Effects of Non-Lethal High-Temperature Stress on Bradysia odoriphaga (Diptera: Sciaridae) Larval Development and Offspring
Abstract
1. Introduction
2. Materials and Methods
2.1. Bradysia Odoriphaga
2.2. Effect of Short-Term High-Temperature Stress on Development of Bradysia Odoriphaga Later Stage Larvae and Their Offspring
2.3. Data Analysis
3. Results
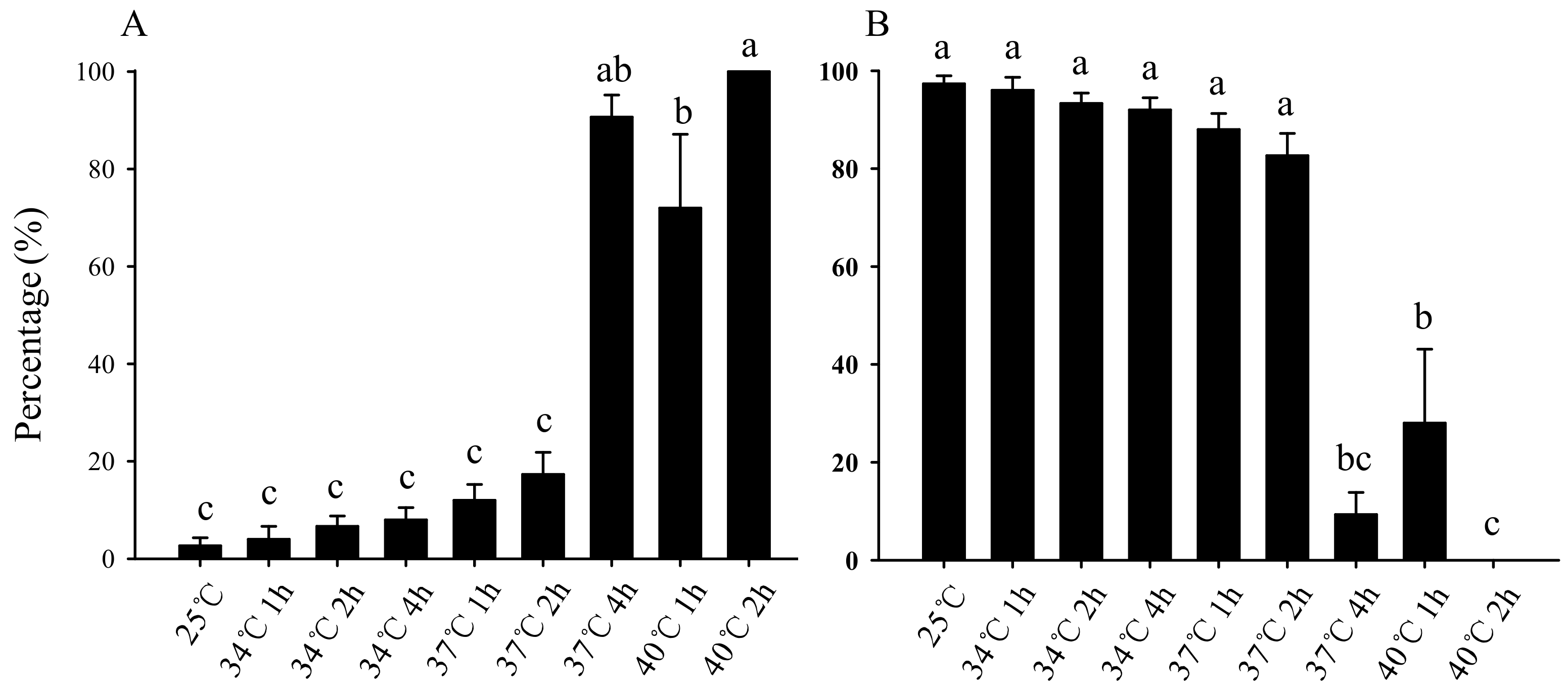
3.1. Effect of Short-Term High-Temperature Stress on Survival and Pupation of Bradysia Odoriphaga Larvae
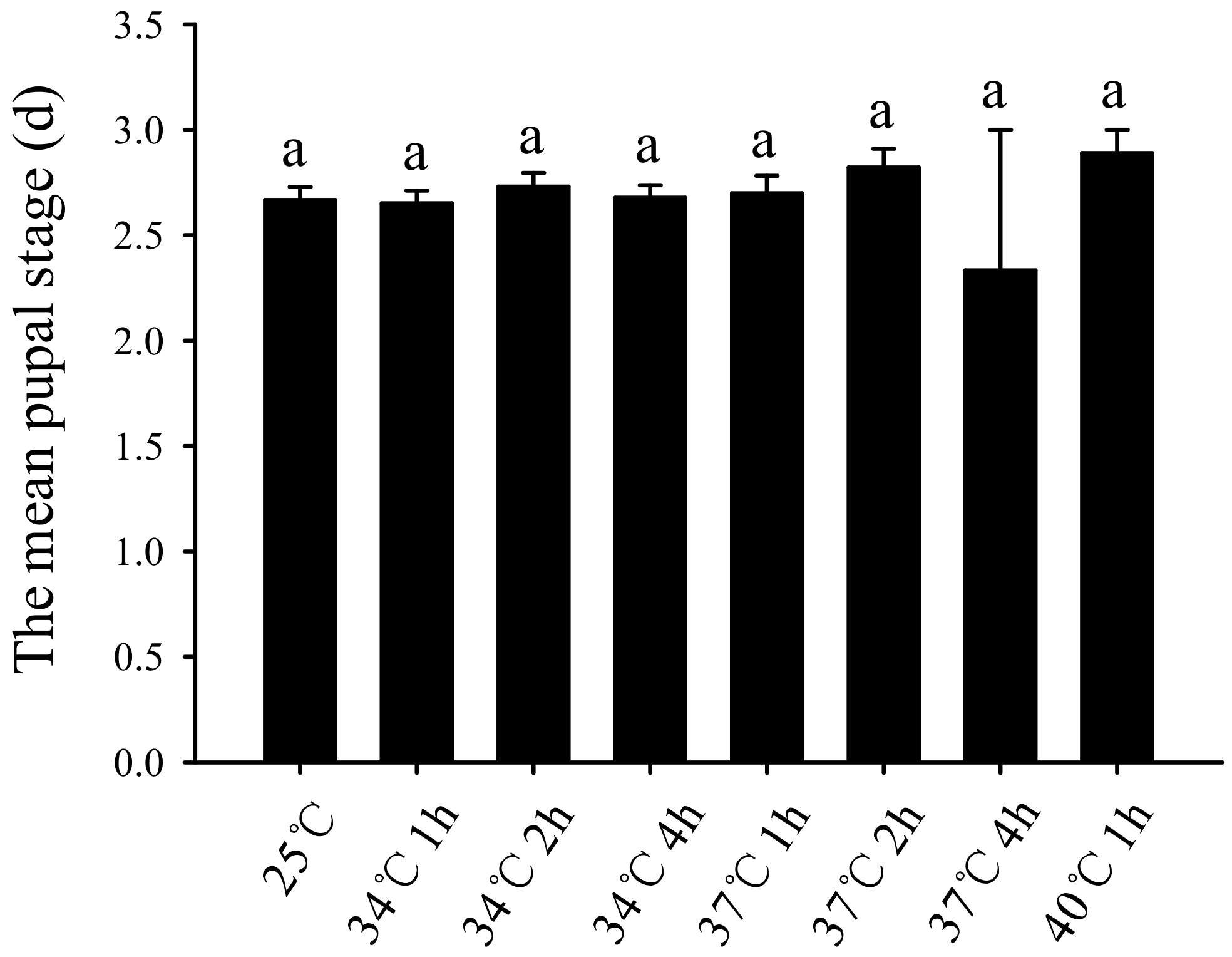
3.2. Effect of Short-Term High-Temperature Stress on the Bradysia Odoriphaga Pupal Stage
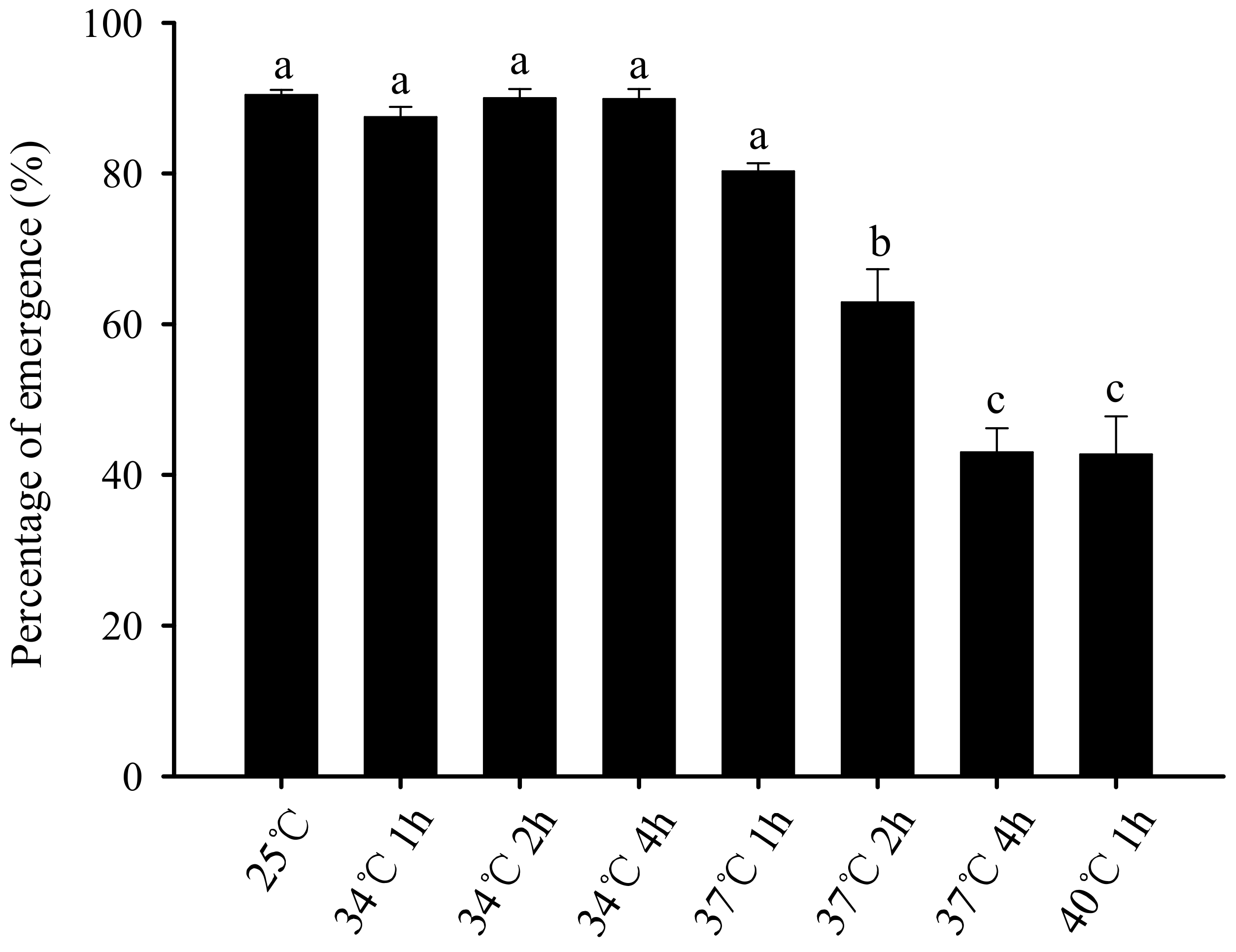
3.3. Effect of Short-Term High-Temperature Stress on the Eclosion Rate of Bradysia Odoriphaga Pupae
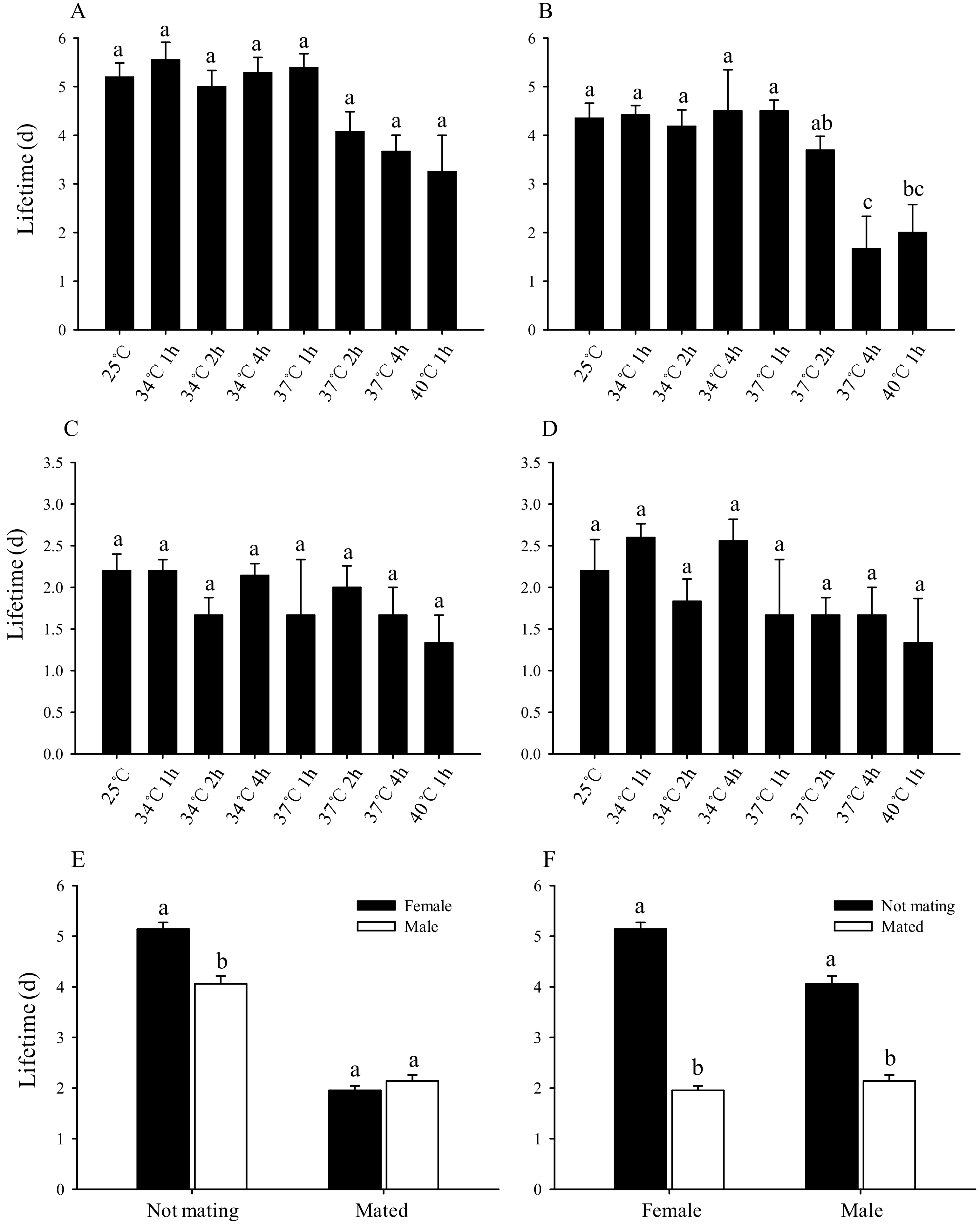
3.4. Effect of Short-Term High-Temperature Stress on the Lifespan of Bradysia Odoriphaga Adults
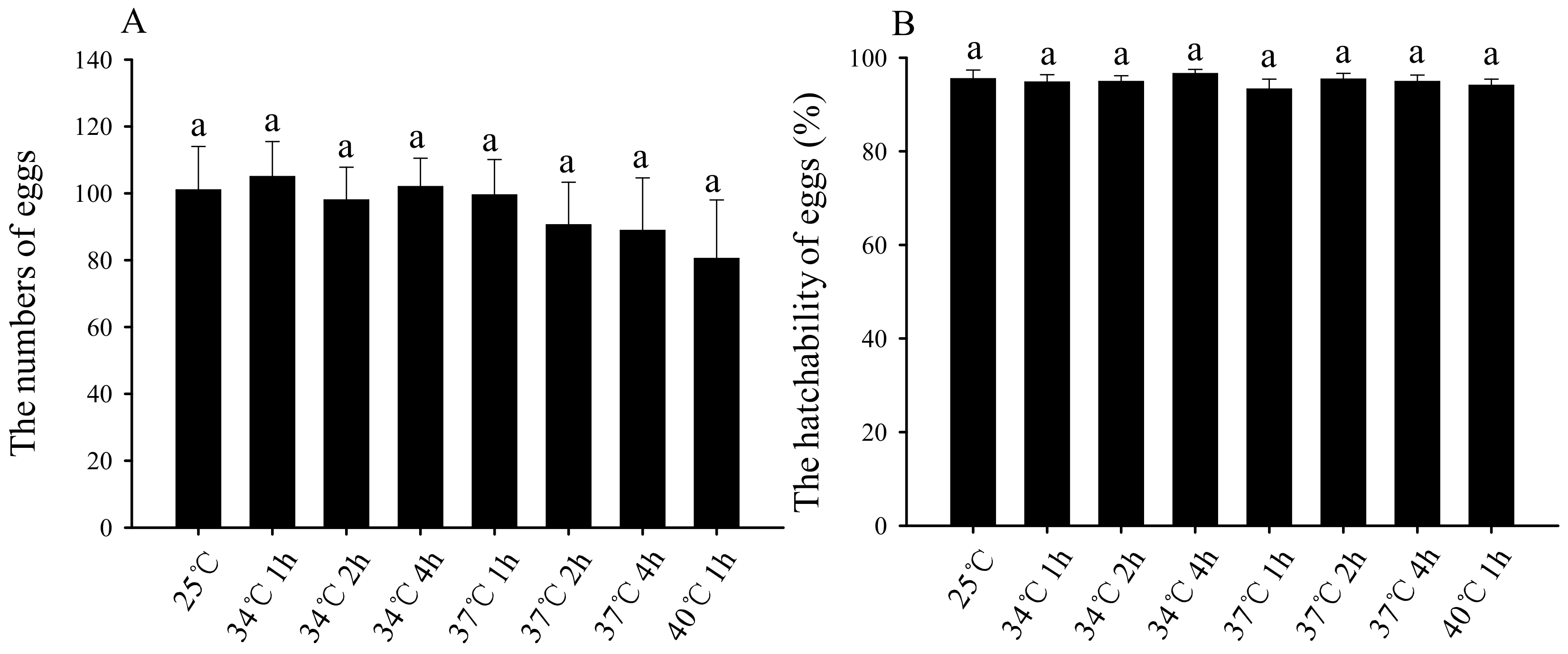
3.5. Effects of Short-Term High-Temperature Stress on the Oviposition Quantity and Hatching Rate of Subsequent Female Adults
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Imahori, Y.; Suzuki, Y.; Uemura, K.; Kishioka, I.; Fujiwara, H.; Ueda, Y.; Chachin, K. Physiological and quality responses of Chinese chive leaves to low oxygen atmospheres. Postharvest Biol. Technol. 2004, 31, 295–303. [Google Scholar] [CrossRef]
