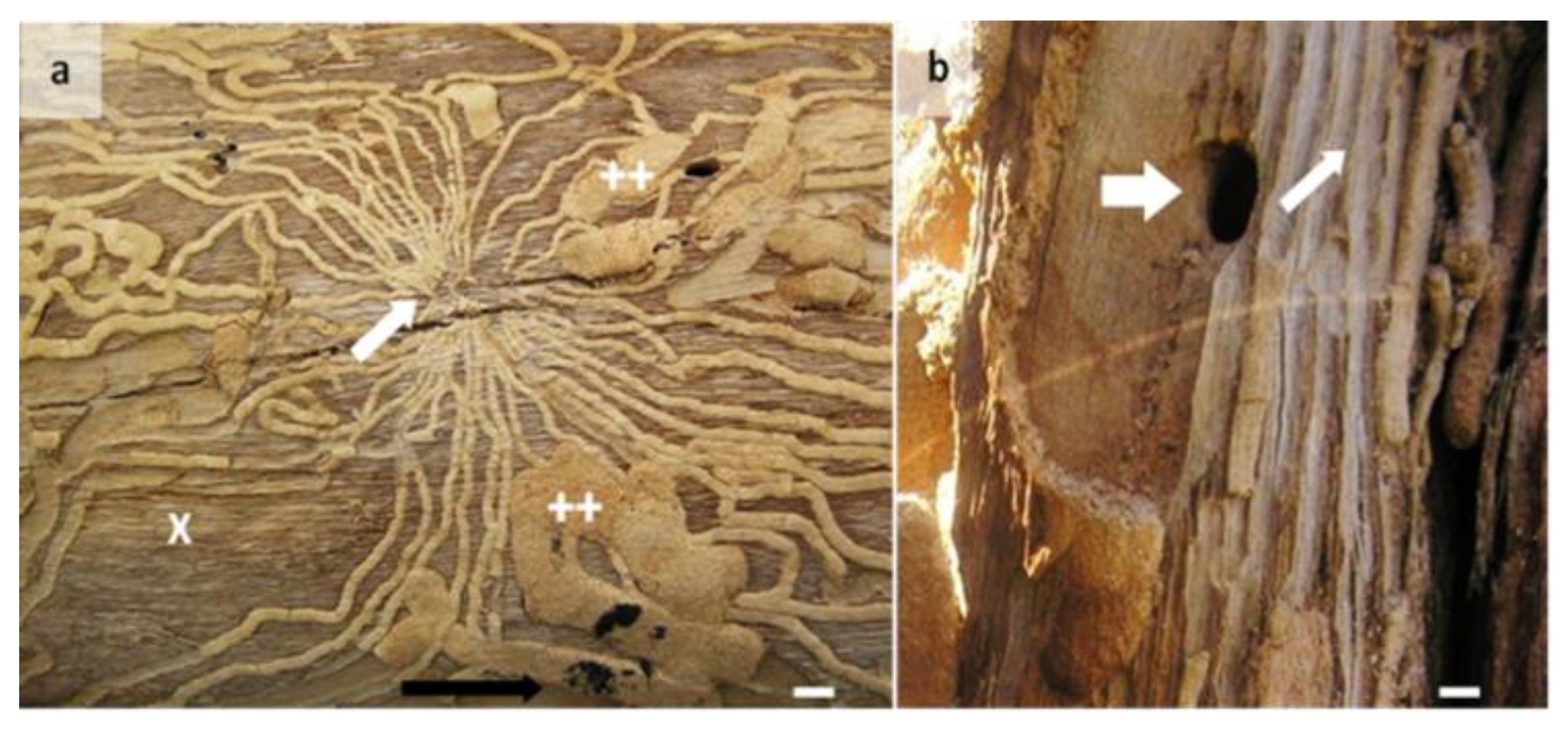
3.2. Incidence of Larval Galleries in Sapwood and Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hanks, L.M.; McElfresh, J.S.; Millar, J.G.; Paine, T.D. Phoracantha semipunctata (Coleoptera: Cerambycidae), a serious pest of Eucalytpus in California: Biology and laboratory-rearing procedures. Ann. Entomol. Soc. Am. 1993, 86, 95–102. [Google Scholar] [CrossRef]
- Paine, T.D.; Millar, J.G.; Paine, E.O.; Hanks, L.M. Influence of host log age and refuge from natural enemies on colonization and survival of Phoracantha semipunctata. Entomol. Exp. Appl. 2001, 98, 157–163. [Google Scholar] [CrossRef]
- Downes, G.; Hudson, I.; Raymond, C.; Dean, G.; Michell, A.; Schimleck, L.; Evans, R.; Muneri, A. Within-tree variation and sampling point correlations. In Sampling Plantation Eucalypts for Wood and Fibre Properties; CSIRO Publishing: Collingwood, VIC Australia, 1997; p. 144. [Google Scholar]
- Kibblewhite, R.; Evans, R.; Shelbourne, C.; Riddell, M. Changes in density and wood-fibre properties with height position in 15/16-year-old Eucalyptus nitens and E. fastigata. Appita J. 2004, 57, 240–247. [Google Scholar]
- Timms, L.L.; Smith, S.M.; De Groot, P. Patterns in the within-tree distribution of the emerald ash borer Agrilus planipennis (Fairmaire) in young, green-ash plantations of south-western Ontario, Canada. Agric. For. Entomol. 2006, 8, 313–321. [Google Scholar] [CrossRef]
- Albert, J.; Platek, M.; Cizek, L. Vertical stratification and microhabitat selection by the Great Capricorn Beetle (Cerambyx cerdo) (Coleoptera: Cerambycidae) in open-grown, veteran oaks. Eur. J. Entomol. 2012, 109, 553–559. [Google Scholar] [CrossRef]
- Nahrung, H.F.; Smith, T.E.; Wiegand, A.N.; Lawson, S.A.; Debuse, V.J. Host tree influences on longicorn beetle (Coleoptera: Cerambycidae) attack in subtropical Corymbia (Myrtales: Myrtaceae). Environ. Entomol. 2014, 43, 37–46. [Google Scholar] [CrossRef]
