Mobility of Stored Product Beetles after Exposure to a Combination Insecticide Containing Deltamethrin, Methoprene, and a Piperonyl Butoxide Synergist Depends on Species, Concentration, and Exposure Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Commodity
2.2. Insecticide Assay
2.3. Statistical Analysis
3. Results
3.1. Initial Analysis
3.2. Rhyzopertha Dominica
3.3. Cryptolestes Ferrugineus
3.4. Sitophilus Oryzae
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oberlander, H.; Silhacek, D.L.; Shaaya, E.; Ishaaya, I. Current status and future perspectives of the use of insect growth regulators for the control of stored product insects. J. Stored Prod. Res. 1997, 33, 1–6. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Athanassiou, C.G.; Vayias, B.J.; Tomanovic, Z. Efficacy of insect growth regulators as grain protectants against two stored product pests in wheat and maize. J. Food Prot. 2012, 75, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Arthur, F.H. Aerosols and contact insecticides as alternatives to methyl bromide in flour mills, food production facilities, and food warehouses. J. Pest Sci. 2012, 85, 323–329. [Google Scholar] [CrossRef]
- Arthur, F.H. Structural management of stored product pests. In Resent Advances in Stored Product Protection; Athanassiou, C.G., Arthur, C.G., Eds.; Springer: New York, NY, USA, 2019; pp. 65–81. [Google Scholar]
- Wijayaratne, L.K.W.; Arthur, F.H.; Whyard, S. Methoprene and control of stored product insects. J. Stored Prod. Res. 2018, 76, 161–169. [Google Scholar] [CrossRef]
- Daglish, G.J. Impact of resistance on the efficacy of binary combinations of spinosad, chlorpyriphos-methyl and s-methoprene against five stored-grain beetles. J. Stored Prod. Res. 2008, 44, 71–76. [Google Scholar] [CrossRef]
- Arthur, F.H. Residual efficacy of chlorpyrifos-methyl + bioresmethrin and chlorpyrifos-methyl + resmethrin for controlling lesser grain borers (Coleoptera: Bostrichidae), rice weevils (Coleoptera: Curculionidae), and red flour beetles (Coleoptera: Tenebrionidae in stored wheat. J. Econ. Entomol. 1992, 85, 570–575. [Google Scholar]
- Kavallieratos, N.G.; Athanassiou, C.G.; Arthur, F.H. Effectiveness of insecticide-incorporated bags to control stored product beetles. J. Stored Prod. Res. 2016, 70, 18–24. [Google Scholar] [CrossRef]