- Shi, C.H.; Yang, F.S.; Zhu, X.; Du, E.X.; Yang, Y.T.; Wang, S.L.; Wu, Q.J.; Zhang, Y.J. Evaluation of housekeeping genes for quantitative real-time PCR analysis of Bradysia odoriphaga (Diptera: Sciaridae). Int. J. Mol. Sci. 2016, 17, 1034. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K.; Zhang, X.M. Notes on the fragrant onion gnats with descriptions of two new species of Bradysia (Diptera: Sciaridae). Acta Agric. Univ. Pekin. 1985, 11, 153–156. [Google Scholar]
- Yang, Y.T.; Li, W.X.; Xie, W.; Wu, Q.J.; Xu, B.Y.; Wang, S.L.; Zhang, Y.J. Development of Bradysia odoriphaga (Diptera: Sciaridae) as affected by humidity: An age-stage, two-sex, life-table study. Appl. Entomol. Zool. 2015, 50, 3–10. [Google Scholar] [CrossRef]
- Shi, C.H.; Hu, J.R.; Xie, W.; Yang, Y.T.; Wang, S.L.; Zhang, Y.J. Control of the Chive gnat, Bradysia odoriphaga (Diptera: Sciaridae) with Allyl Isothiocyanate under field and green house conditions. J. Econ. Entomol. 2017, 110, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.H.; Yang, Y.T.; Han, H.L.; Chen, J.X.; Wu, Q.J.; Xu, B.Y.; Zhang, Y.J. Population dynamics and summer and winter habitats of Bradysia odoriphaga in the Beijing area. Chin. J. Appl. Entomol. 2016, 53, 1174–1183. [Google Scholar]
- Zhang, P.; Liu, F.; Mu, W.; Wang, Q.; Li, H. Comparison of Bradysia odoriphaga Yang and Zhang reared on artificial diet and different host plants based on an age-stage, two-sex life table. Phytoparasitica 2015, 43, 107–120. [Google Scholar] [CrossRef]
- Li, W.X.; Yang, Y.T.; Xie, W.; Wu, Q.J.; Xu, B.Y.; Wang, S.L.; Zhang, Y.J. Effects of temperature on the age-stage, two-sex life Table of Bradysia odoriphaga (Diptera: Sciaridae). J. Econ. Entomol. 2015, 108, 126–134. [Google Scholar] [CrossRef]