- Hanks, L.M.; Paine, T.D.; Millar, J.G. Influence of the larval environment on performance and adult body size of the wood-boring beetle Phoracantha semipunctata. Entomol. Exp. Appl. 2005, 114, 25–34. [Google Scholar] [CrossRef]
- Mendel, Z. Seasonal development of the eucalypt borer, Phoracantha semipunctata, in Israel. Phytoparasitica 1985, 13, 85–93. [Google Scholar] [CrossRef]
- Hosking, G.; Hutcheson, J. Nutritional basis for feeding zone preference of Arhopalus ferus (Coleoptera: Cerambycidae). N. Z. J. For. Sci. 1979, 9, 185–192. [Google Scholar]
- Haack, R.A.; Slansky, F., Jr. Nutrional ecology of wood-feeding Coleoptera, Lepidoptera, and Hymenoptera. In Nutritional Ecology of Insects, Mites and Spiders and Related Invertebrates; Slansky, F., Jr., Rodrigues, J.G., Eds.; John Wiley and Sons: New York, NY, USA, 1987; pp. 449–485. [Google Scholar]
- Haack, R.; Wilkinson, R.; Foltz, J. Plasticity in life-history traits of the bark beetle Ips calligraphus as influenced by phloem thickness. Oecologia 1987, 72, 32–38. [Google Scholar] [CrossRef]
- Kolb, T.E.; Guerard, N.; Hofstetter, R.W.; Wagner, M.R. Attack preference of Ips pini on Pinus ponderosa in northern Arizona: Tree size and bole position. Agric. For. Entomol. 2006, 8, 295–303. [Google Scholar] [CrossRef]
- Wang, Q. A taxonomic revision of the Australian genus Phoracantha Newman (Coleoptera: Cerambycidae). Invertebr. Taxon. 1995, 9, 865–958. [Google Scholar] [CrossRef]
- Wang, Q. The phoracanthine beetles (Coleoptera: Cerambycidae: Cerambycinae): Taxonomic overview and generic relationships. Invertebr. Syst. 1998, 12, 667–684. [Google Scholar] [CrossRef]
- GBIF. Global Biodiversity Information Facility. 2013. Available online: https://www.gbif.org/species/1114140 (accessed on 20 June 2013).