- Arthur, F.H. Residual efficacy of a deltamethrin emulsifiable concentrate formulation against Rhyzopertha dominica (F.) and Sitotroga cerealella (Oliver) after partial treatment of brown rice. Insects 2019, 10, 95. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Arthur, F.H.; Throne, J.E. Efficacy of layer treatment with methoprene for control of Rhyzopertha dominica (Coleoptera: Bostrychidae) on wheat, rice and maize. Pest Manag. Sci. 2010, 67, 380–384. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Arthur, F.H.; Throne, J.E. Efficacy of methoprene for control of five species of psocids (Psocoptera) on wheat, rice, and maize. J. Food Prot. 2010, 73, 2244–2249. [Google Scholar] [CrossRef]
- Daglish, G.J.; Pulvirenti, C. Reduced fecundity of Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae) following exposure of adults to methoprene. J. Stored Prod. Res. 1997, 34, 201–206. [Google Scholar] [CrossRef]
- Arthur, F.H. Evaluation of methoprence alone or in combination with diatomaceous earth to control Rhyzopertha dominica (Coleoptera: Bostrichidae) on stored wheat. J. Stored Prod. Res. 2004, 40, 485–498. [Google Scholar] [CrossRef]
- Chanbang, Y.; Arthur, F.H.; Wilde, G.E.; Throne, J.E.; Subramanyam, B. Susceptibility of eggs and adult fecundity of the lesser grain borer, Rhyzopertha dominica, exposed to methoprene. J. Insect Sci. 2008, 8, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Guedes, R.N.C.; Campbell, J.F.; Arthur, F.H.; Opit, G.P.; Zhu, K.U.; Throne, J.E. Acute lethal and behavioral sublethal responses of two stored product psocids to surface insecticides. Pest Manag. Sci. 2008, 64, 1314–1322. [Google Scholar] [CrossRef]
- Morrison, W.R., III; Wilkins, R.V.; Gerken, A.R.; Scheff, D.S.; Zhu, K.Y.; Arthur, F.H.; Campbell, J.F. Mobility of adult Tribolium castaneum (Coleoptera: Tenebrionidae) and Rhyzopertha dominica (Coleoptera: Bostrichidae) after exposure to long-lasting insecticide-incorporated netting. J. Econ. Entomol. 2018, 111, 2443–2453. [Google Scholar] [CrossRef]
- Nayak, M.K.; Holloway, J.C.; Emery, R.N.; Pavic, H.; Bartlet, J.; Collins, P.J. Strong resistance to phosphine in the rusty grain beetle, Cryptolestes ferrugineus (Stephens) (Coleoptera: Laemophloeidae): Its characterisation, a rapid assay for diagnosis and its distribution in Australia. Pest Manag. Sci. 2013, 69, 48–53. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Brabec, D.L.; Oppert, B.; Guedes, R.N.C.; Campbell, J.F. From immobilization to recovery: Towards the development of a rapid diagnostic indicator for phosphine resistance. J. Stored Prod. Res. 2019, 80, 28–33. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Brabec, D.L.; Agrafioti, P.; Sakka, M.; Campbell, J.F. Using immobilization as a quick diagnostic indicator for resistance to phosphine. J. Stored Prod. Res. 2019, 82, 17–26. [Google Scholar] [CrossRef]
- Agrafioti, P.; Athanassiou, C.G.; Vassilakos, T.N.; Vlontzos, G.; Arthur, F.H. Development of a lethality index to assess susceptibility of Ttribolium confusum and Oryzaephilus surinamensis to insecticides. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Arthur, F.H. Impact of accumulated food on survival of Tribolium castaneum on concrete treated with cyfluthrin wettable powder. J. Stored Prod. Res. 2000, 36, 15–23. [Google Scholar] [CrossRef]
- Arthur, F.H. Dosage rate, temperature, and food source provisioning affect susceptibility of Tribolium castaneum and Tribolium confusum to chlorfenapyr. J. Pest Sci. 2013, 86, 507–513. [Google Scholar] [CrossRef]
- Morrison, W.R., III; Bruce, A.; Wilkins, R.V.; Albin, C.E.; Arthur, F.H. Sanitation improves stored product insect pest management. Insects 2019, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Arthur, F.H.; Campbell, J.F.; Donaldson, J.E. Laboratory evaluation of particle size, food contamination, and residual efficacy of pyrethrin + methoprene aerosol. J. Stored Prod. Res. 2017, 72, 100–110. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Arthur, F.H.; Kavallieratos, N.G.; Thone, J.E. Efficacy of pyriproxifen for control of stored product psocids (Psocoptera) on concrete surfaces. J. Econ. Entomol. 2011, 104, 1765–1769. [Google Scholar] [CrossRef]
- Lanka, S.K.; Arthur, F.H.; Campbell, J.F.; Zhu, K.Y. Evaluation of residual efficacy of pyrethrin + methoprene aerosol on two dermestids: Impact of particle size, species, and temperature. Insects 2019, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Scheff, D.; Brabec, D.; Campbell, J.F.; Arthur, F.H. Case Study: A practical application of an aerosol treatment in a commercial mill. Insects 2019, 10, 150. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Arthur, F.H.; Campbell, J.F.; Donaldson, J.E. Particle size matters: Efficacy of aerosols for the control of stored product psocids. J. Stored Prod. Res. 2019, 83, 148–152. [Google Scholar] [CrossRef]