- Yang, H.W.; Zhang, G.Y. Infectivity of the entomopathogenic nematode, Heterorhabditis sp. D1 to Bradysia odoriphaga (Diptera: Mycetophilidae). Chin. J. Biol. Control. 1990, 6, 110–112. [Google Scholar]
- Sun, R.H.; Li, A.H.; Han, R.C.; Cao, L.; Liu, X.L. Factors affecting the control of Bradysia odoriphaga with entomopathogenic nematode Heterorhabditis indica LN2. Nat. Enem. Insects 2004, 26, 150–155. [Google Scholar]
- Wu, H.B.; Gong, Q.T.; Zhang, K.P.; Zhang, X.P.; Sun, R.H. The efficacy of synergism of entomopathogenic nematodes and black sticky cards to Bradysia odoriphaga. J. Plant. Prot. 2015, 42, 632–638. [Google Scholar]
- Ma, J.; Chen, S.L.; Moens, M.; Han, R.C.; Clercq, P.D. Efficacy of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) against the chive gnat, Bradysia odoriphaga. J. Pest. Sci. 2013, 86, 551–561. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, C.Y.; Li, H.; Liu, F.; Mu, W. Selective toxicity of seven neonicotinoid insecticides to Bradysia odoriphaga and Eisenia foetida. Acta Phytophy. Sin. 2014, 41, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Fan, F.; Wang, Z.Y.; Han, Y.H.; Yang, X.F.; Wei, G.S. Effects of environmental color on biological characteristics of Bradysia odoriphaga (Diptera: Sciaridae). Acta Entomol. Sin. 2015, 58, 553–558. [Google Scholar]