- Majer, J.D.; Recher, H.F.; Ganesh, S. Diversity patterns of eucalypt canopy arthropods in Eastern and Western Australia. Ecol. Entomol. 2000, 25, 295–306. [Google Scholar] [CrossRef]
- Duffy, E.A.J. A Monograph of the Immature Stages of Australasian Timber Beetles (Cerambycidae); Trustees of the British Museum: British Museum (Natural History): London, UK, 1963; pp. 68–74. [Google Scholar]
- Majer, J.D.; Abbott, I. Invertebrates of the jarrah forest. In The Jarrah Forest: A Complex Mediterranean Ecosystem; Dell, B., Havel, J.J., Malajczuk, N., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; pp. 111–122. [Google Scholar]
- Curry, S. The association of insects with eucalypt dieback in south western Australia. In Eucalypt Dieback in Forests and Woodlands; Old, K.M., Kile, G.A., Ohmart, C.P., Eds.; CSIRO: Melbourne, Australia, 1981; pp. 130–133. [Google Scholar]
- Scriven, G.T.; Reeves, E.L.; Luck, R.F. Beetle from Australia threatens eucalyptus. Calif. Agric. 1986, 40, 4–6. [Google Scholar]
- Belal, G.S.; Chavanon, G.; Chafi, A.; Chaabane, K. Annual evaluation of Phoracantha semipunctata Fabricius, 1775 (Coleoptera: Cerambycidae) in the North Eastern Morocco. J. Mater. Environ. Sci. 2017, 8, 273–288. [Google Scholar]
- Drinkwater, T. The present pest status of eucalyptus borers Phoracantha spp. in South Africa. In Proceedings of the 1st Congress of the Entomological Society of Southern Africa, Pretoria, South Africa, 30 September–3 October 1974; pp. 119–129. [Google Scholar]
- Hanks, L.M.; Paine, T.D.; Millar, J.G. Host species preference and larval performance in the wood-boring beetle Phoracantha semipunctata F. Oecologia 1993, 95, 22–29. [Google Scholar] [CrossRef]
- Hanks, L.M. Influence of the larval host plant on reproductive strategies of Cerambycid beetles. Annu. Rev. Entomol. 1999, 44, 483–505. [Google Scholar] [CrossRef]
- Chararas, C. Biologie et ecologie de Phoracantha semipunctata F. (Coleoptere: Cerambycidae xylophage) ravageur des Eucalyptus en Tunisie, et methodes de protection des peuplements. Annales de l’Institut national de Recherches Forestières de Tunisie 1969, 2, 1–37. [Google Scholar]
- Powell, W. Age-specific life-table data for the Eucalyptus boring beetle, Phoracantha semipunctata F. (Coleoptera: Cerambycidae), in Malawi. Bull. Entomol. Res. 1982, 72, 645–653. [Google Scholar] [CrossRef]
- Shibata, E.I. Oviposition schedules, survivorship curves, and mortality factors within trees of two cerambycid beetles (Coleoptera: Cerambycidae), the Japanese pine sawyer, Monochamus alternatus Hope, and sugi bark borer, Semanotus japonicus Lacordaire. Res. Popul. Ecol. 1987, 29, 347–367. [Google Scholar] [CrossRef]
- Bybee, L.F.; Millar, J.G.; Paine, T.D. Seasonal development of Phoracantha recurva and P. semipunctata (Coleoptera: Cerambycidae) in Southern California. Environ. Entomol. 2004, 33, 1232–1241. [Google Scholar] [CrossRef]
- Hanks, L.M.; Millar, J.G.; Paine, T.D. Biology and ecology of the eucalyptus borer. In Proceedings of the 39th meeting of the Californian forest pest council, California Department of Forestry and Fire Protection, Sacramento, CA, USA, 14–15 November 1990; pp. 12–16. [Google Scholar]
- Hanks, L.M.; Millar, J.G.; Paine, T.D. Dispersal of the Eucalyptus Longhorned Borer (Coleoptera: Cerambycidae) in urban landscapes. Environ. Entomol. 1998, 27, 1418–1424. [Google Scholar] [CrossRef]