- Wilkins, R.; Zhu, K.Y.; Campbell, J.F.; Morrison, W.R. Mobility and dispersal of two cosmopolitan stored product insects are adversely affected by long-lasting insecticide netting in a life stage-dependent manner. J. Econ. Entomol. 2020, in press. [Google Scholar]
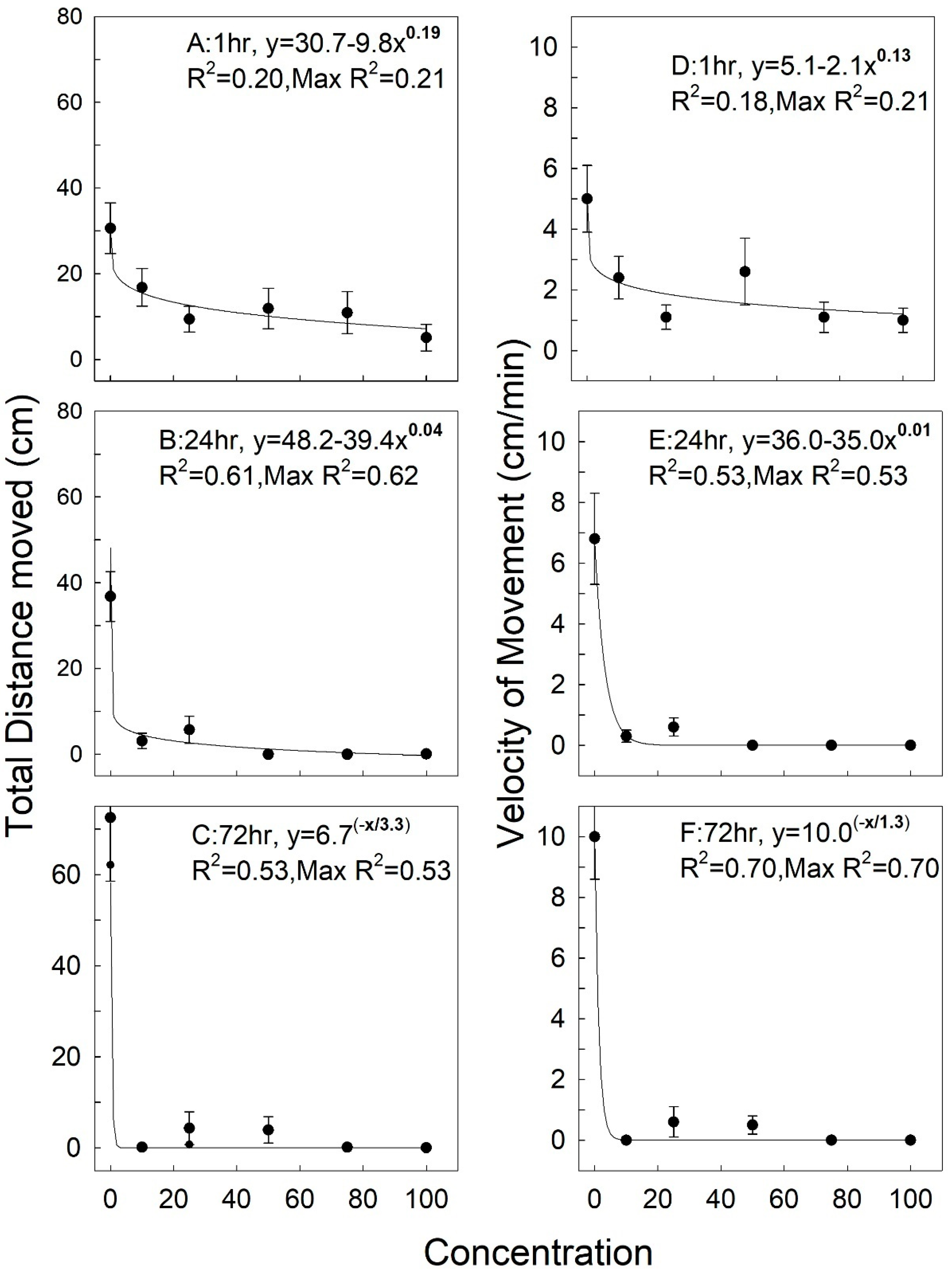
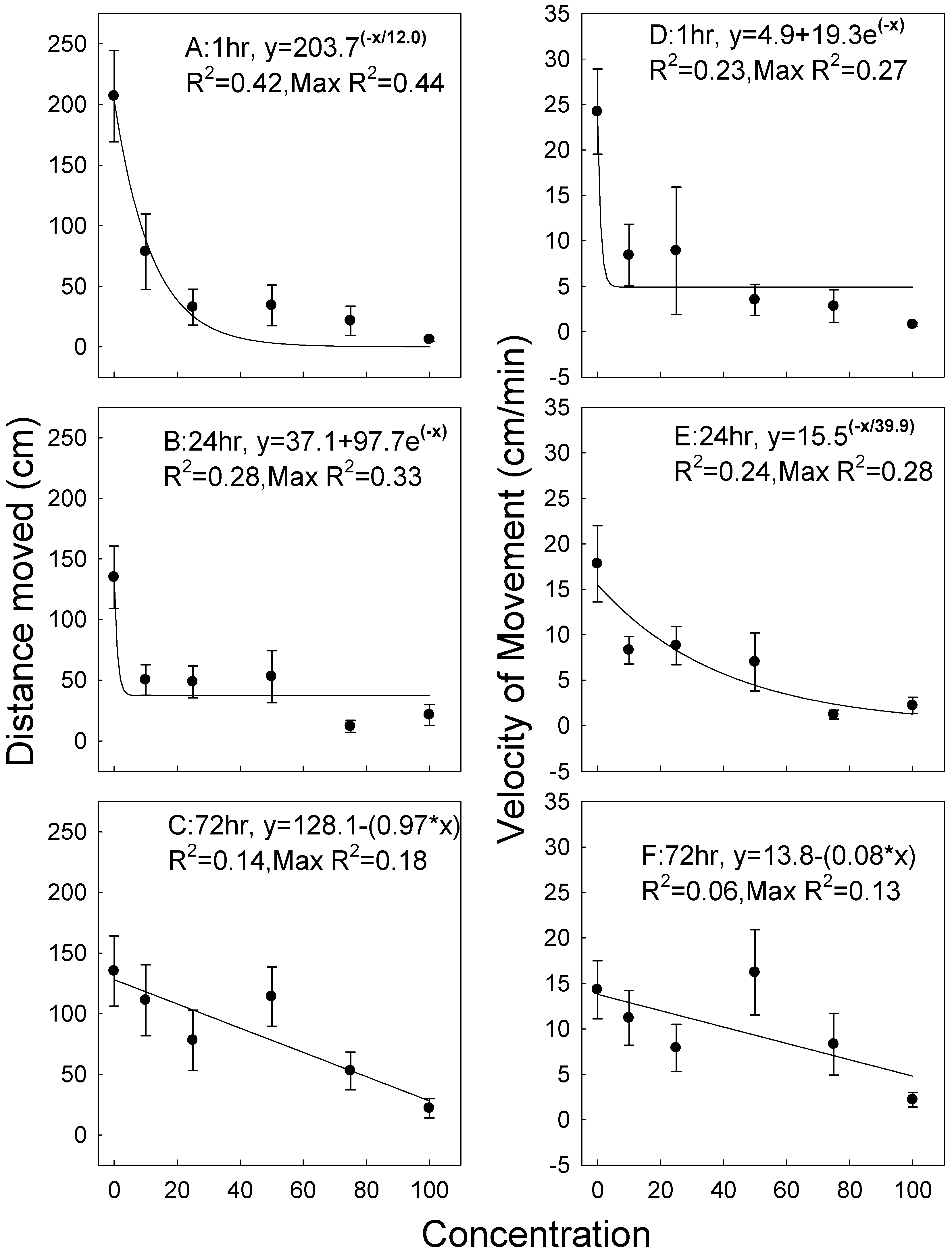

| Response | Factor | df | F | p |
|---|---|---|---|---|
| Distance Moved | Species | 2707 | 92.5 | <0.001 |
| ET | 2707 | 7.4 | <0.001 | |
| Conc. | 5707 | 37.8 | <0.001 | |
| Species*ET | 4707 | 1.6 | 0.162 | |
| Species*Conc. | 10,707 | 5.1 | <0.001 | |
| ET*Conc. | 10,707 | 0.7 | 0.705 | |
| Species*ET*Conc. | 20,707 | 2.4 | <0.001 | |
| Velocity | Species | 2707 | 25.0 | <0.001 |
| ET | 2707 | 7.4 | <0.001 | |
| Conc. | 2707 | 37.8 | 0.039 | |
| Species*ET | 5707 | 1.6 | 0.119 | |
| Species*Conc. | 4707 | 5.1 | <0.001 | |
| ET*Conc. | 10,707 | 0.7 | 0.541 | |
| Species*ET*Conc. | 10,707 | 2.4 | <0.001 |
| Exposure Time | Concentration | Distance Moved | Velocity | ||
|---|---|---|---|---|---|
| Min | Max | Min | Max | ||
| 1 H | 0 | 0.0 | 66.6 | 0.0 | 15.8 |
| 10 | 0.0 | 41.7 | 0.0 | 8.5 | |
| 25 | 0.0 | 42.2 | 0.0 | 4.7 | |
| 50 | 0.0 | 48.4 | 0.0 | 11.9 | |
| 75 | 0.0 | 49.8 | 0.0 | 5.2 | |
| 100 | 0.0 | 37.2 | 0.0 | 3.7 | |
| 24 H | 0 | 5.1 | 66.9 | 0.5 | 18.2 |
| 10 | 0.0 | 24.7 | 0.0 | 2.5 | |
| 25 | 0.0 | 40.9 | 0.0 | 4.1 | |
| 50 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 75 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 100 | 0.0 | 0.5 | 0.1 | 0.0 | |
| 72 H | 0 | 0.0 | 218.4 | 0.0 | 22.8 |
| 10 | 0.0 | 0.5 | 0.0 | 0.1 | |
| 25 | 0.0 | 53.4 | 0.0 | 7.1 | |
| 50 | 0.0 | 30.7 | 0.0 | 4.0 | |
| 75 | 0.0 | 1.0 | 0.0 | 0.1 | |
| 100 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Exposure Time | Concentration | Distance Moved | Velocity | ||
|---|---|---|---|---|---|
| Min | Max | Min | Max | ||
| 1 H | 0 | 0.0 | 416.6 | 0.0 | 55.6 |
| 10 | 0.0 | 318.4 | 0.0 | 34.3 | |
| 25 | 0.0 | 179.0 | 0.0 | 85.1 | |
| 50 | 0.0 | 169.2 | 0.0 | 17.2 | |
| 75 | 0.0 | 151.4 | 0.0 | 3.4 | |
| 100 | 0.0 | 18.5 | 0.0 | 3.2 | |
| 24 H | 0 | 9.1 | 420.5 | 0.9 | 59.2 |
| 10 | 0.5 | 188.7 | 0.1 | 18.9 | |
| 25 | 0.0 | 154.3 | 0.0 | 19.2 | |
| 50 | 0.0 | 190.9 | 0.0 | 33.1 | |
| 75 | 0.0 | 55.0 | 0.0 | 5.5 | |
| 100 | 0.0 | 105.4 | 0.1 | 11.3 | |