- Chen, C.Y.; Mu, W.; Zhao, Y.H.; Li, H.; Zhang, P.; Wang, Q.H.; Liu, F. Biological activity of trans-2-hexenal against Bradysia odoriphaga (Diptera: Sciaridae) at different developmental stages. J. Insect Sci. 2015, 15, iev075. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.L.; Guo, Y.N.; Wang, J.; Li, L.L.; Yu, Y.; Chu, D. Detection and identification of Wolbachia in Bradysia odoriphaga (Diptera: Sciaridae) populations from Shangdong Province, China. Acta Entomol. Sin. 2015, 58, 454–459. [Google Scholar]
- Li, W.X.; Zhou, N.L.; Chen, Y.Y.; Wang, J.; Zhu, X.L.; Wang, Z.J.; Feng, W.M. The effect of the novel “light fertilizer” on the environment factor in high tunnel and growth of Brassica chinensis. Agr. Sci. Tech. 2014, 15, 2245–2248. [Google Scholar]
- Wang, X.L.; Song, X.K.; Han, B. A report of food poisoning with contaminated Chinese chive. J. Chin. Rural Med. 2006, 13, 52. [Google Scholar]
- Lomeli-Flores, J.R.; Barrera, J.F.; Bernal, J.S. Impacts of weather, shade cover and elevation on coffee leafminer Leucoptera coffeella (Lepidoptera: Lyonetiidae) population dynamics and natural enemies. Crop. Prot. 2010, 29, 1039–1048. [Google Scholar] [CrossRef]
- Wang, H.S.; Xu, H.F.; Cui, F. Effect of high temperature on fecundity and ovary development of beet armyworm Spodoptera exigua (Hǜbner). Southwest China J. Agri. Sci. 2006, 19, 916–919. [Google Scholar]
- Liang, L.N.; Zhang, W.; Ma, G.A. A single hot event stimulates adult performance but reduces egg survival in the oriental fruit moth, Grapholitha molesta. PLoS ONE 2014, 9, e116339. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Ma, C.S. Upper critical temperatures for behaviors of threes pecies of cereal aphids in leaf temperature gradients. Acta Ecol. Sin. 2007, 27, 2449–2459. [Google Scholar]