- Hanks, L.M.; Paine, T.D.; Millar, J.G.; Campbell, C.D. Water relations of host trees and resistance to the phloem-boring beetle Phoracantha semipunctata F. (Coleoptera: Cerambycidae). Oecologia 1999, 119, 400–407. [Google Scholar] [CrossRef]
- Ivory, M.H. Preliminary investigations of the pests of exotic forest trees in Zambia. Commonw. For. Rev. 1977, 56, 47–56. [Google Scholar]
- Seaton, S. The Interaction of Drought and the Outbreak of Phoracantha Semipunctata (Coleoptera Cerambcyidae) on Tree Collapse in the Northern Jarrah (Eucalyptus Marginata) Forest; Murdoch University: Perth, Australia, 2012. [Google Scholar]
- Bybee, L.F.; Millar, J.G.; Paine, T.D.; Campbell, K.; Hanlon, C.C. Effects of temperature on fecundity and longevity of Phoracantha recurva and P. semipunctata (Coleoptera: Cerambycidae). Environ. Entomol. 2004, 33, 138–146. [Google Scholar] [CrossRef]
- Seaton, S.; Matusick, G.; Ruthrof, K.; Hardy, G. Outbreak of Phoracantha semipunctata in response to severe drought in a Mediterranean Eucalyptus forest. Forests 2015, 6, 3868–3881. [Google Scholar] [CrossRef]
- Wengert, G. Jarrah: Strong, dark red wood from Australia. FDM Wood Explor. 2003, 75, 24. [Google Scholar]
- Herbarium, W.A. FloraBase-the Western Australian Flora; Department of Biodiversity Conservation and Attractions, Governemnt of Western Australia: Perth, Australia, 2018.
- Chattaway, M.M. The anatomy of bark. II. Oil glands in Eucalyptus species. Aust. J. Bot. 1954, 3, 21–27. [Google Scholar] [CrossRef]
- Abbott, I.; Dell, B.; Loneragan, O. The Jarrah Plant. Chapter 4. In The Jarrah Forest a Complex Mediterranean Ecosystem; Dell, B., Havel, J.J., Malajczuk, N., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; pp. 41–51. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonesca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Robles, G.P.; Hoffman, M.; Pilgrim, J.; Brooks, T.; Mittermeier, C.G.; Lamoreux, J.; Fonseca, G.A.B.D. Hotspots Revisited: Earth’s Biologically Richest and Most Threatened Terrestrial Ecoregions; Conservation International: Mexico City, Mexico, 2004. [Google Scholar]
- Gentilli, J. Climate of the jarrah forest. In The Jarrah Forest: A Complex Mediterranean Ecosystem; Dell, B., Havel, J.J., Malazcuk, N., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; pp. 23–40. [Google Scholar]
- Bell, B.; Heddle, J. Floristic, morphologic and vegetational diversity. In The Jarrah Forest. A Complex Mediterranean Ecosystem; Dell, B., Havel, J.J., Malajczuk, N., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; pp. 53–66. [Google Scholar]
- Matusick, G.; Ruthrof, K.X.; Brouwers, N.C.; Dell, B.; Hardy, G.S.J. Sudden forest canopy collapse corresponding with extreme drought and heat in a mediterranean-type eucalypt forest in southwestern Australia. Euro. J. For. Res. 2013, 132, 497–510. [Google Scholar] [CrossRef]
- Morelli, E.; Bianchi, M.; Sanchez, A. The immature stages of Phoracantha recurva Newman, 1842 and Phoracantha semipunctata Fabricius, 1775 (Coleoptera, Cerambycidae) and a key to larvae of these species. Braz. J. Biol. 2002, 62, 853–860. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crook, D.J.; Fierke, M.K.; Mauromoustakos, A.; Kinney, D.L.; Stephen, F.M. Optimization of sampling methods for within-tree populations of red oak borer, Enaphalodes rufulus (Haldeman) (Coleoptera: Cerambycidae). Environ. Entomol. 2007, 36, 589–594. [Google Scholar] [CrossRef]