| 72 H | 0 | 4.1 | 314.6 | 0.4 | 37.3 |
| 10 | 5.2 | 335.8 | 0.0 | 34.1 | |
| 25 | 0.5 | 242.3 | 0.0 | 25.7 | |
| 50 | 6.2 | 240.7 | 0.0 | 65.5 | |
| 75 | 0.0 | 158.3 | 0.0 | 50.2 | |
| 100 | 0.0 | 97.0 | 0.0 | 9.7 | |
| Exposure Time | Concentration | Distance Moved | Velocity | ||
|---|---|---|---|---|---|
| Min | Max | Min | Max | ||
| 1 H | 0 | 13.5 | 283.0 | 5.5 | 29.2 |
| 10 | 17.6 | 198.4 | 6.7 | 30.8 | |
| 25 | 12.1 | 205.8 | 4.9 | 22.2 | |
| 50 | 8.1 | 259.2 | 1.9 | 25.9 | |
| 75 | 15.1 | 169.9 | 6.2 | 24.9 | |
| 100 | 11.6 | 191.9 | 2.9 | 19.9 | |
| 24 H | 0 | 0.0 | 339.0 | 0.9 | 59.2 |
| 10 | 0.5 | 188.7 | 0.1 | 18.9 | |
| 25 | 6.6 | 180.5 | 0.0 | 19.2 | |
| 50 | 0.0 | 8 7.4 | 0.0 | 33.1 | |
| 75 | 0.5 | 88.8 | 0.0 | 5.5 | |
| 100 | 0.0 | 103.2 | 0.1 | 11.3 | |
| 72 H | 0 | 52.4 | 272.2 | 12.4 | 30.2 |
| 10 | 0.5 | 233.8 | 0.7 | 25.7 | |
| 25 | 0.0 | 227.0 | 0.0 | 22.7 | |
| 50 | 0.0 | 258.1 | 0.0 | 25.8 | |
| 75 | 0.0 | 147.7 | 0.0 | 22.0 | |
| 100 | 0.0 | 174.7 | 0.0 | 17.5 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arthur, F.H.; Athanassiou, C.G.; Morrison, W.R., III. Mobility of Stored Product Beetles after Exposure to a Combination Insecticide Containing Deltamethrin, Methoprene, and a Piperonyl Butoxide Synergist Depends on Species, Concentration, and Exposure Time. Insects 2020, 11, 151. https://doi.org/10.3390/insects11030151
Arthur FH, Athanassiou CG, Morrison WR III. Mobility of Stored Product Beetles after Exposure to a Combination Insecticide Containing Deltamethrin, Methoprene, and a Piperonyl Butoxide Synergist Depends on Species, Concentration, and Exposure Time. Insects. 2020; 11(3):151. https://doi.org/10.3390/insects11030151
Chicago/Turabian StyleArthur, Frank H., Christos G. Athanassiou, and W. Robert Morrison, III. 2020. "Mobility of Stored Product Beetles after Exposure to a Combination Insecticide Containing Deltamethrin, Methoprene, and a Piperonyl Butoxide Synergist Depends on Species, Concentration, and Exposure Time" Insects 11, no. 3: 151. https://doi.org/10.3390/insects11030151
APA StyleArthur, F. H., Athanassiou, C. G., & Morrison, W. R., III. (2020). Mobility of Stored Product Beetles after Exposure to a Combination Insecticide Containing Deltamethrin, Methoprene, and a Piperonyl Butoxide Synergist Depends on Species, Concentration, and Exposure Time. Insects, 11(3), 151. https://doi.org/10.3390/insects11030151