- Zeng, B.; Zhu, W.J.; Fu, Y.G.; Zhou, S.H. Influence of high-temperature exposure on the mating, oviposition and thermotaxis of Bactrocera cucurbitae (Coquillet) (Diptera: Tephritidae). PLoS ONE 2018, 13, e0204065. [Google Scholar] [CrossRef] [PubMed]
- Fields, P.G. The control of stored-product insects and mites with extreme temperatures. J. Stored Prod. Res. 1992, 28, 89–118. [Google Scholar] [CrossRef]
- Shi, C.H.; Hu, J.R.; Wei, Q.W.; Yang, Y.T.; Cheng, J.X.; Han, H.L.; Wu, Q.J.; Wang, S.L.; Xu, B.Y.; Su, Q.; et al. Control of Bradysia odoriphaga (Diptera: Sciaridae) by soil solarization. Crop. Prot. 2018, 114, 76–82. [Google Scholar] [CrossRef]
- Cheng, J.X.; Su, Q.; Jiao, X.G.; Shi, C.H.; Yang, Y.T.; Han, H.L.; Xie, W.; Guo, Z.J.; Wu, Q.J.; Xu, B.Y.; et al. Effects of heat shock on the Bradysia odoriphaga (Diptera: Sciaridae). J. Econ. Entomol. 2017, 110, 1630–1638. [Google Scholar] [CrossRef]
- Cui, X.H.; Wan, F.H.; Xie, M.; Liu, T.X. Effects of heat shock on survival and reproduction of two whitefly species, Trialeurodes vaporariorum and Bemisia tabaci biotype B. J. Insect. Sci. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Ma, C.S.; Ma, G.; Zhao, F. Impact of global warming on cereal aphids. Chin. J. Appl. Entomol. 2014, 51, 1435–1443. [Google Scholar]