- VSNi. Genstat for Windows, 18th ed.; VSN International: Hemel Hempstead, UK, 2017. [Google Scholar]
- Dodds, K.J.; Graber, C.; Stephen, F.M. Facultative intraguild predation by larval Cerambycidae (Coleoptera) on bark beetle larvae (Coleoptera: Scolytidae). Environ. Entomol. 2001, 30, 17–22. [Google Scholar] [CrossRef]
- Ware, V.L.; Stephen, F.M. Facultative intraguild predation of red oak borer larvae (Coleoptera: Cerambycidae). Environ. Entomol. 2006, 35, 443–447. [Google Scholar] [CrossRef]
- Victorsson, J.; Wikars, L.O. Sound production and cannibalism in larvae of the pine-sawyer beetle Monochamus sutor L. (Coleoptera: Cerambycidae). Entomol. Tidskr. 1996, 117, 29–33. [Google Scholar]
- Rose, A.H. Some notes on the biology of Monochamus scutellatus (Say) (Coleoptera: Cerambycidae). Canad. Entomol. 1957, 89, 547–553. [Google Scholar] [CrossRef]
- Ikeda, K. Consumption and food utilization by individual larvae and the population of a wood borer Phymatodes maaki Kraatz (Coleoptera: Cerambycidae). Oecologia 1979, 40, 287–298. [Google Scholar] [CrossRef]
- Shibata, E.I. Effects of Japanese cedar inner bark nutritional quality on development of Semanotus japonicus (Coleoptera: Cerambycidae). Environ. Entomol. 1998, 27, 1431–1436. [Google Scholar] [CrossRef]
- Dunn, J.P.; Potter, D.A.; Kimmerer, T.W. Carbohydrate reserves, radial growth, and mechanisms of resitance of oak trees to phloem-boring insects. Oecologia 1990, 83, 458–468. [Google Scholar] [CrossRef]
- Merchant, A.; Tausz, M.; Arndt, S.K.; Adams, M.A. Cyclitols and carbohydrates in leaves and roots of 13 Eucalyptus species suggests contrasting physiological responses to water defecit. Plant. Cell Environ. 2006, 29, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Banno, H.; Yamagami, A. Life cycle and larval survival rate of the redspotted longicorn beetle, Eupromus ruber (Dalman) (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 1991, 26, 195–204. [Google Scholar] [CrossRef][Green Version]
- FPC. Species Information. Available online: http://www.fpc.wa.gov.au/about-us/publications/species (accessed on 12 April 2017).
- Zhang, Q.H.; Byers, J.A.; Zhang, X.D. Influence of bark thickness, trunk diameter and height on reproduction of the longhorned beetle, Monochamus sutor (Coleoptera: Cerambycidae) in burned larch and pine. J. Appl. Entomol. 1993, 115, 145–154. [Google Scholar] [CrossRef]
- Fierke, M.K.; Kinney, D.L.; Salisbury, V.B.; Crook, D.J.; Stephen, F.M. Development and comparison of intensive and extensive sampling methods and preliminary within-tree population estimates of red oak borer (Coleoptera: Cerambycidae) in the Ozark Mountains of Arkansas. Environ. Entomol. 2005, 34, 184–192. [Google Scholar] [CrossRef][Green Version]
- Wetzel, W. Variability in plant nutrients reduces insect herbivore performance. Nature 2016, 539, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Helal, H.; El-Sebay, Y. Effect of the moisture content of eucalyptus wood on the different stages of Phoracantha semipunctata. Agric. Res. Rev. 1980, 58, 39–46. [Google Scholar]


| Associations | Species | Regression Equation | p-Value |
|---|---|---|---|