- Pelletier, Y. Determination of the lethal high temperature for the Colorado potato beetle (Coleoptera: Chrysomelidae). Can. Agric. Eng. 1998, 40, 185–189. [Google Scholar]
- Ma, C.S.; Hau, B.; Poehling, H.M. The effect of heat stress on the survival of the rose grain aphid, Metopolophium dirhodum (Hemiptera: Aphididae). Eur. J. Entomol. 2004, 101, 327–331. [Google Scholar] [CrossRef]
- Shi, C.H. Application of solarization high temperature film mulching in the control of Bradysia odoriphaga. China Veg. 2017, 1, 90. [Google Scholar]
- Jeffs, C.T.; Leather, S.R. Effects of extreme, fluctuating temperature events on life history traits of the grain aphid, Sitobion avenae. Entomol. Exp. Appl. 2014, 150, 240–249. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, S.; Han, H.L.; Xu, B.Y.; Zhang, Y.J. Effects of male age on its mating ability and female reproduction in Bradysia odoriphaga. J. Environ. Entomol. 2019, 41, 657–663. [Google Scholar]
- Uddin, M.D.K.; Yin, X.W.; Zhang, L. Courtship and mating behavior of the Chinese Chive fly, Bradysia odoriphaga (Diptera: Sciaridae) and evidence of female sex pheromone. Pak. J. Zool. 2016, 48, 1543–1548. [Google Scholar]
- Mazzucco, R.; Van Nguyen, T.; Kim, D.H.; Chon, T.S.; Dieckmann, U. Adaptation of aquatic insects to the current flow in streams. Ecol. Model. 2015, 309, 143–152. [Google Scholar] [CrossRef]
- Pooraiiouby, R.; Sharma, A.; Beard, J.; Reyes, J.; Nuss, A.; Gulia-Nuss, M. Nutritional quality during development alters insulin-Like peptides’ expression and physiology of the adult yellow fever mosquito, Aedes aegypti. Insects 2018, 9, 110. [Google Scholar] [CrossRef]
- Rodríguez-Muňoz, R.; Boonekamp, J.J.; Liu, X.P.; Skicko, I.; Fisher, D.N.; Hopwood, P.; Tregenza, T. Testing the effect of early-life reproductive effort on age-related decline in a wild insect. Evolution 2019, 73, 317–328. [Google Scholar] [CrossRef]
- Llandres, A.L.; Marques, G.M.; Maino, J.L.; Kooijman, S.A.L.M.; Kearney, M.R.; Casas, J. A dynamic energy budget for the whole life-cycle of holometabolous insects. Ecol. Monogr. 2015, 85, 353–371. [Google Scholar] [CrossRef]
- Hu, J.R.; Xie, C.; Shi, C.H.; Wang, S.L.; Wu, Q.J.; Li, C.R.; Zhang, Y.J. Effect of sex and air temperature on the flight capacity of Bradysia odoriphaga (Diptera: Sciaridae). J. Econ. Entomol. 2019, 112, 2161–2166. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, Z.; Wang, Q.L.; Zhang, F.; Liu, T.X. Exposing eggs to high temperatures affects the development, survival and reproduction of Harmonia axyridis. J. Therm. Biol. 2014, 39, 40–44. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, F.; Hoffmann, A.A.; Ma, C.S. A single hot event that does not affect survival but decreases reproduction in the diamondback moth, Plutella xylostella. PLoS ONE 2013, 8, e75923. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.L.; Xue, Z.Y.; Hong, C.L.; Zhu, F.X.; Chen, X.Y.; Wang, W.P.; Cai, Z.C.; Huang, N.; Yang, X.Q. Effciency of different solarization-based ecological soil treatments on the control of Fusarium wilt and their impacts on the soil microbial community. Appl. Soil Ecol. 2016, 108, 341–351. [Google Scholar] [CrossRef]