| Diameter × Height | Jarrah | y = −0.973x + 21.63 | <0.001 |
| Marri | y = −1.372x + 22.76 | <0.001 | |
| Bark thickness × Height | Jarrah | y = −0.720x + 12.56 | <0.001 |
| Marri | y = −1.016x + 17.69 | <0.001 | |
| Bark thickness × Diameter | Jarrah | y = −0.153x + 6.14 | <0.001 |
| Marri | y = −0.595x + 3.69 | <0.001 |
| Associations | Species | Regression Equation | p-Value |
|---|---|---|---|
| Borer emergence × Height | Jarrah | y = −0.700x + 16.36 | <0.001 |
| Marri | y = −1.542x + 25.13 | <0.001 | |
| Incidence × Height | Jarrah | y = −2.403x + 62.86 | <0.001 |
| Marri | y = −4.561x + 75.81 | <0.001 | |
| Larval survival × Height | Jarrah | y = −1.297x + 58.04 | 0.072 |
| Marri | y = −4.532x + 78.05 | <0.001 | |
| Borer emergence × Bark thickness | Jarrah | y = 0.359x + 6.56 | 0.268 |
| Marri | y = 0.706x + 6.88 | <0.001 | |
| Incidence × Bark thickness | Jarrah | y = 2.606x + 22.41 | 0.038 |
| Marri | y = 3.825x + 13.70 | <0.001 |
| Correlation Coefficient | SE | F-Value | p-Value | |
|---|---|---|---|---|
| (a) Number of borers emerged | ||||
| Species | 0.082 | 0.076 | 102.13 | <0.001 |
| Height | −0.298 | 0.106 | 59.28 | <0.001 |
| Diameter | 0.506 | 0.008 | 949.40 | <0.001 |
| Species × Height | 0.512 | 0.217 | 4.50 | <0.001 |
| Species × Diameter | 0.140 | 0.010 | 1.48 | 0.224 |
| Height × Diameter | −0.494 | 0.013 | 20.53 | <0.001 |
| Species × Height × Diameter | 0.443 | 0.001 | 7.03 | <0.001 |
| (b) Borer emergence (m−2) | ||||
| Species | 0.142 | 0.192 | 18.92 | <0.001 |
| Height | −0.266 | 0.458 | 3.8 | <0.001 |
| Diameter | 0.264 | 0.018 | 21.22 | <0.001 |
| Species × Height | 0.134 | 0.979 | 0.76 | 0.697 |
| Species × Diameter | 0.002 | 0.023 | 1.19 | 0.276 |
| Height × Diameter | −0.476 | 0.045 | 2.21 | 0.01 |
| Species × Height × Diameter | 0.047 | 0.103 | 0.64 | 0.813 |
| (c) Incidence (%) | ||||
| Species | 0.024 | 0.017 | 3.36 | 0.067 |
| Height | −0.277 | 0.297 | 3.92 | <0.001 |
| Diameter | 0.365 | 0.015 | 129.62 | <0.001 |
| Species × Height | −0.016 | 9.408 | 0.44 | 0.853 |
| Height × Diameter | −0.526 | 0.135 | 9.16 | <0.001 |
| Species × Diameter | −0.069 | 0.022 | 0.49 | 0.485 |
| Species × Height × Diameter | 0.045 | 1.600 | 1.46 | 0.187 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seaton, S.; Matusick, G.; Hardy, G. Within-Tree Distribution and Survival of the Eucalyptus Longhorned Borer Phoracantha semipunctata (Coleoptera: Cerambycidae) in a Mediterranean-Type Ecosystem. Insects 2020, 11, 225. https://doi.org/10.3390/insects11040225
Seaton S, Matusick G, Hardy G. Within-Tree Distribution and Survival of the Eucalyptus Longhorned Borer Phoracantha semipunctata (Coleoptera: Cerambycidae) in a Mediterranean-Type Ecosystem. Insects. 2020; 11(4):225. https://doi.org/10.3390/insects11040225
Chicago/Turabian StyleSeaton, Stephen, George Matusick, and Giles Hardy. 2020. "Within-Tree Distribution and Survival of the Eucalyptus Longhorned Borer Phoracantha semipunctata (Coleoptera: Cerambycidae) in a Mediterranean-Type Ecosystem" Insects 11, no. 4: 225. https://doi.org/10.3390/insects11040225
APA StyleSeaton, S., Matusick, G., & Hardy, G. (2020). Within-Tree Distribution and Survival of the Eucalyptus Longhorned Borer Phoracantha semipunctata (Coleoptera: Cerambycidae) in a Mediterranean-Type Ecosystem. Insects, 11(4), 225. https://doi.org/10.3390/insects11040225