- Kokalis-Burelle, N.; McSorley, R.; Wang, K.H.; Saha, S.K.; McGovern, R.J. Rhizosphere microorganisms affected by soil solarization and cover cropping in Capsicum annuum and Phaseolus lunatus agroecosystems. Appl. Soil Ecol. 2017, 119, 64–71. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Bressac, C.; Chevrier, C. Heat stress affects male reproduction in a parasitoid wasp. J. Insect Physiol. 2013, 59, 248–254. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, C.; Zhang, S.; Hu, J.; Zhang, Y. Effects of Non-Lethal High-Temperature Stress on Bradysia odoriphaga (Diptera: Sciaridae) Larval Development and Offspring. Insects 2020, 11, 159. https://doi.org/10.3390/insects11030159
Shi C, Zhang S, Hu J, Zhang Y. Effects of Non-Lethal High-Temperature Stress on Bradysia odoriphaga (Diptera: Sciaridae) Larval Development and Offspring. Insects. 2020; 11(3):159. https://doi.org/10.3390/insects11030159
Chicago/Turabian StyleShi, Caihua, Seng Zhang, Jingrong Hu, and Youjun Zhang. 2020. "Effects of Non-Lethal High-Temperature Stress on Bradysia odoriphaga (Diptera: Sciaridae) Larval Development and Offspring" Insects 11, no. 3: 159. https://doi.org/10.3390/insects11030159
APA StyleShi, C., Zhang, S., Hu, J., & Zhang, Y. (2020). Effects of Non-Lethal High-Temperature Stress on Bradysia odoriphaga (Diptera: Sciaridae) Larval Development and Offspring. Insects, 11(3), 159. https://doi.org/10.3390/insects11030159





